Abstract
Recent studies suggest that stress can amplify the harm of air pollution. We examined whether experience of racism and exposure to particulate matter with an aerodynamic diameter of less than 2.5 µm and 10 µm (PM2.5 and PM10) had a synergistic influence on ethnic differences in asthma and lung function across adolescence. Analyses using multilevel models showed lower forced expiratory volume (FEV1), forced vital capacity (FVC) and lower rates of asthma among some ethnic minorities compared to Whites, but higher exposure to PM2.5, PM10 and racism. Racism appeared to amplify the relationship between asthma and air pollution for all ethnic groups, but did not explain ethnic differences in respiratory health.
Keywords: Ethnicity, air pollution, Racism, Lung function
1. Introduction
The global burden of asthma is rising (Asher et al., 2006), with 4.2 million adults and 1.1 million children in the United Kingdom (UK) alone costing over £2.3 billion a year to treat (Asthma, 2011). In the UK (Whitrow and Harding, 2008), ethnic minorities have lower lung function, which is an early predictor of cardiovascular mortality. Asthma is less commonly reported among ethnic minorities than Whites in the UK (Netuveli et al., 2005; Whitrow and Harding, 2010). While some of the determinants are known (Dezateux and Stocks, 1997), ethnic differences in respiratory health remain unexplained.
Environmental factors, such as air pollution (Pope, 2000; Samet et al., 2000), may play a key role in determining ethnic differences in respiratory health (Clougherty and Kubzansky, 2009; Gee and Payne-Sturges, 2004). Some ethnic minority adolescents in the UK are more likely than their White peers to reside in deprived neighbourhoods (Astell-Burt et al., 2012), which experience higher levels of air pollution as measured by a number of different indicators (Wheeler and Ben-Shlomo, 2005). There is increasing evidence that air pollution has a harmful impact, in particular, on lung function development during childhood. Studies have reported negative association between Forced Expiratory Volume in the first second (FEV1) and Forced Vital Capacity (FVC) with pollutants (Rojas-Martinez et al., 2007), especially particulate matter with an aerodynamic diameter of less than 2.5 µm (PM2.5) (Gauderman et al., 2004) and particulate matter with an aerodynamic diameter of less than 10 µm (PM10) (Kulkarni et al., 2006). There is also increasing evidence for positive association between these pollutants and asthma (Anderson et al., 2011). However few studies have examined the role of air pollution on ethnic differences in respiratory health in childhood.
Psychosocial stress may also contribute to ethnic differences in respiratory health either as a direct cause or via enhancing neuroimmune and hypersensitivity response to air pollution (O’Neill et al., 2003). For example, stress related to low socioeconomic circumstances has been reported to increase the risk of asthma (Chen et al., 2006). Studies have also found a higher risk of asthma and lower lung function associated with traffic-related air pollution among children reporting stress in the home environment (Islam et al., 2011; Shankardass et al., 2009) and exposure to violence or domestic verbal abuse (Clougherty et al., 2007). Racism, more commonly experienced by ethnic minority than majority groups and a significant psychosocial stressor regardless of ethnicity (Astell-Burt et al., 2012; Krieger and Sidney, 1996), may have a similar modifying influence on pollution driven ethnic differences in respiratory health (Clougherty and Kubzansky, 2009; Gee and Payne-Sturges, 2004).
While some studies in the UK (Netuveli et al., 2005; Whitrow and Harding, 2008, 2010) have reported ethnic differences in asthma and lung function, and an extensive literature concerning adverse associations between respiratory health and air pollution has developed (Anderson et al., 2011; Dezateux and Stocks, 1997; Gauderman et al., 2004; McConnell et al., 2006; Pope, 2000; Samet et al., 2000), very few studies have investigated the role of air pollution on ethnic differences in lung function and asthma, nor has this been extended to examine the potentially modifying role of psychosocial stress. Using a UK-based multiethnic cohort study of adolescents, we investigate the influence of air pollution on ethnic differences in lung function and asthma and the extent to which racism modifies these associations.
2. Methods
2.1. Data and sampling
The Determinants of Adolescent Social wellbeing and Health (DASH) study received approval from the Multicentre Research Ethics Committee and local education authorities (Harding et al., 2007). Pupils (N=6645) aged 11–13 years old were recruited at Wave 1 in 2003/04 from 52 schools that spanned above and below the national average for academic performance and contained at least 5% of pupils of Black Caribbean ethnicity according to the Department for Education (known then as the ‘Department of Education and Skills’). These schools were located within 10 of London′s 32 boroughs known to have at least 5% of ethnic minority groups. These boroughs were Brent, Croydon, Hackney, Hammersmith & Fulham, Haringey, Lambeth, Newham, Southwark, Waltham Forest and Wandsworth (see Fig. 1).
Fig. 1.
Map of the London boroughs (shaded in grey and labelled) in which schools were sampled for the Determinants of Adolescent Social wellbeing and Health study.
A self-complete questionnaire was administered in classrooms asking a range of questions on socioeconomic circumstances, health status, lifestyles and parenting. A substantive part of the survey was attributed to the identification of ethnic identity, which facilitated the cross-checking of participants′ responses against parental ethnicity and the parental and grandparental countries of birth. The Wave 1 response rate was 83%, with generally better response rates for ethnic minority pupils than White UK pupils. In the Wave 2 survey during 2005 and 2006, 4775 adolescents aged 14–16 years participated (approximately 72% of the Wave 1 sample). The main source of attrition was due to non-participation by two schools because of construction work. The Wave 2 survey repeated the majority of self-reported questions from Wave 1, though physical measures did not include lung function. Survey assistants were trained for a week prior to the survey and recertified at regular intervals. DASH fieldwork protocols can be found at http://dash.sphsu.mrc.ac.uk/. Anthropometry was measured according to World Health Organisation recommendations (Report of a WHO Expert Committee, 1995) and lung function according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (Miller et al. 2005), including training by respiratory physiologists, daily checks and regular calibration of spirometers, and at least 3 satisfactory attempts by pupils.
The longitudinal sample used in this study included White UK (n=873), Black Caribbean (778), Nigerian and Ghanaian (504), Other African (386), Indian (419), Pakistani and Bangladeshi (446), and mixed White/Black Caribbean (262) boys and girls. Adolescents identifying with an ethnic group numerically too small for individual analysis were aggregated into an ‘Other’ category (n=1107). Pupils classified as ‘Other’ included those of several ethnic groups which makes interpretation difficult. We report the results for this group in the text rather than in the tables. Missing data for independent variables was resolved via single imputation of the ethnic- and gender-group specific mean.
2.2. Dependent variables: asthma and lung function
Affirmative asthma status (‘asthma’ hereafter) was identified among adolescents self-reporting to the questions “have you ever had asthma?”, and “in the last month, have you had breathing difficulties or wheeze?”, in line with guidelines set out by the International Study of Asthma and Allergies in Childhood (ISAAC) (Asher et al., 2006). Each of these questions on asthma was asked at 11–13 yr and 14–16 yr.
Forced Expiratory Volume in one second (FEV1) and Forced Vital Capacity (FVC) were measured at 11–13 yr only. FEV1 and FVC are sensitive indices of lung growth, and strongly related to anthropometry (height, sitting height and chest shape) and age. FEV1 is the volume exhaled during the first second of a forced expiratory maneuver started from the level of total lung capacity. FEV1 is by far the most frequently used index for assessing airway obstruction, bronchoconstriction or bronchodilatation. FVC is the volume change of the lung between a full inspiration to total lung capacity and a maximal expiration to residual volume.
The best FEV1 and FVC for each child were selected for analysis, according to ATS/ERS guidelines. Repeatability of the measures was assured by specifying that the difference between the largest and next largest FEV1 and FVC be less than 0.15 l (L); or less than 0.1 L if the FVC is less than 1 L. Of 4775 children at Wave 1, 3768 had a satisfactory measure of FEV1 and 3097 for FVC. We found no ethnic differences in satisfactory measures of FEV1. Compared to Whites, Black Caribbeans (odds ratio (OR): 1.27, (95% confidence interval (95%CI) 1.04, 1.56), Nigerians and Ghanaians (OR: 1.56, 95%CI 1.24, 1.95) and Other Africans (OR: 1.33, 95%CI 1.04, 1.71) were significantly more likely to have unsatisfactory measures of FVC. For the statistical analyses involving lung function measures as the dependent variables, pupils with missing FEV1 or FVC data were omitted. Adolescents with cystic fibrosis (n=8) were also omitted from the analysis.
2.3. Independent variables
Our main focus of investigation was the level of objectively-measured air pollution within urban neighbourhoods of residence. Pupils′ residential addresses were obtained at the time of each survey and coded to Output Areas (OA) using look-up tables provided by
EDINA UKBORDERS, with the support of the Economic and Social Research Council (ESRC) and the Joint Information Systems Committee (JISC), and using boundary material which is copyright of the Crown and the Post Office. OA boundaries (approximately 297 residents on average) were used as proxies for neighbourhoods at 11–13y and 14–16y. Measures of annual mean air pollution per km2, including PM2.5 and PM10, were provided by AEA Technology for 2003, 2004, 2005 and 2006 (http://www.aeat.co.uk/cms/). PM2.5 and PM10 were measured in micrograms per cubic metre (µg/m3). Measures were averaged across 2003/04 (11–13 yr) and 2005/06 (14–16 yr) and point estimates were linked to the centroids of OAs via a Geographic Information System (GIS). As with residential addresses at time of the surveys, school addresses were linked to OAs and assigned air pollution levels via GIS. Given the substantial time spent in school, pollution levels associated with school addresses were also examined. All pollution measures were derived as quartiles for the analyses.
Experiences of interpersonal racism were used as an indicator of psychosocial stress. These experiences were assessed with the question: “Has anyone made you feel bad or hassled you because of your race, skin colour or where you were born?” (Krieger and Sidney, 1996). Adolescents responded ‘yes’ or ‘no’. Previous work has shown this variable to be strongly negatively correlated with psychological wellbeing (Astell-Burt et al., 2012). Interpersonal racism refers to interactions between people that are perceived to be discriminatory with respect to ethnicity (Paradies, 2006). This measure does not directly cover other levels of racism that could also be important, such as institutional racism (that which occurs through laws, policies and practices), or internalised racism (when people hold prejudicial views concerning particular ethnic groups).
Neighbourhood deprivation was measured by the Carstairs index (Carstairs and Morris, 1989), calculated for residential OAs using four variables from the 2001 census (http://www.neighbourhood.statistics.gov.uk): (a) percentage car ownership; (b) percentage male unemployed; (c) percentage homeownership; (d) percentage low social class. Higher scores on the Carstairs index denote more deprived neighbourhoods.
Other key variables included age, sex, ambient (i.e. indoor) air temperature in the school room where anthropometric measurements took place, upper body segment (i.e. the ‘trunk’ of the body, measured by subtracting the pupil′s sitting height from the height of the seat), pubertal stage, Body Mass Index (BMI), place of birth, socioeconomic circumstances (standard of living, parental employment, household overcrowding), individual and household smoking status, family type and (grand)parental asthma status. To take account for the contribution of anthropometric differences between ethnic groups, we used upper body segment. Our previous work has shown that this measure explains more of variation in lung function than standing height (Whitrow and Harding, 2008). Details of these independent variables have been published elsewhere (Harding et al., 2007; Whitrow and Harding, 2008, 2010).
2.4. Statistical methods
Ethnic group-specific means of asthma, lung function, air pollution and experience of racism were measured. Pearson coefficients were used to investigate the correlation between air pollution, neighbourhood deprivation, and other variables (e.g. upper body segment).
For lung function analyses, linear regression models were fitted on Wave 1 data to investigate association with FEV1 and FVC separately. Mixed models estimated with Generalised Estimating Equations (Liang and Zeger, 1986) were used to account for clustering of individuals within schools. The natural logarithms of FEV1, FVC, upper body segment and age were used, as recommended (Chinn and Rona, 1992). Mean lung function (in L) is presented for Whites, with the percentage difference from Whites reported for other ethnic groups.
Multivariate logistic regression models were used to examine association between independent variables and the prevalence of each asthma status. Mixed models were used to take into account clustering from repeated measures of asthma status and independent variables at 11–13 yr and 14–16 yr. Preliminary analyses showed no significant clustering of asthma within schools or OAs.
Ethnic differences in each of the dependent variables (asthma status, FEV1 and FVC) were investigated separately, adjusting for sex and age, plus room temperature, upper body segment and pubertal stage for FEV1 and FVC (hereafter referred to as ‘baseline’ models). Each air pollution measure was added to baseline separately. Each model was then adjusted, first for neighbourhood deprivation, then by experience of racism and all remaining independent variables. Interactions between air pollution and experience of racism were investigated. Analyses were re-run using measures of air pollution within areas in the school OAs to test the sensitivity of exposure in different environments. Models were run in STATA V.11.
3. Results
Table 1 shows ethnic specific distributions of lung function, asthma and key independent variables. Ethnic minority groups lived in more deprived, polluted neighbourhoods and were more likely to experience racism compared to Whites. Compared to Whites, FEV1 and FVC were significantly lower among all ethnic minority groups (including the ‘Other’ group), except for FVC among mixed White/Black Caribbeans. FEV1 and FVC were lower for Indians than all other ethnic groups. At age 11–13 yr, asthma prevalence was significantly lower among Nigerians and Ghanaians compared to Whites. This was also the case for the ‘Other’ group (31.3%, 95%CI 28.6, 34.0). By 14–16 yr, asthma prevalence was lower than Whites among ethnic minority groups, except for Black Caribbean and mixed White/Black Caribbean. Asthma prevalence increased between 11–13 yr and 14–16 yr across all ethnic groups, except for Indians.
Table 1.
Ethnic differences in respiratory health and key explanatory variables.
| White | Black Caribbean | Nigerian and Ghanaian | Other African | Indian | Pakistani and Bangladeshi | Mixed White / Black Caribbean | ||
|---|---|---|---|---|---|---|---|---|
| N | Age | 873 | 778 | 504 | 386 | 419 | 446 | 262 |
| Mean or percentage (95%CI) | ||||||||
| FEV1 (L)a | 11–13 | 2.56 (2.52, 2.60) | 2.35 (2.31, 2.39)⁎ | 2.28 (2.23, 2.32)⁎ | 2.31 (2.25, 2.36)⁎ | 2.15 (2.10, 2.20)⁎ | 2.32 (2.27, 2.37)⁎ | 2.46 (2.40, 2.53)⁎ |
| FVC (L)a | 11–13 | 2.90 (2.85, 2.94) | 2.68 (2.63, 2.73)⁎ | 2.62 (2.56, 2.68)⁎ | 2.54 (2.47, 2.60)⁎ | 2.41 (2.35, 2.47)⁎ | 2.59 (2.53, 2.65)⁎ | 2.81 (2.73, 2.89) |
| Asthmab | 11–13 | 36.1 (33.0, 39.3) | 38.6 (35.2, 42.0) | 29.6 (25.7, 33.7)⁎ | 33.9 (29.4, 38.8) | 35.8 (31.3, 40.5) | 35.4 (31.1, 40.0) | 39.7 (33.9, 45.7) |
| 14–16 | 47.9 (44.6, 51.2)† | 48.3 (44.8, 51.8)† | 39.3 (35.1, 43.6)⁎† | 38.6 (33.9, 43.6)⁎† | 35.8 (31.3, 40.5)⁎ | 40.6 (36.1, 45.2)⁎† | 54.6 (48.5, 60.5)† | |
| PM2.5 (µg/m3)a | 11–13 | 14.3 (14.3, 14.3) | 14.5 (14.4, 14.5)⁎ | 14.6 (14.6, 14.6)⁎ | 14.6 (14.5, 14.6)⁎ | 14.4 (14.3, 14.4)⁎ | 14.5 (14.5, 14.5)⁎ | 14.5 (14.4, 14.6)⁎ |
| 14–16 | 20.1 (20.0, 20.2)† | 20.6 (20.5, 20.7)⁎† | 21.0 (20.9, 21.1)⁎† | 20.9 (20.8, 21.1)⁎† | 20.3 (20.2, 20.5)⁎† | 20.8 (20.6, 20.9)⁎† | 20.7 (20.6, 20.9)⁎† | |
| PM10 (µg/m3)a | 11–13 | 24.6 (24.5, 24.6) | 25.0 (24.9, 25.0)⁎ | 25.2 (25.1, 25.3)⁎ | 25.2 (25.1, 25.2)⁎ | 24.8 (24.7, 24.8)⁎ | 25.0 (24.9, 25.1)⁎ | 25.0 (24.9, 25.1)⁎ |
| 14–16 | 27.3 (27.2, 27.4)† | 27.9 (27.8, 28.0)⁎† | 28.3 (28.2, 28.4)⁎† | 28.3 (28.1, 28.4)⁎† | 27.7 (27.6, 27.8)⁎† | 28.2 (28.0, 28.3)⁎† | 28.0 (27.9, 28.2)⁎† | |
| N’hood Deprivation a | 11–13 | 1.77 (1.57, 1.98) | 3.59 (3.37, 3.80)⁎ | 4.88 (4.62, 5.15)⁎ | 4.56 (4.25, 4.86)⁎ | 3.37 (3.07, 3.67)⁎ | 4.03 (3.75, 4.32)⁎ | 3.42 (3.05, 3.79)⁎ |
| 14–16 | 1.69 (1.48, 1.90)† | 3.44 (3.22, 3.66)⁎† | 4.48 (4.21, 4.75)⁎† | 4.24 (3.93, 4.55)⁎† | 3.15 (2.86, 3.45)⁎† | 4.04 (3.75, 4.33)⁎ | 3.38 (3.01, 3.76)⁎ | |
| Racism b | 11–13 | 13.3 (11.2, 15.7) | 15.9 (13.5, 18.7) | 19.4 (16.2, 23.1)⁎ | 17.4 (13.9, 21.5) | 19.3 (15.8, 23.4)⁎ | 27.1 (23.2, 31.4)⁎ | 22.5 (17.9, 28.0)⁎ |
| 14–16 | 18.6 (16.1, 21.3)† | 28.1 (25.1, 31.4)⁎† | 35.7 (31.6, 40.0)⁎† | 29.5 (25.2, 34.3)⁎† | 31.0 (26.8, 35.6)⁎† | 27.8 (23.8, 32.1)⁎ | 32.1 (26.7, 38.0)⁎† | |
P<0.05 (compared to White).
Mean.
Percentage; L=Litres; µg/m3=microgram per cubic metre; 95%CI=95% Confidence Interval. Particulate matter with an aerodynamic diameter of less than 2.5 µm, PM10: particulate matter with an aerodynamic diameter of less than 10 µm, FEV1: Forced Expiratory Volume in the first second, FVC: Forced Vital Capacity.
P<0.05 (compared to 11–13 yr).
In univariate analyses, FEV1 was negatively associated with PM2.5 (coefficient: −0.009, 95%CI: −0.016, −0.002), and PM10 (−0.010, −0.018, −0.003). Significant negative association was also found between FVC and PM10 (−0.008, −0.017, −0.001). Univariate analyses showed significant negative association between neighbourhood deprivation and FEV1 (−0.003, −0.006, −0.001) and FVC (−0.003, −0.006, −0.001).
3.1. Ethnic differences in lung function
Table 2 shows ethnic differences in FEV1 and FVC and the influence of adjusting for measures of air pollution, neighbourhood deprivation, and other key independent variables on these differences. In baseline models including sex, age, upper body segment, temperature and pubertal stage as covariates, ethnic minority groups had lower FEV1 and FVC compared to their White peers, with the largest decrease from Whites among Nigerians and Ghanaians and the smallest decrease among mixed White/Black Caribbeans.
Table 2.
Association between neighbourhood air pollution and deprivation measures with ethnic differences in FEV1 and FVC.
| White UKa | Black Caribbean | Nigerian and Ghanaian | Other African | Indian | Pakistani and Bangladeshi | Mixed White/Black Caribbean | R2 | |
|---|---|---|---|---|---|---|---|---|
| L (95%CI) | % Decrease from White (95%CI) | |||||||
| FEV1 | ||||||||
| Baseline | 2.45 (2.42, 2.49) | –6.1 (–6.2, –6.4)⁎ | –8.2 (–8.7, –8.0)⁎ | –6.5 (–6.6, –6.4)⁎ | –6.5 (–7.0, –6.4)⁎ | –4.1 (–4.5, –4.0)⁎ | –3.3 (–4.1, –2.8)⁎ | 0.57 |
| Baseline+PM2.5 | 2.45 (2.42, 2.49) | –6.1 (–6.2, –6.4)⁎ | –8.2 (–8.7, –8.0)⁎ | –6.1 (–6.6, –6.0)⁎ | –6.5 (–7.0, –6.4)⁎ | –4.1 (–4.5, –4.0)⁎ | –3.3 (–4.1, –2.8)⁎ | 0.57 |
| + N’hood Deprivation | 2.45 (2.42, 2.48) | –6.1 (–6.2, –6.0)⁎ | –8.2 (–8.7, –7.7)⁎ | –6.1 (–6.6, –5.6)⁎ | –6.5 (–7.0, –6.0)⁎ | –4.1 (–4.5, –3.6)⁎ | –3.3 (–4.1, –2.4)⁎ | 0.57 |
| + Individual and family characteristics | 2.45 (2.42, 2.48) | –5.7 (–5.8, –5.6)⁎ | –8.2 (–8.3, –7.7)⁎ | –6.5 (–6.6, –6.0)⁎ | –7.8 (–7.9, –7.3)⁎ | –4.9 (–5.4, –4.8)⁎ | –2.9 (–3.7, –2.4)⁎ | 0.59 |
| Baseline+PM10 | 2.45 (2.42, 2.48) | –6.1 (–6.2, –6.0)⁎ | –8.2 (–8.7, –7.7)⁎ | –6.1 (–6.6, –5.6)⁎ | –6.5 (–7.0, –6.0)⁎ | –4.1 (–4.5, –3.6)⁎ | –3.3 (–4.1, –2.4)⁎ | 0.57 |
| + N’hood Deprivation | 2.45 (2.42, 2.48) | –6.1 (–6.2, –6.0)⁎ | –8.2 (–8.3, –7.7)⁎ | –6.1 (–6.6, –5.6)⁎ | –6.5 (–7.0, –6.0)⁎ | –4.1 (–4.5, –3.6)⁎ | –3.3 (–4.1, –2.4)⁎ | 0.57 |
| + Individual and family characteristics | 2.45 (2.42, 2.48) | –5.7 (–5.8, –5.6)⁎ | –8.2 (–8.3, –7.7)⁎ | –6.5 (–6.6, –6.0)⁎ | –7.8 (–7.9, –7.3)⁎ | –4.9 (–5.4, –4.8)⁎ | –2.9 (–3.7, –2.4)⁎ | 0.59 |
| FVC | ||||||||
| Baseline | 2.78 (2.74, 2.81) | –5.8 (–5.5, –5.3)⁎ | –8.3 (–8.4, –7.5)⁎ | –7.9 (–8.4, –7.1)⁎ | –7.6 (–8.0, –6.8)⁎ | –6.5 (–6.6, –5.7)⁎ | –2.2 (–2.9, –1.1) | 0.57 |
| Baseline+PM2.5 | 2.77 (2.74, 2.81) | –5.4 (–5.5, –5.3)⁎ | –7.9 (–8.4, –7.5)⁎ | –7.6 (–8.0, –7.1)⁎ | –7.2 (–8.0, –6.8)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.8 (–2.9, –1.1) | 0.57 |
| + N’hood Deprivation | 2.77 (2.74, 2.81) | –5.4 (–5.5, –5.3)⁎ | –7.9 (–8.4, –7.5)⁎ | –7.2 (–8.0, –7.1)⁎ | –7.2 (–8.0, –6.8)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.8 (–2.9, –1.1) | 0.57 |
| + Individual and family characteristics | 2.77 (2.73, 2.81) | –5.1 (–5.1, –5.0)⁎ | –7.6 (–8.1, –7.1)⁎ | –7.2 (–7.7, –6.4)⁎ | –7.6 (–8.1, –7.1)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.4 (–2.2, –0.7) | 0.59 |
| Baseline+PM10 | 2.77 (2.74, 2.81) | –5.4 (–5.5, –5.3)⁎ | –7.9 (–8.4, –7.5)⁎ | –7.6 (–8.0, –7.1)⁎ | –7.2 (–8.0, –6.8)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.8 (–2.9, –1.1) | 0.57 |
| + N’hood Deprivation | 2.77 (2.74, 2.81) | –5.4 (–5.5, –5.3)⁎ | –7.9 (–8.4, –7.5)⁎ | –7.2 (–8.0, –7.1)⁎ | –7.2 (–8.0, –6.8)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.8 (–2.9, –1.1) | 0.57 |
| + Individual and family characteristics | 2.77 (2.73, 2.81) | –5.1 (–5.1, –5.0)⁎ | –7.6 (–8.1, –7.1)⁎ | –7.2 (–7.7, –6.8)⁎ | –7.6 (–8.1, –7.1)⁎ | –6.1 (–6.6, –5.7)⁎ | –1.4 (–2.2, –0.7) | 0.59 |
Baseline: ethnicity, sex, age (log), upper body segment (log), temperature, pubertal stage; individual and family characteristics: migrant generational status, tobacco exposure (self and parental), socioeconomic status (standard of living, parental employment, household overcrowding), asthma status (individual and (grand)parental)); PM2.5: particulate matter with an aerodynamic diameter of less than 2.5 µm, PM10: particulate matter with an aerodynamic diameter of less than 10 µm, FEV1: Forced Expiratory Volume in the first second, FVC: Forced Vital Capacity
P<0.05 (compared to White); L=Litres; 95%CI=95% Confidence Interval.
Reference group.
Adjusting baseline models in Table 2 for PM2.5 and PM10, and subsequently for neighbourhood deprivation, explained less than 1% of the ethnic minority – White difference in FEV1 or FVC for any group. Neither PM2.5, PM10 or neighbourhood deprivation was associated with FEV1 or FVC when added to baseline models. In line with previous findings (Whitrow and Harding, 2008), adjustment for individual measures of socioeconomic circumstances, migrant generational status, history of asthma and exposure to tobacco smoke increased the model fit for FEV1 and FVC by approximately 2%. Racism was not associated with FEV1 or FVC, and it did not influence the ethnic differences in lung function. Interactions of racism with ethnicity, and racism with either measure of air pollution were also not significant, suggesting no ethnic specific association between racism and FEV1 or FVC, and no amplification of the effect of air pollution.
3.2. Ethnic differences in asthma
In univariate analyses, increased risk of asthma was associated with a quartile increase in PM2.5 (odds ratio (OR): 1.35, 95%CI: 1.32, 1.38) and PM10 (1.35, 1.32, 1.38). Neighbourhood deprivation was not associated with asthma (1.00, 0.98, 1.01).
Table 3 shows ethnic differences in asthma adjusting for measures of air pollution, neighbourhood deprivation, and other key independent variables. Most ethnic minority groups had significantly lower prevalence of asthma in baseline models, including the ‘Other’ group (Odds Ratio: 0.74, 95%CI 0.64, 0.86). Exceptions to this pattern were notable for Black Caribbeans and Mixed White/Black Caribbeans.
Table 3.
Association between neighbourhood air pollution and deprivation measures with ethnic differences in asthma.
| White UKa | Black Caribbean | Nigerian and Ghanaian | Other African | Indian | Pakistani and Bangladeshi | Mixed White/Black Caribbean | |
|---|---|---|---|---|---|---|---|
| % (95%CI) | Odds Ratios compared to White (95%CI) | ||||||
| Baseline | 39.9 (36.9, 43.0) | 1.00 (0.83, 1.20) | 0.69 (0.55, 0.85)⁎ | 0.62 (0.49, 0.80)⁎ | 0.61 (0.48, 0.77)⁎ | 0.76 (0.61, 0.95)⁎ | 1.22 (0.94, 1.59) |
| Baseline+PM2.5 (quartiles) | 40.4 (37.4, 43.6) | 0.97 (0.80, 1.17) | 0.66 (0.53, 0.82)⁎ | 0.60 (0.47, 0.77)⁎ | 0.60 (0.47, 0.75)⁎ | 0.73 (0.58, 0.92)⁎ | 1.19 (0.91, 1.55) |
| + N’hood Deprivation | 40.3 (37.2, 43.4) | 0.98 (0.81, 1.18) | 0.66 (0.53, 0.83)⁎ | 0.61 (0.48, 0.78)⁎ | 0.60 (0.47, 0.76)⁎ | 0.74 (0.59, 0.93)⁎ | 1.20 (0.92, 1.56) |
| + Individual and family characteristics | 37.9 (34.8, 41.2) | 1.05 (0.86, 1.28) | 0.78 (0.62, 0.99)⁎ | 0.78 (0.60, 1.02) | 0.69 (0.54, 0.88)⁎ | 0.81 (0.64, 1.02) | 1.16 (0.89, 1.52) |
| Baseline+PM10 (quartiles) | 40.5 (37.4, 43.6) | 0.97 (0.80, 1.17) | 0.66 (0.53, 0.82)⁎ | 0.60 (0.47, 0.77)⁎ | 0.59 (0.47, 0.75)⁎ | 0.73 (0.58, 0.91)⁎ | 1.19 (0.91, 1.55) |
| + N’hood Deprivation | 40.4 (37.3, 43.5) | 0.97 (0.81, 1.18) | 0.66 (0.53, 0.83)⁎ | 0.61 (0.48, 0.78)⁎ | 0.60 (0.47, 0.75)⁎ | 0.73 (0.58, 0.92)⁎ | 1.20 (0.92, 1.56) |
| + Individual and family characteristics | 38.1 (34.9, 41.3) | 1.05 (0.86, 1.27) | 0.78 (0.62, 0.99)⁎ | 0.78 (0.60, 1.01)⁎ | 0.68 (0.54, 0.87)⁎ | 0.80 (0.63, 1.01) | 1.16 (0.89, 1.52) |
Baseline: ethnicity, sex, age.
Individual and family characteristics: migrant generational status, tobacco exposure (self and parental), socioeconomic status (standard of living, parental employment, household overcrowding), family history of asthma (parental and grandparental), BMI, family type; PM2.5: particulate matter with an aerodynamic diameter of less than 2.5 µm, PM10: particulate matter with an aerodynamic diameter of less than 10 µm.
P<0.05 (compared to White); 95%CI=95% Confidence Interval.
Reference group.
Adjusting baseline models for each measure of air pollution separately had little influence on the ethnic differences in asthma. In baseline+air pollution models, statistical associations remained for PM2.5 (1.16, 1.09, 1.24) and PM10 (1.11, 1.05, 1.17). Controlling for other independent variables partially attenuated the ethnic differences, though the positive association between asthma, PM2.5 and PM10 remained significant.
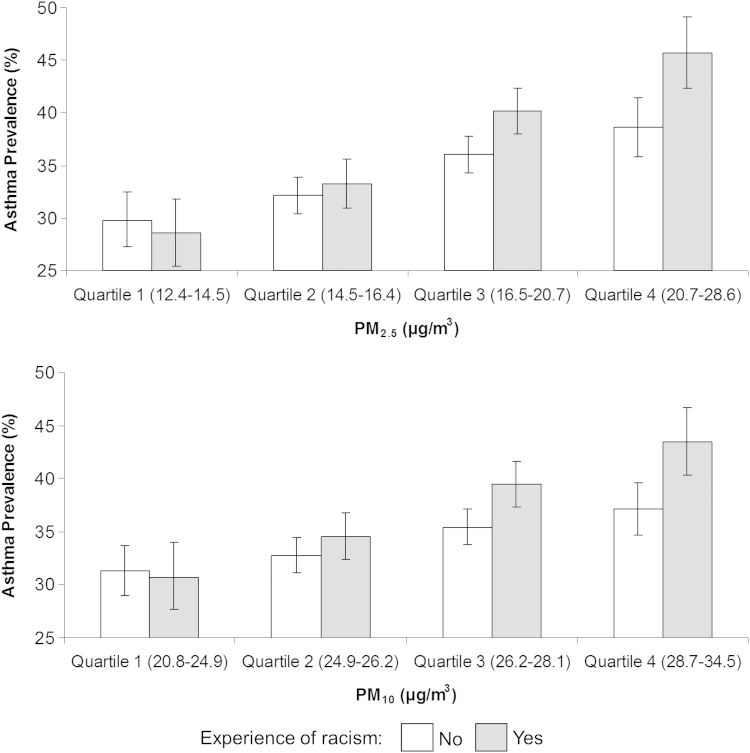
Racism was positively associated with asthma (1.16, 1.07, 1.26), though adjustment thereof did not substantially change the ethnic differences reported. No significant interaction between racism and ethnicity was observed, suggesting there was not an ethnic-specific influence of racism on the risk of having asthma. There were, however, significant interactions between racism and PM2.5 (p=0.002) and PM10 (p=0.012) on asthma. This meant that the risk of having asthma was significantly higher for adolescents exposed to both air pollution and racism, in comparison to those who were exposed to just one of these measures (Fig. 2). The interaction between racism and air pollution on asthma did not vary within ethnic groups.
Fig. 2.
Synergistic influence of racism and traffic pollution (PM2.5, PM10) on the prevalence of asthma. PM2.5: particulate matter with an aerodynamic diameter of less than 2.5 µm, PM10: particulate matter with an aerodynamic diameter of less than 10 µm, µg/m3: microgram per cubic metre, bars represent predicted probabilities, exponentiated and multiplied by 100, lines represent 95% Confidence Limits.
Re-fitting all models with measures of air pollution in areas around schools in substitution for those assessing the neighbourhood environment yielded similar results to those reported above.
4. Discussion
4.1. Key findings
This is the first study to systematically investigate the associations between objectively-measured urban air pollution and ethnic differences in respiratory health across adolescence. We found that neighbourhood levels of PM2.5 and PM10 were associated with both poorer lung function and higher asthma prevalence. However, while ethnic minorities tended to reside in areas with higher concentrations of each of these pollutants, this did not appear to contribute to their lower lung function. Furthermore, and somewhat paradoxically, some minority groups tended to have lower prevalence of asthma compared with their White UK counterparts, in spite of the more polluted environs in which they lived and attended school.
Racism, included as a marker of psychosocial stress and potential modifier of the association between air pollution and respiratory health, was not associated with lung function, but was positively associated with the risk of asthma. Importantly, we found the risk of asthma associated with PM2.5 and PM10 was exacerbated if adolescents reported they had experienced racism. This latter finding is in line with the ‘stress-exposure-disease’ framework (Clougherty and Kubzansky, 2009; Gee and Payne-Sturges, 2004) and joins a small, but growing number of US-based studies (Clougherty et al., 2007; Islam et al., 2011; Shankardass et al., 2009) reporting differential susceptibility in the association between air pollution and respiratory health via psychosocial stress.
4.2. Interpretation
We previously reported that ethnic minority adolescents tend to have lower lung function in comparison to their White peers, and that a substantial proportion of these differences are explained by anthropometry, shorter upper body segment in particular (Whitrow and Harding, 2008). In this study, we hypothesised that exposure to higher levels of PM2.5 and PM10 in neighbourhoods and areas around schools would provide further explanation for lower lung function among ethnic minorities. However, no supportive evidence of this hypothesis was procured, nor did negative associations between lung function and air pollution remain significant after controlling for anthropometric and socio-demographic measures, which was unexpected in light of the studies reporting independent negative influences of air pollution on FEV1 (Gauderman et al., 2004; Kulkarni et al., 2006; Rojas-Martinez et al., 2007). Each of these discrepancies may be due to the timing of our pollution measures in relation to lung function assessment. High levels of air pollution have been proposed to hinder the development of alveoli (which determines the FVC measure). The peak number alveoli is usually reached by age 10 (Gauderman et al., 2004), younger than the age of the DASH cohort. The identification of pollution effects on ethnic differences in lung function may also have been compromised by the availability of lung function data only at ages 11–13 yr (and not at younger ages); it is plausible that substantial physiological responses to air pollution might occur across time, as indicated by longitudinal studies spanning several years in duration (Gauderman et al., 2004).
Despite having longitudinal data on asthma status and a significant association with PM2.5 and PM10, controlling for local air pollution also had no more than a marginal influence on ethnic differences in the risk of having asthma. However, an interesting paradox was demonstrable in that while ethnic minorities were over-represented in more polluted local environs than their White peers, they also tended to have significantly lower asthma prevalence. Lower prevalence among South Asians has been previously reported (Netuveli et al., 2005) and the explanation for these and other ethnic differences may be entwined with issues such as community knowledge of respiratory health, and potentially also reduced access to culturally appropriate primary and specialist healthcare services (Whitrow and Harding, 2010). These issues could result in reporting bias, however, ethnic differences in other phenomena within households that we were unable to measure, such as wood burning fireplaces and pet ownership, may also play an important role. It is also plausible, though not possible to examine with our data, that the impact of pollution on respiratory health would be a function of the amount of time spent in the neighbourhood of residence. This is not only in reference to the duration of residence between household relocations, but also the day-to-day activities across a range of places that leads to varying levels of exposure. Further work using objective methods to track ethnic differences in activity spaces (e.g. via Global Positioning Systems) and levels of air pollution encountered would be a novel step towards enhancing exposure ascertainment in this regard.
Nevertheless, previous studies (entirely from the US) have also tended to report that ethnic differences in asthma risk persist even after controlling for measures of air pollution (Akinbami et al., 2005; Pearlman et al., 2006; Rosenbaum, 2008), though not in all cases (Aligne et al., 2000). Unlike our study, most of these have relied upon proxies for air pollution rather than objective measures. For example, Rosenbaum (Rosenbaum, 2008) examined the local presence of transportation facilities and manufacturing activity, whereas Akinbami et al., 2005 investigated the association for inner-city neighbourhood of residence. Results have been equivocal among studies focussing on neighbourhood deprivation, which is associated with various measures of air pollution (Wheeler and Ben-Shlomo, 2005) and could therefore also be considered a proxy. One study reported persisting ethnic differences in asthma after controlling for neighbourhood deprivation (Pearlman et al., 2006), whereas another found that deprivation (among other characteristics) explained the ethnic differences (Aligne et al., 2000). Critically, all of these proxy measures are unlikely to perfectly correlate with air pollution and some (e.g. deprivation) may also be considered sources of psychosocial stress (O’Neill et al., 2003). Thus our findings are in broad agreement, and the use of objective measures of air pollution in neighbourhood and school areas presents an important methodological extension.
Although ethnic minority groups are more likely to experience racial discrimination, this exposure has previously been shown to be detrimental to a range of health outcomes regardless of ethnic group status (Paradies, 2006). A limitation of the measure of racism used in our study was the potential for minimisation or recall bias, wherein experiences of racism are likely to be under-reported. Nevertheless, we found a consistent association between asthma and racism across all ethnic groups and a non-ethnic group-specific amplification of asthma risk for adolescents in more polluted environs having experienced racial discrimination. This is in line with and extends previous reports linking chronic stress with asthma symptoms (Oh et al., 2004; Wright et al., 2002), acute stress with asthma episodes (Sandberg et al., 2000, 2004), and other reports of association between low socioeconomic circumstances among adolescents (as a marker of psychosocial stress and a lack of control (Marmot, 2006)) with stress-linked immune mediators interleukin (IL)-5 and interferon-γ (Chen et al., 2003, 2006). According to Clougherty and Kubzansky, a potential explanation for this interaction follows that repeated exposure to psychosocial stress may weaken the body′s potential to regulate allostasis, resulting in ‘wear and tear’, diminished immune function, and increased sensitivity to environmental exposures such as air pollution (Clougherty and Kubzansky, 2009). The accumulation of these experiences over a sustained period of the early lifecourse may help to explain ethnic differences in health and wellbeing which persist in later adulthood (Astell-Burt et al., 2013; Feng et al., 2013). Although there have been reports of an increased rate of lung function decline among older people suffering psychological distress (Kubzansky et al., 2002), there are generally fewer studies which have examined this ‘stress-exposure-disease’ framework (Clougherty and Kubzansky, 2009; Gee and Payne-Sturges, 2004) in the context of lung function among adolescents compared to those concerned with asthma symptomology. Our study did not observe any significant association between racism and FEV1 or FVC, or modification of this association with air pollution, though data on lung function was limited to the Wave 1 survey only and it may be that the detection of such physiological responses require longer follow-up.
An important strength of this study was the prospective follow-up of a large multiethnic cohort of adolescents, with measures of individual socioeconomic and psychosocial factors. Respiratory statuses were measured in line with international standards (Asher et al., 2006; Miller et al., 2005). We were able to take into account change in asthma and air pollution measures across the study period and in multiple contexts in line with previous recommendations (Kulkarni and Grigg, 2008). This is important as not only can asthma and individual social circumstances change over time, but so can the level of PM2.5 and PM10 in the local environs (Fuller and Green, 2006). The use of objective measures of air pollution has also meant that our findings were not influenced by the issue of self-reported air pollution measures, known to exaggerate the relationship with respiratory health (Kuehni et al., 2006). In addition, we ran sensitivity analyses on the measurement of lung function using the new Global Lung Initiative (GLI) equations, which are based on multi-ethnic populations (Quanjer et al., 2012). Similar patterns of lower FEV1 and FVC relative to White pupils were found with no effect from pollution. Based on the GLI equation, the proportion of lung function decrement was greatest for Nigerian Ghanaians (FEV: z-score −1.63, 95%CI −1.74, −1.52) and smallest in Mixed White Black Caribbean (−0.98, −1.1,−0.84).
5. Conclusions
Despite ethnic minority adolescents living in neighbourhoods and attending schools within areas containing higher levels of local PM2.5 and PM10 than their White contemporaries, ethnic differences in respiratory health persisted largely unabated by ethnic differences in local air pollution. However, the amplification of pollution effects on asthma via psychosocial stress suggests that interventions aiming to eliminate discrimination might have benefits for ameliorating a wider range of health problems than initially supposed (i.e. not just mental health). This would suggest that the reasons for addressing the causes of discrimination are not limited only towards resolving societal and economic injustice, but as part of a multi-dimensional strategy towards tackling health inequalities across the lifecourse.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Akinbami L.J., Rhodes J.C., Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- Aligne C.A., Auinger P., Byrd R.S., Weitzman M. Risk factors for pediatric asthma. Contributions of poverty, race, and urban residence. American Journal of Respiratory and Critical Care Medicine. 2000;162:873–877. doi: 10.1164/ajrccm.162.3.9908085. [DOI] [PubMed] [Google Scholar]
- Anderson H.R., Favarato G., Atkinson R.W. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Quality, Atmosphere and Health. 2011:1–10. [Google Scholar]
- Asher M.I., Montefort S., Björkstén B., Lai C.K.W., Strachan D.P., Weiland S.K., Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. The Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Astell-Burt T., Feng X., Croteau K.K., G.S. Influence of neighbourhood ethnic density, diet and physical activity on ethnic differences in weight status: a study of 214,807 adults in Australia. Social Science and Medicine. 2013 doi: 10.1016/j.socscimed.2013.06.006. (in press) [DOI] [PubMed] [Google Scholar]
- Astell-Burt T., Maynard M.J., Lenguerrand E., Harding S. Racism, ethnic density and psychological well-being through adolescence: evidence from the Determinants of Adolescent Social well-being and Health longitudinal study. Ethnicity and Health. 2012;17:71–87. doi: 10.1080/13557858.2011.645153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthma UK Where Do We Stand? Asthma in the UK Today. Asthma UK. 2011 [Google Scholar]
- Carstairs V., Morris R. Deprivation and mortality: an alternative to social class? Journal of Public Health. 1989;11:210–219. doi: 10.1093/oxfordjournals.pubmed.a042469. [DOI] [PubMed] [Google Scholar]
- Chen E., Fisher E.B., Bacharier L.B., Strunk R.C. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- Chen E., Hanson M.D., Paterson L.Q., Griffin M.J., Walker H.A., Miller G.E. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chinn S., Rona R. Height and age adjustment for cross sectional studies of lung function in children aged 6–11 years. Thorax. 1992;47:707–714. doi: 10.1136/thx.47.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J.E., Kubzansky L.D. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environmental Health Perspectives. 2009;117:1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J.E., Levy J.I., Kubzansky L.D., Ryan P.B., Suglia S.F., Canner M.J., Wright R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental Health Perspectives. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezateux C., Stocks J. Lung development and early origins of childhood respiratory illness. British Medical Bulletin. 1997;53:40–57. doi: 10.1093/oxfordjournals.bmb.a011605. [DOI] [PubMed] [Google Scholar]
- Feng X., Astell-Burt T., Kolt G.S. Ethnic density, social interactions and psychological distress: evidence from 226,487 Australian adults. BMJ Open. 2013;3:e002713. doi: 10.1136/bmjopen-2013-002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G.W., Green D. Evidence for increasing concentrations of primary PM10 in London. Atmospheric Environment. 2006;40:6134–6145. [Google Scholar]
- Gauderman W.J., Avol E., Gilliland F., Vora H., Thomas D., Berhane K., McConnell R., Kuenzli N., Lurmann F., Rappaport E. The effect of air pollution on lung development from 10 to 18 years of age. New England Journal of Medicine. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Gee G.C., Payne-Sturges D.C. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental Health Perspectives. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S., Whitrow M., Maynard M.J., Teyhan A. Cohort profile: the DASH (Determinants of Adolescent Social well-being and Health) study, an ethnically diverse cohort. International Journal of Epidemiology. 2007;36:512–517. doi: 10.1093/ije/dym094. [DOI] [PubMed] [Google Scholar]
- Islam T., Urman R., Gauderman W.J., Milam J., Lurmann F., Shankardass K., Avol E., Gilliland F., McConnell R. Parental stress increases the detrimental effect of traffic exposure on children′s lung function. American Journal of Respiratory and Critical Care Medicine. 2011;184:822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N., Sidney S. Racial discrimination and blood pressure: the CARDIA study of young black and white adults. American Journal of Public Health. 1996;86:1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky L.D., Wright R.J., Cohen S., Weiss S.T., Rosner B., Sparrow D. Breathing easy: a prospective study of optimism and pulmonary function in the Normative Aging Study. Annals of Behavioral Medicine. 2002;24:345–353. doi: 10.1207/S15324796ABM2404_11. [DOI] [PubMed] [Google Scholar]
- Kuehni C.E., Strippoli M.P.F., Zwahlen M., Silverman M. Association between reported exposure to road traffic and respiratory symptoms in children: evidence of bias. International Journal of Epidemiology. 2006;35:779–786. doi: 10.1093/ije/dyl022. [DOI] [PubMed] [Google Scholar]
- Kulkarni N., Grigg J. Effect of air pollution on children. Paediatrics and Child Health. 2008;18:238–243. [Google Scholar]
- Kulkarni N., Pierse N., Rushton L., Grigg J. Carbon in airway macrophages and lung function in children. New England Journal of Medicine. 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Marmot M.G. Status Syndrome. JAMA: The journal of the American Medical Association. 2006;295:1304–1307. doi: 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- McConnell R., Berhane K., Yao L., Jerrett M., Lurmann F., Gilliland F., Künzli N., Gauderman J. Traffic, susceptibility, and childhood asthma. Environmental Health Perspectives. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., van der Grinten C.P.M., Gustafsson P. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Netuveli G., Hurwitz B., Levy M., Fletcher M., Barnes G., Durham S.R., Sheikh A. Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005;365:312–317. doi: 10.1016/S0140-6736(05)17785-X. [DOI] [PubMed] [Google Scholar]
- O’Neill M.S., Jerrett M., Kawachi I., Levy J.I., Cohen A.J., Gouveia N., Wilkinson P., Fletcher T., Cifuentes L.J.S. Health, wealth, and air pollution: advancing theory and methods. Environmental Health Perspectives. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y.-M., Kim Y.S., Yoo S.H., Kim S.K., Kim D.S. Association between stress and asthma symptoms: a population-based study. Respirology. 2004;9:363–368. doi: 10.1111/j.1440-1843.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Pearlman D.N., Zierler S., Meersman S., Kim H.K., Viner-Brown S.I., Caron C. Race disparities in childhood asthma: does where you live matter? Journal of the National Medical Association. 2006;98:239–247. [PMC free article] [PubMed] [Google Scholar]
- Pope C.A. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who′s at risk? Environmental Health Perspectives. 2000;108:713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer P.H., Stanojevic S., Cole T.J., Baur X., Hall G.L., Culver B.H., Enright P.L., Hankinson J.L., Ip M.S., Zheng J. Multi-ethnic reference values for spirometry for the 3–95yr age range: the global lung function 2012 equations. European Respiratory Journal. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Report of a WHO Expert Committee, 1995. Physical Status: The Uses and Interpretation of Anthropometry. WHO Technical Report Series 854. World Health Organisation, Geneva. [PubMed]
- Rojas-Martinez R., Perez-Padilla R., Olaiz-Fernandez G., Mendoza-Alvarado L., Moreno-Macias H., Fortoul T., McDonnell W., Loomis D., Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. American Journal of Respiratory and Critical Care Medicine. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- Rosenbaum E. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. Journal of Health and Social Behavior. 2008;49:131–145. doi: 10.1177/002214650804900202. [DOI] [PubMed] [Google Scholar]
- Samet J.M., Dominici F., Curriero F.C., Coursac I., Zeger S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. The New England Journal of Medicine. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Sandberg S., Jarvenpaa S., Pettinen A., Paton J., McCann D. Asthma exacerbations in children immediately following stressful life events: a Cox′s hierarchical regression. Thorax. 2004;59:1046–1051. doi: 10.1136/thx.2004.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg S., Paton J., Ahola S., McCann D., McGuinness D., Hillary C.R., Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356:982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- Shankardass K., McConnell R., Jerrett M., Milam J., Richardson J., Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences. 2009;106:1240–1246. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler B.W., Ben-Shlomo Y. Environmental equity, air quality, socioeconomic status, and respiratory health: a linkage analysis of routine data from the Health Survey for England. Journal of Epidemiology and Community Health. 2005;59:948–954. doi: 10.1136/jech.2005.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitrow M.J., Harding S. Ethnic differences in adolescent lung function: anthropometric, socioeconomic, and psychosocial factors. American Journal of Respiratory and Critical Care Medicine. 2008;177:1262–1267. doi: 10.1164/rccm.200706-867OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitrow M.J., Harding S. Asthma in Black African, Black Caribbean and South Asian adolescents in the MRC DASH study: a cross sectional analysis. BMC Pediatrics. 2010;10:18. doi: 10.1186/1471-2431-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R.J., Cohen S., Carey V., Weiss S.T., Gold D.R. Parental stress as a predictor of wheezing in infancy. A prospective birth-cohort study. American Journal of Respiratory and Critical Care Medicine. 2002;165:358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]