Abstract
The effects of environmental tobacco smoke (ETS) are less studied especially on neonates. This study evaluates the clinical and biochemical effects in neonates exposed to ETS during pregnancy. Two hundred pregnant women asked to complete the questioners about their ETS. Ninety from them were enrolled in biochemical assays as two groups according to ETS. The cotinine level determined in saliva and serum of mothers to confirm their tobacco exposure. The routine tracheal suction from the fetus was used to determine the level of neuron specific enolase (NSE), soluble E-cadherin, sApo-1/Fas, nitric oxide (NO) and cotinine. In clinical assessment, the percent of full term babies in non-exposed group (72 %) are higher compared to exposed group (67 %). Apgar score at the first min, admission to intensive care unit (ICU) and morbidity during the first month shows statistical significance increase in exposed compared to non-exposed group (p = 0.03, 0.05, 0.01, respectively). The new born weight in exposed group significantly decreased compared to non-exposed group (2,850 g ± 3.74 vs 2,967.67 g ± 3.34; p = 0.02). In biochemical assessment, NSE and sE-cadherin significantly increased, while NO significantly decreased (p = 0.000) in exposed compared to non-exposed group. There is a positive correlation between level of cotinine and both NSE, sE-cadherin (r = 0.7, 0.9; p = 0.000, 0.006, respectively). To our knowledge, this is the first study link between prenatal tobacco exposure (PTE) and biochemical parameters measured in tracheal suction. PTE will lead to decrease in birth weight most probably by decreasing NO, sFas, and increasing sE-cadherin. While, increased morbidity of neonates in the exposed group could be attributed to cessation of breast feeding and its complication and increased NSE in the studied markers.
Keywords: Neonate, Prenatal tobacco exposure, NSE, sFas, Cotinine, NO, Tracheal suction
Introduction
Passive smoking is the inhalation of smoke from tobacco products used by others. Also, called secondhand smoke (SHS) or environmental tobacco smoke (ETS). SHS has been less well studied and is less consistent in the literature as regard adverse pregnancy outcomes. Moreover, smoking is the most preventable risk for poor pregnancy outcomes. Prenatal tobacco exposure (PTE) defined as any exposure across pregnancy and, or exposure within each trimester. PTE was a significant predictor of increased activity and attention problems in child behavior [1]. Cotinine levels during pregnancy may predict birth weight [2], head circumference [3] and IQ scores [4]. Whereas, reduction in its level associated with improved outcomes. Cotinine concentrations in plasma and urine showed a significant dose-dependent difference among non-smokers, passive and active smokers [5].
Neuron specific enolase (NSE) is a marker of early cellular brain damage [6]. The cut-off threshold for salivary NSE of 3.7 mg/l was optimum, for differentiation of normal from abnormal individuals with 80 % accuracy [7]. Increased sFas serum concentration in prenatal and early postnatal, possibly indicates gradual decrease of proliferation and apoptosis in early postnatal life [8, 9]. The maternal nicotine exposure during last trimester of pregnancy alters a key component of lung innate immunity in offspring [10]. Specific cell adhesion molecules are important to lung development and airway morphogenesis. E-cadherin protein level was significantly upregulated in congenital lung lesions with abnormal airway branching [11]. Whereas, reduced production of the vasodilator nitric oxide (NO) in fetal vessels in pregnant smokers may lower the blood flow to the fetus and result in lower birth weight, length, and head circumference [12].
Hence, the aim of this study is to determine the cotinine level, NSE, soluble human epithelial cadherin (sE-cadherin), sApo-1/Fas and NO in tracheal suction of exposed and non-exposed neonate to PTE just after delivery as it could reflect the internal environment of the fetus. Also, to explain some possible mechanisms of adverse effects of PTE.
Subjects and Methods
This study carried out among primiparous women in Maternal Health Center of Assiut University Hospital in Assiut city. It is prospective, cross sectional, hospital based study. Women with mal position baby, or ante-partum hemorrhage were excluded. At the start of the work, 200 pregnant women with a singleton pregnancy and gestational age more than 28 weeks were asked to complete the questionnaires (maternal sheet) by the help of well-trained nurse, also, another sheet after delivery to determine the morbidity of the fetus (neonatal assessment sheet). From those, only 90 women with their infants were submitted to biochemical analysis. The women were divided into two groups according to exposure to ETS each group includes 45 women. Apgar score at 1 and 5 min and assessment observation sheet used to determine the fetal condition after birth and 1 month later. Before labor, two samples from the mother (urine by catheter and saliva) obtained to confirm exposure or non-exposure to ETS and one sample from newborn as routine tracheal suction to measure specific biochemical markers. Suctions contained meconium were excluded. Ethical approval for the study was obtained from the Institutional Ethics Committee and Review Board of the Faculty of Medicine, Assiut University. Informed and written consent was obtained from all participants.
Biochemical Assays
Tracheal suction obtained from all new born just after delivery for determination of specific biochemical markers. NSE (EC 4.2.1.11) determined by two monoclonal antibodies using EIA kit (Sweden). Quantitative sandwich enzyme immunoassay technique used for determination of sE-cadherin and sAPO-1/Fas by commercial kits (USA). Serum level of NO measured by colorimetric method as total nitrite concentration [13]. Cotinine measured by chemical method according to Peach et al. [14]. Briefly, 0.5 ml of sample pipetted into small test tube with 4 M acetate buffer (0.2 ml), pH 4.7. Then, the mixture reacted by the sequential addition at 15 s intervals of freshly prepared 10 % aqueous KCN (0.1 ml), 10 % aqueous chloramine-T (0.l ml), and 0.5 ml of 1 % barbituric acid in acetone and water (1: 1 v/v). A positive result was indicated by the appearance of an orange color within 20 min. The samples were measured at 506 nm 20 min after reaction and compared with the reading given by an aqueous solution of 10 μg/ml cotinine.
Statistical Analysis
Data were statistically analyzed using SPSS version 16 for Windows and expressed as mean ± SE. Statistical analysis performed using Mann–Whitney U test, Chi square and Pearson’s test. A p value of ≤0.05 was considered statistically significant.
Results
Clinical Assessment
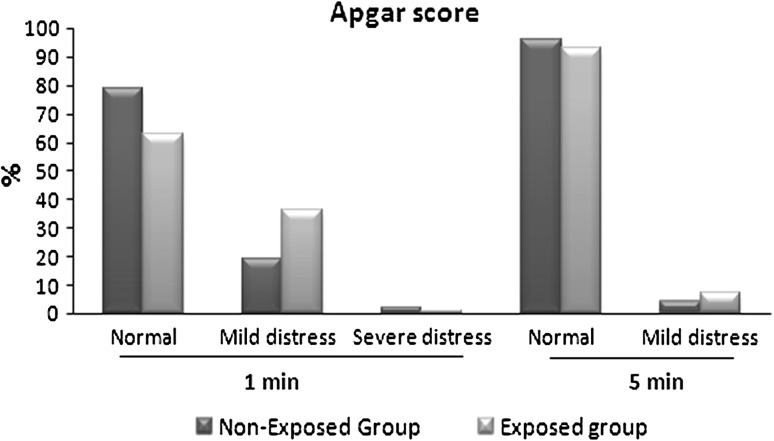
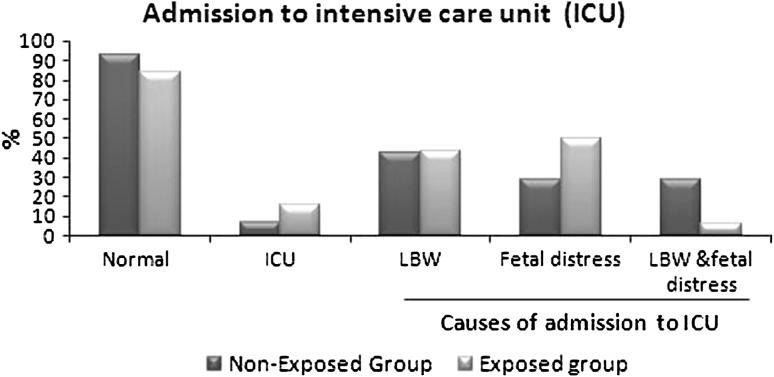
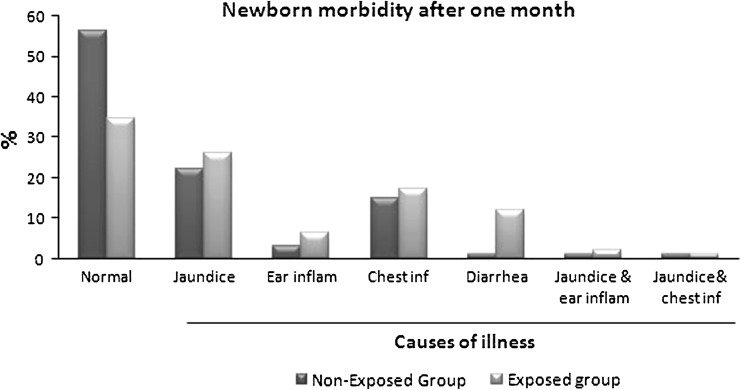
In this study, no significant differences between maternal age in exposed and non-exposed groups (22.56 ± 3.9 vs 22.83 ± 3.5, respectively) was observed. There is increased percent of full term babies in non-exposed group (72 %) compared to exposed group (67 %). The new born weight in exposed group significantly decreased (2,850 ± 3.74 vs 2,967.67 ± 3.34 g; p = 0.02) compared to non-exposed group. Demographic data of all mothers shows significant differences in maternal’s education and occupation (Table 1). One of interesting results in this study, is the increased rate of cesarean section in non-exposed group than exposed group (p = 0.000; Table 2). Apgar score at the first min after labor shows significant differences between two groups (p = 0.03; Fig. 1), but after 5 min Apgar score became insignificant (Table 3). Also, there is significant differences between both groups as regard admission to intensive care unit (ICU) (p = 0.05), but the cause of admission as low birth weight, fetal distress or both did not differ significantly (Fig. 2). In follow up after 1 month, there was significant increase in morbidity in exposed group (X2 = 15.16, p = 0. 01). As regard the cause of illness, only diarrhea that show significant increase (p = 0.05) in exposed group (Fig. 3). The effects of PTE on fetus (Table 4) show significant decreased in breast-feeding continuation in exposed compared to non-exposed group (p = 0.008; Table 5).
Table 1.
Socio-demographic data of all participants
| Characteristics | Non-exposed group n = 100 | Exposed group n = 100 | X2 value | p value |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Residence | ||||
| Rural | 67 | 78 | 3.03 | 0.08 |
| Urban | 33 | 22 | ||
| Maternal education | ||||
| Illiterate | 32 | 47 | 7.61 | 0.05* |
| Basic education | 19 | 9 | ||
| Secondary | 36 | 36 | ||
| University | 13 | 8 | ||
| Maternal occupation | ||||
| Employed | 13 | 3 | 6.79 | 0.009* |
| Unemployeda | 87 | 97 | ||
| Husband’s education | ||||
| Illiterate | 25 | 24 | 4.24 | 0.2 |
| Basic education | 16 | 24 | ||
| Secondary | 34 | 37 | ||
| University | 25 | 15 | ||
| Husband’s occupation | ||||
| Employee | 24 | 8 | 10.98 | 0.02* |
| Manual | 46 | 58 | ||
| Farmer | 12 | 16 | ||
| Professional | 17 | 18 | ||
| Worker | 1 | – | ||
*Significantly difference. As the total number of each group is 100, so the number is the same as percent
aUnemployed includes irregular temporary jobs
Table 2.
The effects of environmental tobacco smoking on current labor of both groups
| Items | Non-exposed group n = 100 | Exposed group n = 100 | X2 value | p value | ||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | |||||
| Membrane rupture | ||||||
| Spontaneous | 83 | 73 | 2.91 | 0.08 | ||
| Induced | 17 | 27 | ||||
| Time of membrane rupture | ||||||
| At first stage | 45 | 41 | 0.33 | 0.5 | ||
| At second stage | 55 | 59 | ||||
| Amniotic fluid status | ||||||
| Clear | 99 | 98 | 0.33 | 0.5 | ||
| Stain with meconium | 1 | 2 | ||||
| Mode of delivery | ||||||
| Normal vaginal delivery | 85 | 97 | 8.79 | 0.000* | ||
| C.S. | 15 | 3 | ||||
| Causes of C.S. | Total No: 15 | Total No: 3 | ||||
| Failure of progress | 7 | 46.7 | 2 | 66.7 | 0.5 | N.A |
| Fetal distress | 7 | 46.7 | 1 | 37.3 | ||
| Leakage of amniotic fluid. | 1 | 6.7 | 0 | 0 | ||
*Significantly difference. As the total number of each group is 100, so the number is the same as percent except in causes of Caesarian section (C.S)
Fig. 1.
Apgar score at the first min after labor, showed significant differences between exposed and non-exposed groups to ETS by Chi square, (X2 = 6.96, p = 0.03). While majority of new born in both groups had normal Apgar score after 5 min (X2 = 0.803, p = 0. 3)
Table 3.
The effect of PTE on newborn in both groups
| Items | Non-exposed group | Exposed group | X2 value | p value | ||
|---|---|---|---|---|---|---|
| No | % | No. | % | |||
| Vital status | n = 100 | n = 100 | 2.202 | 0.1 | ||
| Live | 98 | 98 | 100 | 100 | ||
| Still birth | 2 | 2 | 0 | 0 | ||
| Admission to NICU | n = 98 | n = 100 | ||||
| Yes | 7 | 7.1 | 16 | 16 | 3.78 | 0.05* |
| No | 91 | 92.9 | 84 | 84 | ||
| Apgar score at 1st min | n = 98 | n = 100 | ||||
| Normal | 77 | 78.6 | 63 | 63 | 6.96 | 0.03* |
| Mild distress | 19 | 19.4 | 36 | 36 | ||
| Severe distress | 2 | 2 | 1 | 1 | ||
| Apgar score after 5 min | n = 98 | n = 100 | ||||
| Normal | 94 | 95.9 | 93 | 93 | 0.803 | 0.3 |
| Mild distress | 4 | 4.1 | 7 | 7 | ||
| Newborn examination | n = 98 | n = 100 | ||||
| Normal | 97 | 99 | 98 | 98 | 0.318 | 0.5 |
| Abnormal | 1 | 1 | 2 | 2 | ||
| Causes of admission to NICU | n = 7 | n = 16 | ||||
| LBW | 3 | 42.8 | 7 | 43.8 | 2.38 | 0.3 |
| Fetal distress | 2 | 28.6 | 8 | 50 | ||
| LBW and fetal distress | 2 | 28.6 | 1 | 6.2 | ||
NICU neonatal intensive care unit, LBW Low birth weight
*Significantly difference
Fig. 2.
There is significant differences between exposed and non-exposed groups to ETS as regard admission to ICU by Chi square (X2 = 3.78, p = 0.05). But the cause of admission as low birth weight, fetal distress or both did not differ significantly (X2 = 2.38, p = 0. 3)
Fig. 3.
The main morbidity of infants were jaundice, chest infection, diarrhea and ear inflammation with statistically significant differences between exposed and non-exposed groups to ETS (X2 = 15.16, p = 0. 01)
Table 4.
The effects of PTE on new born within one month after labor
| Items | Non-exposed group A | Exposed group | X2 value | p value | ||
|---|---|---|---|---|---|---|
| No. | % | No | % | |||
| Vital status | n = 98 | n = 100 | 2.30 | 0.1 | ||
| Alive | 95 | 96.9 | 92 | 92 | ||
| Dead | 3 | 3.1 | 8 | 8 | ||
| Newborn morbidity | n = 95 | n = 92 | 15.16 | 0.01* | ||
| Jaundice | 22 | 22.3 | 24 | 26.1 | ||
| Ear inflammation | 3 | 3.2 | 6 | 6.5 | ||
| Chest infection | 14 | 14.9 | 16 | 17.4 | ||
| Diarrhea | 1 | 1.1 | 11 | 12 | ||
| Jaundice and ear inflammation | 1 | 1.1 | 2 | 2.2 | ||
| Jaundice and chest infection | 1 | 1.1 | 1 | 1.1 | ||
| None | 53 | 56.4 | 32 | 34.8 | ||
| Breast feeding | ||||||
| Continue | 75 | 62 | 9.71 | 0.008* | ||
| Need supplementation | 19 | 22 | ||||
| Stopped | 1 | 8 | ||||
*Significantly difference
Table 5.
The effects of environmental tobacco smoking on mother after labor in both groups
| Items | Non-exposed group n = 100 | Exposed group n = 100 | X2 value | p value | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Puerperium | ||||||
| Normal | 95 | 95 | 90 | 90 | 1.80 | 0.1 |
| Complicated | 5 | 5 | 10 | 10 | ||
| Type or complication | ||||||
| Bleeding | 2 | 40 | 6 | 60 | 1.50 | 0.4 |
| Infection | 3 | 60 | 3 | 30 | ||
| Others | 1 | 10 | ||||
Biochemical Assessment
There is significant increase in cotinine level in maternal urine, saliva and neonatal suction of exposed group compared to non-exposed (p = 0.000; Table 4). A correlation between level of cotinine in mother and neonates (r = 0.5; p = 0.000) was detected. Also, a significant increase of NSE and sE-cadherin (p = 0.000, 0.001, respectively) but decrease of NO (p = 0.000) in exposed compared to non-exposed group was detected. However, no significant difference in sFas level detected (Table 4). There is a negative correlation between birth weight and sFas and NSE (r = −0.9, −0.3; p = 0.01, 0.03), but, a positive correlation between level of cotinine in neonatal suction and NSE, sE-cadherin (r = 0.7, 0.9; p = 0.006, 0.000, respectively). By regression analysis, sE-cadherin is a constant predictor for prognosis in neonate when level of cotinine is a dependent variable (F = 26.74; p = 0.004).
Discussion
ETS represents a potentially large public health problem as 22–30 % of non-smoking pregnant women are exposed to it. ETS-exposed women have increased risks of infants with lower birth weight, congenital anomalies, longer lengths, and trends towards smaller head circumferences [15].
Cotinine level in children’s urine statistically differentiated children from exposed homes compared with non-exposed homes [16]. Most of the children considered non-exposed if cotinine levels below 1 ng/ml in their urine [17]. In this study, the presence of ETS exposure confirmed by questionnaires and cotinine level in urine and saliva of pregnant women. The new born weight in exposed group significantly decreased (2,850 ± 3.74 vs 2,967.67 ± 3.34; p = 0.02) while the cotinine level (17.28 ± 0.81, 1.81 ± 0.43) significantly increased compared to non-exposed group, the mean weight decrease is ~117 g corresponding to ~15 ng/ml increase in the cotinine level. This in agreement with El-Mohandes et al. [2] as reported that the urinary cotinine was the best predictor of infant irritability (r = 0.7) and salivary cotinine level of smoking mothers (20 to ≥100 ng/ml) was associated with significant reduction in birth weight ranged from 88 to 205 g, respectively [2]. In this study, there is significant increased level of NSE in exposed group to PTE and a positive correlation between level of cotinine and NSE (r = 0.7; p = 0.006) that may suggest some neuronal affection most probably in the form of irritability as El-Mohandes et al. [2] reported (Table 6).
Table 6.
Biochemical markers in tracheal suction of neonates after delivery
| ETS | Min–Max | Mean ± SE | p | |
|---|---|---|---|---|
| Markers in maternal samples | ||||
| Cotinine in saliva (ng/ml) | Exposed | 3.14–24.51 | 14.28 ± 0.70 | 0.000* |
| Non-exposed | 0.9–1.24 | 1.63 ± 0.45 | ||
| Cotinine in urine (ng/ml) | Exposed | 2.57–20.92 | 11.09 ± 0.27 | 0.000 |
| Non-exposed | 0.80–1.50 | 1.06 ± 0.07 | ||
| Markers in neonatal suction | ||||
| Cotinine (ng/ml) | Exposed | 3.66–32.56 | 17.28 ± 0.81 | 0.000* |
| Non-exposed | 0.30–2.16 | 1.81 ± 0.43 | ||
| NSE (μ/l) | Exposed | 3.70–8.94 | 5.65 ± 0.19 | 0.000* |
| Non-exposed | 0.47–1.40 | 0.77 ± 0.11 | ||
| E-cadherin (ng/ml) | Exposed | 2.40–4.40 | 3.12 ± .25 | 0.001* |
| Non-exposed | 0.80–1.50 | 1.07 ± 0.22 | ||
| sAPO-1/Fas (pg/ml) | Exposed | 803–2445 | 1560 ± 276 | 0.1 |
| Non-exposed | 1248–2411 | 2078 ± 148 | ||
| NO (μmol/ml) | Exposed | 0.424–1.854 | 0.79 ± 0.04 | 0.01* |
| Non-exposed | 0.96–1.03 | 0.99 ± 0.02 | ||
ETS environmental tobacco smoking, Min–Max Minimum–Maximum, NSE neuron specific enolase, NO nitric oxide
*Significantly difference
Small for gestational age pregnancies had lower sFas levels than control subjects in the second trimester, but not in the first trimester [18]. This in agreement with this study as there is a decrease in the level of sFas in exposed group to ETS compared to non-exposed but not reach a significant level (1,560 ± 276 vs 2,078 ± 148 pg/ml, p = 0.1). This could be explained as intrauterine fetal development is characterized by increased rates of proliferation and apoptosis, while both these processes may be attenuated in post-natal period. A higher apoptosis rate in the first trimester of pregnancy, possibly affecting maternal immuno-tolerance, followed by a down-regulation during the post-natal period [19]. There is an acceleration of Fas-FasL-mediated apoptosis during delivery and a respective decrease postpartum in both normal and IUGR pregnancies [20].
Maternal smoking reduced endothelial nitric oxide synthase (eNOS) activity in the fetal vascular bed lead to reduction of vasodilator capacity and retard fetal growth. So that, smoking cessation early in pregnancy may prevent these effects in newborns [12]. This in agreement with this study, as there is significant decrease in the level of the vasodilator, NO in exposed neonates. Reduced production of NO in fetal vessels may lower the blood flow to the fetus and result in lower birth weight. To our knowledge, this is the first time to use tracheal suction in the biochemical assay that can reflects the true internal environment of the fetus. However, this need further studies to measure hormones and other biochemical markers in neonatal tracheal suction that will help in screening and early detection of some diseases. One of interesting results, in this study, is the increased rate of cesarean section in non-exposed group than exposed group.
In conclusion, PTE decreased the birth weight most probably by decreasing NO. The decrease in sFas level may indicate decrease proliferation and consequently birth weight. While, increased morbidity of neonates in the exposed group could be attributed to increased NSE and sE-cadherin in the studied markers. Also, cessation of breast feeding and its sequel. So that, neonates should be more protected from ETS exposure in prenatal and postnatal periods.
References
- 1.Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9(7):739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Mohandes AA, Kiely M, Gantz MG, Blake SM, El-Khorazaty MN. Prediction of birth weight by cotinine levels during pregnancy in a population of black smokers. Pediatrics. 2009;124(4):e671–e680. doi: 10.1542/peds.2008-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leviton A, Kuban K, Allred EN, Hecht JL, Onderdonk A, O’Shea TM, McElrath T. ELGAN study investigators. Antenatal antecedents of a small head circumference at age 24-months post-term equivalent in a sample of infants born before the 28th post-menstrual week. Early Hum Dev. 2010;86(8):515–521. doi: 10.1016/j.earlhumdev.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahu K, Rahu M, Pullmann H, Allik J. Effect of birth weight, maternal education and prenatal smoking on offspring intelligence at school age. Early Hum Dev. 2010;86(8):493–497. doi: 10.1016/j.earlhumdev.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Wu FY, Chiu HT, Wu HD, Lin CJ, Lai JS, Kuo HW. Comparison of urinary and plasma cotinine levels during the three trimesters of pregnancy. Paediatr Perinat Epidemiol. 2008;22(3):296–301. doi: 10.1111/j.1365-3016.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 6.Sterk M, Oenings A, Eymann E, Roos W. Development of a new automated enzyme immunoassay for the determination of neuron-specific enolase. Anticancer Res. 1999;19(4A):2759–2762. [PubMed] [Google Scholar]
- 7.Al-Rawi NH, Atiyah KM. Salivary neuron specific enolase: an indicator for neuronal damage in patients with ischemic stroke and stroke-prone patients. Clin Chem Lab Med. 2009;47(12):1519–1524. doi: 10.1515/CCLM.2009.345. [DOI] [PubMed] [Google Scholar]
- 8.Malamitsi-Puchner A, Sarandakou A, Tziotis J, Trikka P, Creatsas G. Evidence for a suppression of apoptosis in early postnatal life. Acta Obstet Gynecol Scand. 2001;80(11):994–997. doi: 10.1034/j.1600-0412.2001.801104.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarandakou A, Malamitsi-Puchner A, Protonotariou E, Rigopoulou U, Papagianni B, Creatsas G. Indicative markers of cell proliferation and apoptosis during the perinatal period. Am J Perinatol. 2003;20(6):283–288. doi: 10.1055/s-2003-42774. [DOI] [PubMed] [Google Scholar]
- 10.Lazic T, Matic M, Gallup JM, Van Geelen A, Meyerholz DK, Grubor B, Imerman PM, de-Macedo MM, Ackermann MR. Effects of nicotine on pulmonary surfactant proteins A and D in ovine lung epithelia. Pediatr Pulmonol. 2010;45(3):255–262. doi: 10.1002/ppul.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpe MV, Chung E, Ulm JP, Gilchrist BF, Ralston S, Wang KT, Nielsen HC. Aberrant cell adhesion molecule expression in human bronchopulmonary sequestration and congenital cystic adenomatoid malformation. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L143–L152. doi: 10.1152/ajplung.90618.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen MR, Simonsen U, Uldbjerg N, Aalkjaer C, Stender S. Smoking cessation early in pregnancy and birth weight, length, head circumference, and endothelial nitric oxide synthase activity in umbilical and chorionic vessels: an observational study of healthy singleton pregnancies. Circulation. 2009;119(6):857–864. doi: 10.1161/CIRCULATIONAHA.107.755769. [DOI] [PubMed] [Google Scholar]
- 13.Van Bezooijen RL, Que I, Ederveen AG, Kloosterboer HJ, Papapoulos SE, Lowik CW. Plasma nitrate + nitrite levels are regulated by ovarian steroids but do not correlate with trabecular bone mineral density in rats. J Endocrinol. 1998;159:27–34. doi: 10.1677/joe.0.1590027. [DOI] [PubMed] [Google Scholar]
- 14.Peach H, Ellard GA, Jenner PJ, Morris RW. A simple inexpensive urine test of smoking. Thorax. 1985;40:351–357. doi: 10.1136/thx.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmasi G, Grady R, Jones J, Mcdonald S. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstetr Gynecol. 2010;89:423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 16.PolaÅ, ska K, Hanke W, Sobala W, Ligocka D. Prenatal and postnatal child exposure to environmental tobacco smoke. Przegl Lek. 2009;66(10):554–557. [PubMed] [Google Scholar]
- 17.Puig C, Garcia-Algar O, Monleon T, Pacifici R, Zuccaro P, Sunyer J, Figueroa C, Pichini S, Vall O. A longitudinal study of environmental tobacco smoke exposure in children: parental self reports versus age dependent biomarkers. BMC Public Health. 2008;6(8):47. doi: 10.1186/1471-2458-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg G, Lee C, Rauh-Hain JA, Ecker J, Khankin EV, Hsu CD, Cohen B, Rana S, Karumanchi SA, Thadhani R, Hacker MR, Lim KH. Early-pregnancy soluble Fas levels in idiopathic small-for-gestational-age pregnancies. Am J Obstet Gynecol. 2010;202(3):299.e1–299.e7. doi: 10.1016/j.ajog.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Protonotariou E, Rizos D, Malamitsi-Puchner A, Sarandakou A, Botsis D. Tissue polypeptide specific antigen and soluble Fas during normal pregnancy and early life. In Vivo. 2006;20(6B):901–905. [PubMed] [Google Scholar]
- 20.Briana DD, Baka S, Boutsikou M, Liosi S, Vraila VM, Gourgiotis D, Hassiakos D, Malamitsi-Puchner A. Soluble fas antigen and soluble fas ligand in intrauterine growth restriction. Neonatology. 2010;97(1):31–35. doi: 10.1159/000227290. [DOI] [PubMed] [Google Scholar]