Abstract
This study aimed to elucidate the mechanisms of melatonin to manage neurological damage in Alzheimer’s disease (AD) induced in ovariectomized rats. Forty adult female rats were enrolled in our study and were classified as; gonad intact control, ovariectomized control group, ovariectomized rats received melatonin, ovariectomized rats injected with AlCl3 to induce AD and AD-induced rats treated with melatonin. Hydrogen peroxide (H2O2), malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), B cell lymphoma 2 (Bcl-2), brain derived neurotrophic factor (BDNF), acetylcholinesterase (AchE) and acetylcholine (Ach) were estimated in the brain tissues of the different groups. Treatment of AD-induced rats with melatonin produced marked improvement in the most studied biomarkers which was confirmed by histological investigation of the brain. In Conclusion, melatonin significantly ameliorates the neurodegeneration characteristic of AD in experimental animal model due to its antioxidant, antiapoptotic, neurotrophic and anti-amyloidogenic activities.
Keywords: Alzheimer’s disease, Melatonin, Neurogenesis, Anti-amyloidogenesis, Rats
Introduction
Alzheimer’s disease (AD) is a progressive neurological disorder associated with aging. At autopsy, when the brain of Alzheimer’s patient is examined under the microscope, abnormal cells structures appear particularly in the brain areas related to memory including the entorhinal cortex and hippocampus. Neuropathological features of this disease are plaques of amyloid-beta (Aβ) surrounded by dying neurons and inflammatory cells and also neurofibrillary tangles present in cerebral cortex [1].
Alzheimer’s disease is oftenly associated with abnormal localization of iron regulatory proteins (IRPs). Specifically, alteration in IRP-2 might be directly linked to impaired iron homeostasis leading to neurofibrillary tangles, senile plaques and neuropil threads in Alzheimer’s patients [2].
Aluminum exposure has been reported to be a risk factor for Alzheimer’s disease. Aluminum is a neurotoxic agent inducing the production of free radicals in the brain which may cause degenerative events and leads to formation of amyloid plaques and intraneuronal neurofilamentous aggregates that are tau positive in the brain of AD’s patients [3].
It has been reported that deprivation in estrogen levels during postmenopause may increase the risk of Alzheimer’s disease. Multifaceted effects of estrogens include improvement of cerebral metabolic profile and the reduction of oxidative stress through actions on mitochondria [4].
Melatonin is mainly found in the pineal gland and is usually decreased with age in all humans, but this decline is more pronounced in Alzheimer’s patients. Melatonin has several biological effects, acting as an anti-amyloid-beta aggregation, anti-inflammatory, antioxidant and neuroprotective agent. It can directly act as free radical scavenger and indirectly induce the expression of some genes linked to the antioxidant defense system [5]. Melatonin could serve as a promising modulator for Alzheimer-like pathological changes and behavioral abnormalities [6].
Materials and Methods
Melatonin (N-acetyl-5-methoxytryptamine) was purchased from Sigma Co., USA and aluminum chloride was supplied from BDH Laboratory Supplies Poole, UK.
Experimental Animals
Fifty young adult female Sprague-Dawley rats weighing 100–120 g were obtained from the Animal House Colony of the National Research Centre (NRC), Cairo, September, 2010 and acclimated in a specific pathogen free (SPF) barrier area at 25 ± 1 °C and humidity (55 %) and controlled constantly with a 12 h light/dark cycle. The rats were ovariectomized surgically in Hormones Department, N.R.C. and were housed with ad libitum access standard laboratory diet consisting of casein 10 %, salts mixture 4 %, vitamins mixture 1 %, corn oil 10 % and cellulose 5 % completed to 100 g with corn starch [7]. Animals cared for according to the guidelines for animal experiments by the Ethical Committee of N.R.C.
Experimental Design
The animals were classified into five main groups each with 10 rats.
Group one Gonad intact control (non-ovariectomized) group-treated with vehicle [(Dimethylsulfoxide (DMSO) 5 % in saline] three times weekly for 18 weeks after 6 months of starting the experiment.
Group two Ovariectomized control group-treated with vehicle (DMSO 5 % in saline) three times weekly for 18 weeks after 6 months of surgical operation.
Group three Ovariectomized rats received melatonin orally three times weekly in a dose of 20 mg/Kg b wt dissolved in DMSO 5 % in saline [8] at 9 pm for 18 weeks, after 6 months of surgical operation.
Group four Ovariectomized rats were injected intraperitoneally (i.p.) with aluminum chloride (AlCl3) dissolved in distilled water daily for 3 months in a dose of 4.2 mg/Kg b wt [9], after 3 months of surgical operation and served as Al-intoxicated control group.
Group five Ovariectomized rats were injected intraperitoneally with AlCl3 (4.2 mg/Kg b wt) daily for 3 months, after 3 months of surgical operation. Then, they received melatonin orally in a dose of 20 mg/Kg b wt , at 9 p.m., three times weekly for 18 weeks.
Samples Collection
At the end of the experiment, the rats were fasted overnight, subjected to anesthesia by diethyl ether and sacrificed. The whole brain of each rat was rapidly dissected and washed with isotonic saline and dried on a filter paper.
Preparation of homogenates
Each brain was sagitally divided into two portions. The first portion of each brain was weighed and homogenized to give 10 % (w/v) homogenate in ice cold medium containing 50 mM Tris–HCl and 300 mM sucrose at pH 7.4 [10]. The homogenate was centrifuged at 3,000 rpm for 10 min in cooling centrifuge at 4 °C. The supernatant (10 %) was stored at −80 °C until using for the biochemical analysis including oxidative stress biomarkers [Hydrogen peroxide (H2O2) and malondialdehyde (MDA)], antioxidant status (TAC), superoxide dismutase (SOD) and catalase (CAT), antiapoptotic marker [B cell lymphoma 2 (Bcl-2)], neurotrophic factor [brain derived neurotrophic factor (BDNF)] and cholinergic markers [acetylcholine-esterase (AchE) and acetylcholine (Ach)]. Also, brain total protein concentration was measured to express the concentration of different brain parameters per mg protein. The second portion of the brain was fixed in formalin buffer (10 %) for histological investigation.
Biochemical Analysis
Brain H2O2 and MDA levels were determined colorimetrically using the kit purchased from Biodiagnostic Co., Egypt, according to the methods of Aebi [11] and Satoh [12], respectively. Also, brain TAC was assayed colorimetrically using the kit purchased from Biodiagnostic Co., Egypt, according to the method of Koracevic et al. [13]. Meanwhile, brain SOD and CAT activities were determined colorimetrically using the kit purchased from Biodiagnostic Co., Egypt, according to the methods of Nishikimi et al. [14] and Aebi [11], respectively. Brain Bcl-2 level was detected by ELISA technique using the kit purchased from Bender Med Systems Co., Vienna, Europe, according to the method of Barbareschi et al. [15]. While, brain BDNF level was detected by ELISA technique using the kit purchased from R and D system Co., UK, Europe, according to the method of Barakat-Walter [16]. Brain AchE activity was determined colorimetrically using the kit purchased from Quimica Clinica Aplicada S.A Co., Amposta, Spain, according to the method of Den Blaauwen et al. [17]. While, brain Ach level was measured colorimetrically using the kit purchased from Biovision Research Products Co., Linda Vista Avenue, USA, according to the method of Oswald et al. [18]. Quantitative estimation of brain total protein homogenate was carried out according to the method of Lowry et al. [19].
Histopathological Investigation
The brain tissues fixed in formalin buffer for 1 week, were washed in running tap water for 24 h and dehydrated in ascending series of ethyl alcohol (50–90), then in absolute alcohol. The samples were cleared in xylol and immersed in a mixture of xylol and paraffin in the oven at 60 °C. The tissues were transported to pure paraffin wax with melting point 58 °C in the oven and then mounted in blocks and left at 4 °C. The paraffin blocks were sectioned on the microtome at thickness of 5 μm and mounted on clean glass slides and left in the oven at 40 °C for dryness. The slides were deparaffinized in xylol and then immersed in descending series of ethyl alcohol (90–50). The ordinary haematoxylin and eosin stain was used to stain the slides [20].
Statistical Analysis
The results were expressed as Mean ± SE of the mean. Data were analyzed by one way analysis of variance (ANOVA) and was performed using the Statistical Package for the Social Science (SPSS) program, version 11 followed by least significant difference (LSD) to compare significance between groups [21]. Difference was considered significant when P value was <0.05.
Results
The data in Table 1 shows insignificant increase in brain H2O2 and MDA levels in the ovariectomized (Ovx) control group in comparison with the gonad intact control group. While, treatment of Ovx rats with melatonin produces insignificant decrease in brain H2O2 and MDA levels in comparison with the Ovx control group. Daily administration of AlCl3 to Ovx rats results in significant elevation of brain H2O2 and MDA levels in comparison with the Ovx control group. In contrast, treatment of Al-intoxicated Ovx rats with melatonin reveals significant decrease in brain H2O2 and brain MDA levels in comparison with the Al-intoxicated control group.
Table 1.
Effect of treatment with melatonin on brain oxidative stress biomarkers in ovariectomized and Al-intoxicated ovariectomized rats
| Groups | Parameters | |
|---|---|---|
| H2O2 (mmol/mg protein) | MDA (nmol/mg protein) | |
| Gonad intact control | 6.90 × 10−3±3.23 × 10−4 | 5.60 ± 0.30 |
| Ovx control | 9.30 × 10−3 ± 4.00 × 10−4 (34.78 %) | 6.40 ± 0.34 (14.28 %) |
| Ovx + melatonin | 7.31 × 10−3±3.39 × 10−4 (−21.37 %) | 5.70 ± 0.29 (−10.93 %) |
| Al-intoxicated control | 3.40 × 10−2±1.86 × 10−3b (265.59 %) | 9.60 ± 0.40b (50 %) |
| Ovx + Al + melatonin | 1.80 × 10−2 ± 1.90 × 10−3c (−47.05 %) | 6.55 ± 0.34c (−31.77 %) |
Data are represented as Mean ± SE (10 rats/group)
Ovx ovariectomized
bsignificant change at p < 0.05 in comparison with ovariectomized control group
csignificant change at p < 0.05 in comparison with Al-intoxicated control group
(%) percent of difference with respect to corresponding control value
Table 2 indicates that ovariectomy causes significant reduction in brain TAC and SOD activity as well as insignificant decrease in brain CAT activity in comparison with the gonad intact control group. On the other hand, treatment of Ovx rats with melatonin induces significant increase in brain TAC and insignificant increase in brain SOD and CAT activities in comparison with the Ovx control group. Daily administration of AlCl3 to Ovx rats produces significant reduction in brain TAC and insignificant inhibition of brain SOD and CAT activities in comparison with the Ovx control group. While, treatment of Al-intoxicated Ovx rats with melatonin induces significant elevation in brain TAC and brain CAT activity associated with insignificant increase in brain SOD activity in comparison with the Al-intoxicated control group.
Table 2.
Effect of treatment with melatonin on brain antioxidant status in ovariectomized and Al-intoxicated ovariectomized rats
| Groups | Parameters | ||
|---|---|---|---|
| TAC (mmol/mg protein) | SOD (U/mg protein) | CAT (U/mg protein) | |
| Gonad intact control | 14.20 ± 0.81 | 2.81 ± 0.18 | 5.79 ± 0.31 |
| Ovx control | 8.86 ± 0.43a (−37.62 %) | 2.06 ± 0.19a (−26.60 %) | 4.95 ± 0.29 (−14.55 %) |
| Ovx + melatonin | 10.57 ± 0.53b (19.30 %) | 2.50 ± 0.15 (21.21 %) | 5.56 ± 0.29 (12.32 %) |
| Al-intoxicated control | 6.33 ± 0.35b (−28.55 %) | 1.80 ± 0.17 (−12.72 %) | 4.16 ± 0.26 (−15.95 %) |
| Ovx + Al + melatonin | 9.91 ± 0.51c (56.63 %) | 2.21 ± 0.12 (22.91 %) | 5.28 ± 0.31c (27.09 %) |
Data are represented as Mean ± SE (10 rats/group)
Ovx ovariectomized
asignificant change at p < 0.05 in comparison with gonad intact control group
bsignificant change at p < 0.05 in comparison with ovariectomized control group
csignificant change at p < 0.05 in comparison with Al-intoxicated control group
(%) percent of difference with respect to corresponding control value
Table 3 shows that ovariectomy results in significant decrease in brain Bcl-2 and BDNF levels in comparison with the gonad intact control group. While, the Ovx rats which were treated with melatonin exerts significant increase in brain Bcl-2 and BDNF levels in comparison with the Ovx control group. Administration of AlCl3 to Ovx rats leads to significant reduction in brain Bcl-2 and BDNF levels in comparison with the Ovx control group. On the other hand, treatment of Al-intoxicated Ovx rats with melatonin reveals significant increase in brain Bcl-2 and BDNF levels in comparison with the Al-intoxicated control group.
Table 3.
Effect of treatment with melatonin on brain antiapoptotic marker (Bcl-2) and neurotrophic factor (BDNF) in ovariectomized and Al-intoxicated ovariectomized rats
| Groups | Parameters | |
|---|---|---|
| Bcl-2 (ng/mg protein) | BDNF (pg/mg protein) | |
| Gonad intact control | 1.15 × 10−1 ± 5.74 × 10−3 | 88.26 ± 3.31 |
| Ovx control | 6.90 × 10−2 ± 3.00 × 10−3a (−40.00 %) | 62.38 ± 3.15a (−29.32 %) |
| Ovx + melatonin | 8.95 × 10−2 ± 3.39 × 10−3b (29.71 %) | 75.15 ± 3.12b (20.47 %) |
| Al-intoxicated control | 5.82 × 10−2 ± 3.70 × 10−3b (−15.57 %) | 45.50 ± 2.49b (−27.05 %) |
| Ovx + Al + melatonin | 8.05 × 10−2 ± 4.06 × 10−3c (38.19 %) | 64.61 ± 2.87c (42.00 %) |
Data are represented as Mean ± SE (10 rats/group)
Ovx ovariectomized
asignificant change at p < 0.05 in comparison with gonad intact control group
bsignificant change at p < 0.05 in comparison with ovariectomized control group
csignificant change at p < 0.05 in comparison with Al-intoxicated control group
(%) percent of difference with respect to corresponding control value
Table 4 represents that ovariectomy induces insignificant increase in brain AchE activity associated with insignificant decrease in brain Ach level in comparison with the gonad intact control group. In contrast, treatment of Ovx rats with melatonin results in insignificant decrease in brain AchE activity accompanied with insignificant increase in brain Ach level in comparison with the Ovx control group. Aluminum administration to Ovx rats induces significant elevation in brain AchE activity and significant decrease in brain Ach level in comparison with the Ovx control group. On the other hand, treatment of Al-intoxicated Ovx rats with melatonin produces significant decrease in brain AchE activity accompanied with significant increase in brain Ach level in comparison with the Al-intoxicated control group.
Table 4.
Effect of treatment with melatonin on brain acetylcholinesterase (AchE) and acetylcholine (Ach) in ovariectomized and Al-intoxicated ovariectomized rats
| Groups | Parameters | |
|---|---|---|
| AchE (U/mg protein) | Ach (nmol/mg protein) | |
| Gonad intact control | 568.96 ± 26.11 | 8.50 × 10−2 ± 3.42 × 10−3 |
| Ovx control | 608.55 ± 33.75 (6.95 %) | 8.10 × 10−2 ± 3.25 × 10−3 (−4.70 %) |
| Ovx + melatonin | 580.92 ± 28.96 (−4.53 %) | 8.70 × 10−2 ± 3.46 × 10−3 (7.40 %) |
| Al-intoxicated control | 858.30 ± 43.82b (41.04 %) | 6.00 × 10−2 ± 2.55 × 10−3b (−25.92 %) |
| Ovx + Al + melatonin | 699.67 ± 37.78c (−18.48 %) | 7.80 × 10−2 ± 4.30 × 10−3c (30.00 %) |
Data are represented as Mean ± S.E (10 rats/group)
Ovx ovariectomized
bsignificant change at p < 0.05 in comparison with ovariectomized control group
csignificant change at p < 0.05 in comparison with Al-intoxicated control group
(%) percent of difference with respect to corresponding control value
Microscopic examination of brain sections of gonad intact control rats (Fig. 1) shows normal structure of neurons. While, microscopic examination of brain sections of Ovx control rats (Fig. 2) shows the appearance of degenerated neurons. Melatonin hormone administration in Ovx rats shows more or less normal structure of the neurons (Fig. 3). On the other hand, microscopic investigation of brain sections of Al-intoxicated Ovx rats demonstrates various sizes of amyloid plaques formation in the cerebral cortex and in the hippocampus (Fig. 4). While, treatment of Al-intoxicated Ovx rats with melatonin hormone shows more or less normal neurons with appearance of some dark neurons in the cortex area (Fig. 5).
Fig. 1.
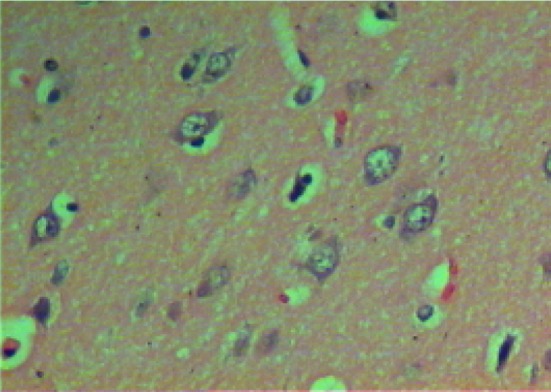
Micrograph of brain section of gonad intact control rat showing the highly active nerve cells that having huge nuclei with relatively pale-stained, the nuclear chromatin and prominent nuclei disappeared. The surrounding support cells having small nuclei with densely stained, condensed chromatin with no visible nucleoli are shown in the cortex (H and E ×400)
Fig. 2.
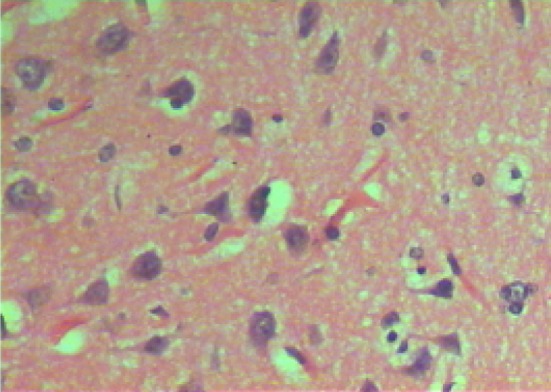
Micrograph of brain section of ovariectomized control rat showing dark neurons with corkscrew dendrites and many other neurons appeared in a degenerative form in the cortex (H and E ×400)
Fig. 3.
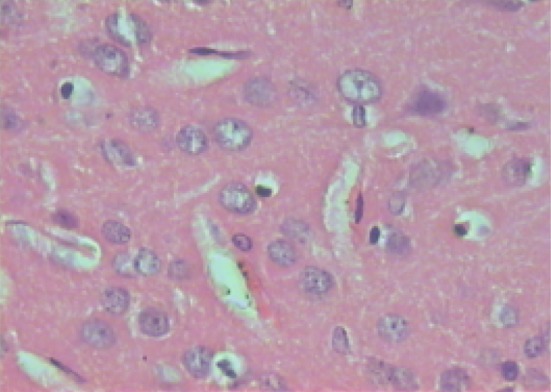
Micrograph of brain section of melatonin-treated ovariectomized rat showing more or less normal structure of neurons beside some dark neurons appeared in the cortex (H and E ×400)
Fig. 4.
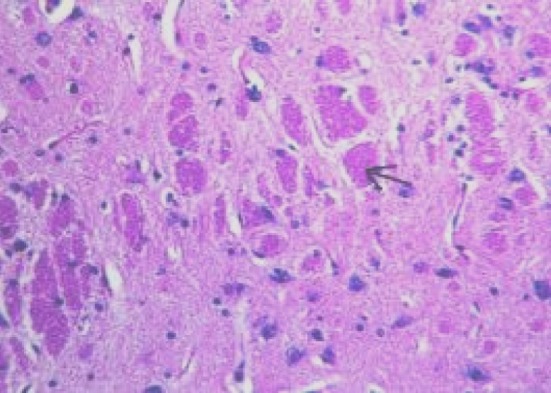
Micrograph of brain section of Al-intoxicated ovariectomized rat showing various sizes of amyloid plaques formation (arrow) in the cerebral cortex and hippocampus (H and E ×40)
Fig. 5.
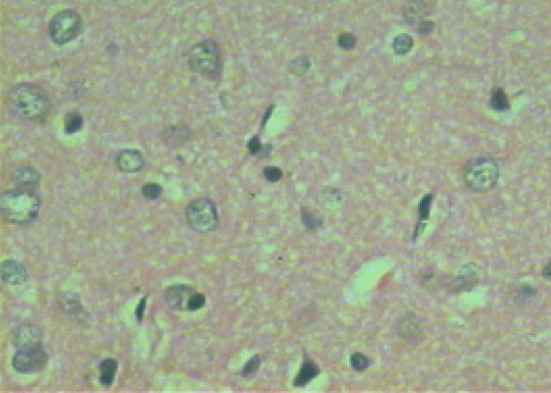
Micrograph of brain section of melatonin-treated Al-intoxicated ovariectomized rat showing some dark neurons and the remaining appears more or less as normal in the cortex (H and E ×400)
Discussion
There is growing evidence that oxidative stress and estrogen deprivation after menopause or ovariectomy are two main risk factors which are closely related to the pathological development of Alzheimer’s disease. Also, aluminum has been implicated in aging-related changes and particularly in neurodegenerative diseases as it promotes the formation of amyloid-β (Aβ) plaques [22].
Our results showed that each of H2O2 and MDA in the brain of either Ovx or Al-intoxicated Ovx rats has significantly increased. Tuneva et al. [23] reported that in vitro and in vivo studies demonstrated the increased ROS including H2O2 production in different brain areas due to Al exposure. Aluminum has a strong prooxidant activity in spite of its non redox status [24]. Kumar et al. [25] observed that Al exposure is associated with impairment of antioxidant defense system that may lead to oxidative stress. Also, Al could increase the activity of monoamine oxidase enzyme (MAO) in the brain which leads to increase generation of H2O2 [26]. Aluminum can induce lipid peroxidation and alter physiological as well as biochemical behavior of living organism, a matter implicated in increased brain MDA level which is the major aldehyde formed after breakdown of lipid hydroperoxides [27].
Administration of melatonin to both Ovx and Al-intoxicated Ovx rats could significantly decrease both of brain H2O2 and MDA levels. Martinez et al. [28] reported that melatonin reduces the neuronal damage mediated by ROS. Melatonin has a powerful scavenging activity for hydroxyl and peroxyl radicals over that of both glutathione and vitamin E. Melatonin also conserves the activities of the antioxidant enzymes via enhancing gene expression of these enzymes [29]. Melatonin has a phenol group that provides a proton to detoxify hydroxyl or lipid peroxy radicals and thus can reduce lipid peroxidation induced by Al in Alzheimer’s patients [30]. Also, melatonin has the ability to serve as a metal chelator to reduce metal toxicity [31]. Melatonin stabilizes membranes against free radicals and can resist the rigidity of the biological membranes caused by Al [32]. Moreover, Yang et al. [6] reported that melatonin effectively reduces lipid peroxidation induced by Al in Alzheimer’s patients. Melatonin is a potent antioxidant that protects DNA, lipids and proteins from free radical damage [33]. The ability of melatonin to serve as a metal chelator for Fe3+ ions provides an evidence supporting its role as a neuroprotective agent [5].
Moreover, the data of this study showed significant decrease in each of brain TAC, SOD and CAT activities in both of Ovx and Al-intoxicated Ovx rats. Munoz-Castaneda et al. [34] showed that the lack of estrogen by ovariectomy induced a reduction of antioxidant status (GSH, SOD and GPx) and elevated lipid peroxides in rats. Aluminum exposure causes impairment of the antioxidant defense system that may lead to oxidative stress [25, 35]. Aluminum causes brain damage via ROS more than any other organ because of high brain lipid content, high oxygen turnover, low mitotic rate as well as low antioxidant concentration [36].
Treatment of Ovx and Al-intoxicated Ovx rats with melatonin resulted in significant increase in each of brain TAC, SOD and CAT activities. Melatonin is known to increase tissue mRNA levels of both isoforms of Mn-SOD and Cu–Zn SOD. Several melatonin metabolites that are generated when melatonin interacts with toxic reactants are themselves able to increase the efficiency of the electron transport chain in the inner mitochondrial membrane with a consequent impairment of free radical generation [37]. Kumar et al. [25] reported that aluminum altered the cellular redox state of the brain by inhibiting antioxidant enzymes defense, such as SOD and catalase, which function as blockers of free radical. Increased activities of GPx and Cu–Zn SOD/Mn-SOD in the rat brain after exogenous administration of melatonin has been reported [38]. Gomez et al. [39] observed increased levels of each of Cu-ZnSOD, MnSOD, GPx and CAT in the hippocampi of rats after melatonin administration.
The results of our study revealed significant decrease in Bcl-2 and BDNF levels in the brain of Ovx and Al-intoxicated Ovx rats. Sharma and Mehra [40] stated that ovariectomy decreased Bcl-2 expression and increased proapoptotic marker (Bax) expression in the rat hippocampus. Altered Bax/Bcl-2 ratio is critical to Al-induced apoptosis [41] leading to activation of caspase-3 and release of cytochrome c. Kumar et al. [35] reported that aluminum induces oxidative stress on the neuronal cells and increases p53 protein expression by activating p38 MAPK to initiate apoptosis and this is accompanied by a marked inhibition of Bcl-2 and increased Bax expression. It has been reported that amyloid β could activate p53 by direct interaction with the p53 promoter which led to Bax and caspase-6 activation with subsequent reduction of Bcl-2 and execution of the cell death pathway [41].
Takuma et al. [42] showed marked decrease in the BDNF mRNA level in the hippocampus due to ovariectomy in mice. Astrocytes are the principal target for Al toxic action, thus blocking the release of neurotrophic factor. Disruption of the proinflammatory cytokine/neurotrophin balance by Al plays an important role in the neurodegenerative disease [43]. Aluminum intoxication results in increased tumor necrosis factor-α (TNF-α) and macrophage inflammatory protein-1α (MIP-1α), with consequent decrease in NGF and BDNF. BDNF is one of the target genes of phosphorylated CREB so that its mutation or blocking resulted in a dramatic loss of BDNF transcription [44]. Decrease in brain BDNF level could be associated with AD pathogenesis.
Treatment of both Ovx and Al-intoxicated Ovx rats with melatonin resulted in significant elevation in both brain Bcl-2 and BDNF levels. Melatonin acts through the mitochondrial pathway and blocks the spill of cytochrome c to the cytosol and thus prevents activation of caspases, increasing cellular content of Bcl-2 in old rats, thus reduces apoptosis. Melatonin regulates the complex Bax/Bcl-2 and antagonizes apoptosis through the activation of MAPK/ERK pathway and inhibition of the stress kinases JNK and p38 MAPK in neuronal cells [45]. Melatonin and its activated receptors have been linked to the regulation of neurotrophic factors, including BDNF. Both G-proteins mediated signaling and other pathways such as ERK may contribute to melatonin action on BDNF [46].
The data of the current study showed significant increase in brain AchE activity with concomitant decrease in Ach level in both Ovx and Al-intoxicated Ovx rats. Gulya et al. [47] showed that aluminum causes cholinergic system dysfunction that may contribute to learning and memory deficits observed in Alzheimer’s dementia. Zhang et al. [48] reported increased AchE activity in Al overloaded rats. Kaizer et al. [49] suggested that Al exposure increased AchE activity via allosteric interaction between Al and the peripheral anionic site of the enzyme molecule, leading to the etiology of AD pathological deterioration.
Aluminum exerts cholinotoxic effects by blocking the provision of acetyl-CoA which is required for Ach synthesis or by impairing the activities of choline acetyl transferase (ChAT) enzyme itself [50]. Aluminum promotes the formation of amyloid-β plaques (Aβ 1-42) which significantly reduced brain Ach level leading to greater hippocampal Ach reduction accompanied by more memory impairment. The effect of Aβ 1-42 was increased when Aβ 1-42 was combined with Ovx [51].
Significant decrease in brain AchE of Al-intoxicated Ovx rats was observed when treated with melatonin. This finding was in agreement with the results achieved by Agrawal et al. [8]. The ability of melatonin to inhibit AchE activity and improve cognitive functions is related to its effect in balancing oxidant/antioxidant status and regulating the generation of inflammatory mediators particularly TNF-α and IL-1β [9].
Treatment of Al-intoxicated Ovx rats with melatonin significantly increased brain Ach level. Melatonin exerts beneficial effects on cholinergic neurotransmission in brain by increasing ChAT activity in the frontal cortex and hippocampus [52]. Melatonin possesses an electron rich aromatic indole ring and functions as donar and directly detoxifies free radicals and thus can enhance brain Ach activity.
Microscopic examination of the brain of gonad intact control group showed normal nerve cells with visible nuclei in the cortex. Examination of brain sections of Ovx rats showed that the hippocampus has not suffered any histological changes, whereas the cortex area showed dark neurons with corkscrew dendrites (tangles) and many of these neurons appeared in a degenerative form. When Ovx rats were treated with melatonin, normal structure of the hippocampus was observed while some dark neurons were detected in the cortex, these findings were in agreement with Hua et al. [53]. Meanwhile, examination of the brain of Al-intoxicated Ovx rats showed formation of amyloid plaques in both the cerebral cortex and in the hippocampus. While, treatment of Al-intoxicated Ovx rats with melatonin revealed almost normal structure of the hippocampus with few dark neurons remaining in the cortex. Yang et al. [6] reported that melatonin effectively reduces lipid peroxidation and ameliorates Alzheimer-like pathological changes.
In conclusion, our present study clearly demonstrated that both oxidative stress and estrogen deprivation are essential factors in promoting the AD pathophysiologic course in experimental animals. Treatment with melatonin hormone significantly ameliorated the neurodegeneration characteristic of Alzheimer’s disease as indicated by the tangible improvement in the biochemical markers. These findings were well confirmed by the remarkable improvement in the histological features of the brain. These effects of melatonin hormone were achieved through its powerful antioxidant, antiapoptotic, and neurotrophic as well as anti-amyloidogenic activities. These results represent good therapeutic approaches for intervention against progressive neurological damage associated with Alzheimer’s disease.
References
- 1.Welsh-Bohmer KA, White CL. Alzheimer’s disease: what changes in the brain cause dementia? Neurology. 2009;72:21–23. doi: 10.1212/01.wnl.0000343818.11392.d9. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Wehr K, Harris PL, Siedlak SL, Connor JR, Perry G. Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res. 1998;788:232–236. doi: 10.1016/S0006-8993(98)00002-X. [DOI] [PubMed] [Google Scholar]
- 3.Walton JR, Wang MX. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J Inorg Biochem. 2009;103:1548–1554. doi: 10.1016/j.jinorgbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia T, Esparza JL, Nogues MR, Romeu M, Domingo JL, Gomez M. Oxidative stress status and RNA expression in hippocampus of an animal model of Alzheimer’s disease after chronic exposure to aluminum. Hippocampus. 2010;20:218–225. doi: 10.1002/hipo.20612. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Yang Y, Fu Z, Li Y, Feng J, Luo J, et al. Melatonin ameliorates Alzheimer like pathological changes and spatial memory retention impairment induced by calyculin A. J Psychopharmacol. 2011;25(8):1118–1125. doi: 10.1177/0269881110367723. [DOI] [PubMed] [Google Scholar]
- 7.AOAC. Official Methods of Analysis. 16th ed. Association of Official Analysis: Washington; 1995.
- 8.Agrawal RE, Tyagi R, Shukla R, Nath C. Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav Brain Res. 2008;189:381–386. doi: 10.1016/j.bbr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Julka D, Gill KD. Effect of aluminum on regional brain antioxidant defense status in Wistar rats. Res Exp Med. 1996;196:187–194. doi: 10.1007/BF02576841. [DOI] [PubMed] [Google Scholar]
- 10.Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of l-cysteine and glutathione on the modulated suckling rat brain Na+, K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res. 2004;49:475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 12.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 13.Koracevic DG, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 15.Barbareschi M, Caffo O, Veronese S, Leek RD, Fina P, Fox S, et al. Bcl-2 and P53 expression in node negative breast carcinoma-a study with long term follow up. Human Pathol. 1996;27:1149–1155. doi: 10.1016/S0046-8177(96)90307-X. [DOI] [PubMed] [Google Scholar]
- 16.Barakat-Walter I. Brain derived neurotrophic factor like immunoreactivity is localized mainly in small sensory neurons of rat dorsal root ganglia. J Neurosci Meth. 1996;68:281–288. doi: 10.1016/0165-0270(96)00093-3. [DOI] [PubMed] [Google Scholar]
- 17.Den Blaauwen DH, Poppe WA, Tritschler W. Acetylcholinesterase with acetylthiocholine iodide as substrate: references depending on age and sex with special reference to hormonal effects and pregnancy. J Clin Chem Clin Biochem. 1983;21:381–386. [PubMed] [Google Scholar]
- 18.Oswald C, Smits SH, Hoing M, Sohn-Bosser L, Dupont L, Le Rudulier D, et al. Crystal structures of choline/acetylcholine substrate-binding protein chox from sinorhizobium meliloti in the liganded and unliganded-closed states. J Biol Chem. 2008;283:32848–32859. doi: 10.1074/jbc.M806021200. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Carleton HM, Drury RAB, Wallington EA. Carleton’s histological technique. 5th ed. New York: Oxford University Press, Oxford; 1980. pp. 188–189, 237–240, 290–291.
- 21.Armitage P, Berry G. Comparison of several groups. In: Armitage P, Berry G, editors. Statistical method in medical research, 2th ed. Oxford: Blackwell Significant Publication; 1987. pp. 186–213.
- 22.Bharathi P, Vasudevaraju P, Govindaraju M, Palanisamy AP, Sambamurti K, Rao KS. Molecular toxicity of aluminum in relation to neurodegeneration. Ind J Med Res. 2008;128:545–556. [PubMed] [Google Scholar]
- 23.Tuneva J, Chittur S, Boldyrev AA, Birman I, Carpenter DO. Cerebellar granule cell death induced by aluminum. Neurotoxicol Res. 2006;9:297–304. doi: 10.1007/BF03033320. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Bal A, Gill KD. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminum. Brain Res. 2008;1232:94–103. doi: 10.1016/j.brainres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Bal A, Gill KD. Susceptibility of mitochondrial superoxide dismutase to aluminum-induced oxidative damage. Toxicology. 2009;255:117–123. doi: 10.1016/j.tox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Huh JW, Choi MM, Lee JH, Yang SJ, Kim MJ, Choi J, et al. Activation of monoamine oxidase isotypes by prolonged intake of aluminum in rat brain. J Inorg Biochem. 2005;99:2088–2091. doi: 10.1016/j.jinorgbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi S, Mahdia AA, Nawaba A, Chandera R, Hasan M, Siddiqui MS, et al. Influence of age on aluminum-induced lipid peroxidation and neurolipofuscin in frontal cortex of rat brain: a behavioral, biochemical and ultrastructural study. Brain Res. 2009;1253:107–116. doi: 10.1016/j.brainres.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Martinez GR, Almeida EA, Klitzke CF, Onuki J, Prado FM, Medeiros MH, Di Mascio P. Measurement of melatonin and its metabolites: importance for the evaluation of their biological roles. Endocrine. 2005;27:111–118. doi: 10.1385/ENDO:27:2:111. [DOI] [PubMed] [Google Scholar]
- 29.Sharman EH, Bondy SC, Sharman KG, Lahiri D, Cotman CW, Perreau VM. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem Int. 2007;50:336–344. doi: 10.1016/j.neuint.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. Melatonin’s interaction with reactive species. J Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079X.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 31.Gulcin I, Buyukokuroglu M, Kufrevioglu OI. Metals chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res. 2003;34:278–281. doi: 10.1034/j.1600-079X.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 32.Albendea CD, Gomez-Trullen EM, Fuentes-Broto L, Miana-Mena FJ, Millan-Plano S, Reyes-Gonzales MC, et al. Melatonin reduces lipid and protein oxidative damage in synaptosomes due to aluminum. J Trace Elem Med Biol. 2007;21:261–268. doi: 10.1016/j.jtemb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ. Oxidative damage to catalase-induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res. 2003;37:543–553. doi: 10.1080/1071576031000083206. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Castaneda JR, Muntane J, Munoz MC, Bujalance I, Montilla P, Tunez I. Estradiol and catecholestrogens protect against adriamycin-induced oxidative stress in erythrocytes of ovariectomized rats. Toxicol Lett. 2006;160:196–203. doi: 10.1016/j.toxlet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Kumar V, Bal A, Gill KD. Aluminum-induced oxidative DNA damage recognition and cell cycle disruption in different regions of rat brain. Toxicology. 2009;264:137–144. doi: 10.1016/j.tox.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Nehru B, Anand P. Oxidative damage following chronic aluminum exposure in adult and pup rat brains. J Trace Elem Med Biol. 2005;19:203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Aging Dev. 2002;123:1007–1019. doi: 10.1016/S0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079X.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 39.Gomez M, Esparza JL, Nogues MR, Giralt M, Cabre M, Domingo JL. Prooxidant activity of aluminum in the rat hippocampus: gene expression of antioxidant enzymes after melatonin administration. Free Radic Biol Med. 2005;38:104–111. doi: 10.1016/j.freeradbiomed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Sharma K, Mehra RD. Long term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res. 2008;1204:1–15. doi: 10.1016/j.brainres.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 41.Johnson VJ, Kim S, Sharma RP. Aluminum maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci. 2005;83:329–339. doi: 10.1093/toxsci/kfi028. [DOI] [PubMed] [Google Scholar]
- 42.Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, et al. 17-β estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146:60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm. 2000;2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 44.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 45.Luchetti F, Betti M, Canonico B, Arcangeletti M, Ferri P, Galli F, Papa S. ERK/MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic Biol Med. 2009;46:339–351. doi: 10.1016/j.freeradbiomed.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Imbesi M, Uz T, Manev H. Melatonin receptor agonist ramelteon activates the extracellular signal regulated kinase 1/2 in mouse cerebellar granule cells. Neuroscience. 2008;155:1160–1164. doi: 10.1016/j.neuroscience.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 47.Gulya K, Rakonczay Z, Kasa P. Cholinotoxic effects of aluminum in rat brain. J Neurochem. 1990;54:1020–1026. doi: 10.1111/j.1471-4159.1990.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Yang JQ, He BC, Zhou QX, Yu HR, Tang Y, Liu BZ. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saud Med J. 2009;30:760–766. [PubMed] [Google Scholar]
- 49.Kaizer RR, Correa MC, Gris LR, Da Rosa CS, Bohrer D, Morsch VM, et al. Effect of long term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res. 2008;33:2294–2301. doi: 10.1007/s11064-008-9725-6. [DOI] [PubMed] [Google Scholar]
- 50.Alleva K, Rankin J, Santucci D. Neurobehavioural alteration in rodents following developmental exposure to aluminum. Toxicol Ind Health. 1998;14:209–221. doi: 10.1177/074823379801400113. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki K, Al-Khatib IH, Egashira N, Akiyoshi Y, Arai T, Mishima K, et al. Ovariectomy combined with amyloid-β1-42 impairs memory by decreasing acetylcholine release and α7nAchR expression without induction of apoptosis in the hippocampus CA1 neurons of rats. Neurotox Res. 2004;6:299–309. doi: 10.1007/BF03033440. [DOI] [PubMed] [Google Scholar]
- 52.Weinstock M, Shoham S. Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J Neural Transm. 2004;111:347–366. doi: 10.1007/s00702-003-0058-y. [DOI] [PubMed] [Google Scholar]
- 53.Hua X, Lei M, Zhang Y, Ding J, Han Q, Hu G, Xiao M. Long term d-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer’s disease. Life Sci. 2007;80:1897–1905. doi: 10.1016/j.lfs.2007.02.030. [DOI] [PubMed] [Google Scholar]


