Abstract
Background
Tall cell variant (TCV) and diffuse sclerosing variant (DSV) of papillary thyroid cancer are aggressive subtypes, for which tumors ≤1 cm have not been exclusively studied.
Methods
The SEER database (1988–2009) was used to compare characteristics of TCV ≤1 cm (mTCV) and DSV ≤1 cm (mDSV) with classic papillary thyroid microcarcinoma (mPTC). Survival was analyzed with the Kaplan–Meier method and log-rank test, and risk factors for nodal metastases with chi-square analysis and binary logistic regression.
Results
There were 97 mTCV, 90 mDSV, and 18,260 mPTC patients. mTCV incidence increased by 79.9% (p=0.153) over the study period, while mDSV incidence decreased by 10.3% (p=0.315). Compared to classic mPTC, mTCV tended to be larger on average (7.1 mm vs. 5.3 mm, p<0.001), with higher rates of multifocality (47.2% vs. 34.0% respectively, p=0.018) and lymph-node examination (63.9% vs. 39.2% respectively, p<0.001), while in mDSV, nodal metastases were more frequent (57.1% vs. 33.1% respectively, p=0.007). Both aggressive variants had higher rates of extrathyroidal extension (27.8% mTCV vs. 13.3% mDSV vs. 6.1% mPTC, p<0.001). Aggressive variants also received radioactive iodine more frequently (39.2% mTCV vs. 40.0% mDSV vs. 29.1% mPTC, p<0.001). However, they were not statistically more likely to receive thyroidectomy over lobectomy compared to classic mPTC. There were no significant differences in overall and disease-specific survival between the histologies. In mTCV, after adjustment, extrathyroidal extension was independently associated with size >7 mm (odds ratio (OR) 4.4 [CI 1.5–13.6]) and nodal metastasis with multifocality (OR 5.4 [CI 1.3–23.4]) and extrathyroidal extension (OR 5.8 [CI 1.3–25.4]). No statistically significant predictors of extrathyroidal extension or nodal metastasis in mDSV were observed.
Conclusions
Aggressive variants of mPTC tend to exhibit more aggressive pathologic characteristics than classic mPTC, but survival appears to be similar. Treatment with total thyroidectomy and central lymphadenectomy may be warranted if the diagnosis can be made pre- or intraoperatively.
Introduction
Papillary thyroid microcarcinoma (mPTC), defined as papillary thyroid carcinoma (PTC) ≤1 cm in size, is rapidly rising in incidence, accounting for 49% of the increase in PTC incidence from 1973 to 2002. Currently, it represents 43% of PTC in patients older than 45 years (1–3). While the course of mPTC is generally indolent, with a 10-year disease-specific survival of 99.5%, a subpopulation of patients with mPTC carry increased risk of mortality. Risk factors such as age, ethnicity, nodal metastases, extrathyroidal invasion, and distant metastasis have helped characterize this population. However, the search continues for additional factors that can be used to identify patients with mPTC who carry a poor prognosis (4,5).
Several histologic variants of PTC have been identified as having aggressive behavior in comparison to classic PTC, including diffuse sclerosing variant (DSV) and tall cell variant (TCV). First described in 1985, DSV is characterized by papillary morphology, diffuse involvement of the thyroid gland, prominent fibrosis, abundant psammoma bodies, squamous metaplasia, and lymphocytic infiltration easily confused with thyroiditis (6–8). It accounts for approximately 2–6% of PTC, classically occurs in young women, and is reported to have increased rates of multifocality, bilaterality, extrathyroidal extension, recurrence, and nodal/distant metastasis (8–17). TCV, first described in 1976, accounts for 3–12% of all PTC, and is characterized by a population of cells at least twice as tall as they are wide, comprising 30–70% of total tumor cells (8,18–22). TCV has been reported to be larger than classic PTC on average, with higher rates of bilaterality, multifocality, extrathyroidal extension, recurrence, lymph-node/distant metastasis, and decreased survival (12,17,23–30).
While it appears clear that DSV and TCV are aggressive, the extent to which DSV ≤1 cm (mDSV) and TCV ≤1 cm (mTSV) exhibit aggressive behavior remains an open question, and the optimal management of these tumors is currently unclear. Studies of aggressive variants of PTC largely consist of case reports and single-center case series, and two population-level studies that have been performed on the topic of DSV and TCV do not specifically address tumors ≤1 cm in size (17,30). Similarly, in studies of mPTC, the inclusion of aggressive morphologies is either variable or unclear, and it appears that no subset analysis of aggressive variants has been undertaken (4,5,31).
To our knowledge, this study represents the first population-level analysis of aggressive variants of mPTC in which we compare incidence, demographic, clinical, and pathologic characteristics of mDSV and mTCV with classic mPTC, in addition to identifying risk factors associated with extrathyroidal extension and nodal metastases.
Materials and Methods
Data source and study participants
The data source for this study was the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) database, which provides population-based data on cancer incidence and survival from 18 registries and represents 28% of the U.S. population (32).
Cases of mPTC, mTCV, and mDSV from 1988 to 2009 were selected from all 18 registries using ICD-O-3 codes 8050, 8260, 8341 (classic mPTC), 8350 (DSV), and 8344 (TCV) in combination with “Extent of Disease” and “Collaborative stage” variables signifying tumors ≤1 cm in size. Of note, the ICD-O-3 code 8344 does not distinguish between tall cell and columnar cell variants of PTC, likely due to ambiguities in definition (33,34). Our study was further restricted to patients ≥18 years of age whose data were informed by active follow-up. Patients with secondary malignancies or other primary thyroid malignancies were excluded.
Incidence data were obtained from years 2001–2009. TCV was first reported in 2001, and incidence analysis was restricted to the years 2001–2009 to allow for comparison between mTCV, mDSV, and mPTC. Rates were age adjusted using the 2000 U.S. standard population. Annual percentage changes (APC) were calculated. Demographic variables of interest included age at diagnosis, sex, and ethnicity. Clinical variables of interest included surgical therapy, lymph-node examination, radiation therapy, and survival status as of December 31, 2009. Survival time was calculated as time in years from diagnosis until death, date last known to be alive, or December 31, 2009, whichever came first. Overall and disease-specific survival rates were calculated. Pathologic variables of interest included tumor size, multifocality, extrathyroidal extension, nodal metastasis, and distant metastasis. Multifocality was introduced as a disease-specific variable in 2004, and data were available for 61%, 49%, and 72% of cases of mPTC, mDSV, and mTCV diagnosed from 1988 to 2009. Extrathyroidal extension was defined as tumor invasion beyond the thyroid capsule. Location of nodal metastasis were grouped corresponding to American Joint Committee on Cancer stages N1a (level VI) and N1b (levels I, II, III, IV, V, VII).
Statistical analysis
Summary statistics were used to describe baseline characteristics. Chi-square test and analysis of variance were used to analyze categorical and continuous variables respectively. Fischer's exact test was used to analyze categorical variables with expected values less than five. Survival was analyzed using the Kaplan–Meier method, and the log-rank test was used to determine if differences in survival were statistically significant. Stepwise binary logistic regression was used to identify factors independently associated with nodal metastases and extrathyroidal extension. Variables with p<0.1 on univariate analysis were included in multivariate analysis. All tests were two-sided, and p<0.05 was considered statistically significant.
Incidence and trend analyses were performed by SEER*Stat v7.1.0 obtained from SEER (Bethesda, MD). All other analyses were performed with SPSS v19 (SPSS, Inc., Chicago, IL). Because SEER data are publicly available and de-identified, our study was deemed to be exempt from institutional review board approval.
Results
There were 90 cases of mDSV, 97 cases of mTCV, and 18,260 cases of classic mPTC diagnosed during the study period. Patients with mTCV were followed for up to 9 years, while mDSV and classic mPTC patients were followed for up to 22 years. Mean follow-up for mDSV, mTCV, and classic mPTC was 7.0 years, 3.8 years, and 5.3 years respectively.
Incidence
Incidence of classic mPTC increased from 1.42 per 100,000 in 2001 to 3.47 per 100,000 in 2009, representing an annual percentage change (APC) of +11.8% (ptrend<0.001). mTCV increased in incidence from 0.010 to 0.019 per 100,000 (APC +5.7%, ptrend=0.153), and mDSV decreased in incidence from 0.0075 to 0.0067 per 100,000 (APC −4.7%, ptrend=0.315).
Characteristics
Clinical and pathologic characteristics are summarized in Tables 1 and 2. There were no significant demographic differences between patients with mDSV and mTCV compared to classic mPTC with respect to age, sex, or ethnicity. Patients with mTCV had lymph nodes examined more frequently compared to mPTC (63.9 % vs. 39.2%, p<0.001). Aggressive variants were more likely to receive radioiodine ablation (RAI; 40.0% mDSV vs. 39.2% mTCV vs. 29.1% mPTC, pmDSV=0.013, pmTCV<0.001). However, they were not statistically more likely to receive total thyroidectomy versus lobectomy compared to classic mPTC (70.0% mDSV vs. 78.4% mTCV vs. 71.8% mPTC, pmDSV=0.655, pmTCV=0.311).
Table 1.
Clinical Characteristics of Classic Papillary Thyroid Microcarcinoma Compared with Tall Cell and Diffuse Sclerosing Variants
| Classic mPTC[n (%)] | mDSV[n (%)] | p-Value | mTCV[n (%)] | p-Value | |
|---|---|---|---|---|---|
| Age | 0.699 | 0.144 | |||
| Mean±SEM (in years) | 47.6±0.1 | 48.2±1.4 | 49.6±1.4 | ||
| Age 18–44 | 7827 (42.9) | 34 (37.8) | 40 (41.2) | ||
| Age 45–64 | 8293 (45.4) | 48 (53.3) | 41 (42.3) | ||
| Age ≥65 | 2140 (11.7) | 8 (8.9) | 16 (16.5) | ||
| Female sex | 15,009 (82.2) | 80 (88.9) | 0.098 | 78 (80.4) | 0.647 |
| Ethnicity | 0.093 | 0.172 | |||
| White | 13,430 (73.5) | 57 (63.3) | 82 (84.5) | ||
| Hispanic | 1816 (9.9) | 13 (14.4) | 5 (5.2) | ||
| Asian/Pacific Islander | 1761 (9.6) | 13 (14.4) | 7 (7.2) | ||
| Black | 919 (5.0) | 7 (7.8) | 3 (3.1) | ||
| Other/Unknown | 334 (1.8) | 0 (0.0) | 0 (0.0) | ||
| Surgery | 0.655 | 0.311 | |||
| No surgery | 117 (0.6) | 0 (0.0) | 0 (0.0) | ||
| Lobectomy | 4993 (27.3) | 27 (30.0) | 21 (21.6) | ||
| Thyroidectomy | 13,114 (71.8) | 63 (70.0) | 76 (78.4) | ||
| Other/unknown | 36 (0.2) | 0 (0.0) | 0 (0.0) | ||
| Lymph nodes examined | 0.113 | <0.001 | |||
| Not examined | 11,503 (60.5) | 62 (68.9) | 35 (36.1) | ||
| Examined | 7156 (39.2) | 28 (31.1) | 62 (63.9) | ||
| Unknown | 51 (0.3) | 0 (0.0) | 0 (0.0) | ||
| Radiation | 0.013 | <0.001 | |||
| None | 12,339 (67.6) | 52 (57.8) | 50 (51.5) | ||
| Radioiodine ablation | 5308 (29.1) | 36 (40.0) | 38 (39.2) | ||
| External beam radiation | 107 (0.6) | 2 (2.2) | 4 (4.1) | ||
| Radioactive implant | 123 (0.7) | 0 (0.0) | 0 (0.0) | ||
| Other/unknown | 383 (2.1) | 0 (0.0) | 5 (5.2) | ||
| Overall survival | 0.720 | 0.532 | |||
| 5-year | 97.5 % | 100.0 % | 98.5 % | ||
| 10-yeara | 94.7 % | 97.6 % | 98.5 % | ||
| Disease-specific survival | 0.410 | 0.173 | |||
| 5-year | 99.6 % | 90.9 % | 98.5 % | ||
| 10-yeara | 99.4 % | 90.9 % | 98.5 % |
Percentages total 100% by column.
Represents 9-year survival for mTCV.
Table 2.
Pathologic Characteristics of Classic Papillary Thyroid Microcarcinoma Compared with Tall Cell and Diffuse Sclerosing Variants
| Classic mPTC[n (%)] | mDSV[n (%)] | p-Value | mTCV[n (%)] | p-Value | |
|---|---|---|---|---|---|
| Size | 0.165 | <0.001 | |||
| Mean (mm) (SEM) | 5.3 (0.02) | 5.8 (0.3) | 7.1 (0.3) | ||
| Size ≤7 mm | 12,427 (68.1) | 63 (70.0) | 49 (50.5) | ||
| Size >7 mm | 5341 (29.2) | 27 (30.0) | 43 (44.3) | ||
| ≤1 cm, NOS | 492 (2.7) | 0 (0.0) | 5 (5.2) | ||
| Multifocality | 0.926 | 0.018 | |||
| Unifocal | 7452 (40.8) | 30 (33.3) | 38 (39.2) | ||
| Multifocal | 3837 (21.0) | 15 (16.7) | 34 (35.0) | ||
| Unknown | 6971 (38.2) | 45 (50.0) | 25 (25.8) | ||
| Extrathyroidal extension | 0.004 | <0.001 | |||
| Intrathyroidal | 17,041 (93.3) | 78 (86.7) | 70 (72.2) | ||
| Extrathyroidal | 1109 (6.1) | 12 (13.3) | 27 (27.8) | ||
| Unknown | 110 (0.6) | 0 (0.0) | 0 (0.0) | ||
| Nodal metastasisa | 0.007 | 0.081 | |||
| No positive lymph nodes | 4785 (66.9) | 12 (42.9) | 35 (56.5) | ||
| ≥1 positive lymph node | 2365 (33.1) | 16 (57.1) | 27 (43.5) | ||
| Unknown | 6 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Median (IQR) | 2 (1–3) | 4 (1–12) | 2 (1–3) | ||
| Distant metastasis | 0.519 | 0.076 | |||
| No distant metastasis | 17,948 (98.3) | 90 (100.0) | 95 (97.9) | ||
| Distant metastasis | 83 (0.5) | 0 (0.0) | 2 (2.1) | ||
| Unknown | 229 (1.3) | 0 (0.0) | 0 (0.0) |
Percentages total 100% by column.
Percentage reflects fraction of patients whose lymph nodes were examined.
Compared to classic mPTC, mDSV had significantly higher rates of extrathyroidal extension (13.3% vs. 6.1%, p=0.004) and nodal metastasis (57.1% vs. 33.1%, p=0.007). In patients with nodal metastasis, the ratio of N1a vs. N1b metastases was similar (50% vs. 50% mDSV, 59.0% vs. 41.0% mPTC, p=0.694). mDSV also tended to be larger (5.8 mm vs. 5.3 mm, p=0.165). There were no differences between mDSV and mPTC with respect to rates of multifocality (33.3% vs. 34.0%, p=0.926) and distant metastasis (0.0% vs. 0.5%, p=0.519).
The mTCV tumors tended to be larger on average compared to mPTC (7.1 mm vs. 5.3 mm, p<0.001), with significantly higher rates of multifocality (47.2% vs. 34.0%, p=0.018) and extrathyroidal extension (27.8% vs. 6.1%, p<0.001). Patients with mTCV tended to have higher rates of nodal metastasis (43.5% vs. 33.1%, p=0.081) and, more than four times the rate of distant metastasis compared to those with mPTC (2.1% vs. 0.5%, p=0.076). In patients with nodal metastasis, patients with mTCV tended to metastasize to the central compartment more frequently than those with mPTC (N1a vs. N1b, 76.5% vs. 23.5% mTCV, 59.0% vs. 41.0% mPTC, p=0.145), although the trend was not statistically significant.
Survival
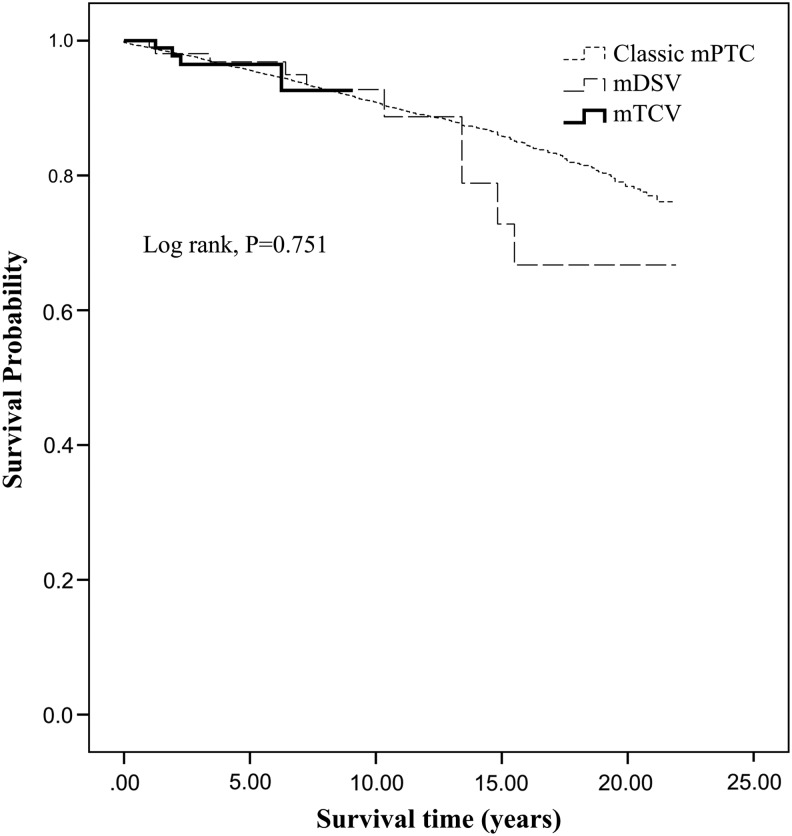
All-cause mortality occurred in 3.3% of mDSV (n=3), 1.0% of mTCV (n=1), and 3.0% of classic mPTC (n=541), while disease specific deaths occurred in 1.1% of mDSV (n=1), 1.0% of mTCV (n=1), and 0.4% (n=69) of classic mPTC. Ten-year disease-specific survival for mDSV, mTCV, and classic mPTC was 100.0%, 98.5%, and 99.4% respectively, and univariate analysis of survival revealed no association between histologic variant and overall or disease-specific survival (Figs. 1 and 2). Due to the limited number of deaths in the mTCV and mDSV cohorts, multivariate analysis could not be performed.
FIG. 1.
Kaplan–Meier analysis of overall survival. mPTC, classic papillary thyroid microcarcinoma; mDSV, diffuse sclerosing variant ≤1 cm; mTCV, tall cell variant ≤1 cm.
FIG. 2.
Kaplan–Meier analysis of disease specific survival.
Predictors of extrathyroidal extension and cervical lymph-node metastasis
No statistically significant predictors of extrathyroidal extension or cervical lymph-node metastases in mDSV were observed. In mTCV, extrathyroidal extension was independently associated with size >7 mm (OR 4.4 [CI 1.5–13.6]), nodal metastasis with multifocality (OR 5.4 [CI 1.3–23.4]) and extrathyroidal extension (OR 5.8, [CI 1.3–25.4]).
Discussion
To our knowledge, this study represents the first population-level analysis of aggressive variants of mPTC. We found the incidences of mTCV and mDSV are not increasing to the same degree as mPTC, and that mTCV and mDSV exhibit aggressive features despite their small size. In comparison to mPTC, mDSV was characterized by higher rates of extrathyroidal extension and nodal metastasis, while mTCV was characterized by higher rates of multifocality and extrathyroidal extension. Both multifocality and extrathyroidal extension predicted nodal metastasis in mTCV. While no association between histologic variant and overall or disease-specific survival was found, our study was underpowered to detect such a difference.
The rapidly rising incidence of mPTC is well established, and is especially salient in patients older than 45 years (1–3). However, no data on the incidence of aggressive variants of mPTC have been reported. In a population-level analysis of DSV and TCV of all sizes, Kazaure et al. reported that the incidence of aggressive variants was outpacing that of PTC in the United States, attributing the increase to improved detection and accuracy in the diagnosis of DSV and TCV (17). A population-level analysis of thyroid cancers in Parma, Italy, similarly showed a significant increase in TCV from 1998 to 2009 (35). Therefore, the 79.9% increase in incidence of mTCV observed in our study is likely real, although our study was limited by sample size. The incidence of mDSV appears to have been more level during the study period.
Management of aggressive variants is controversial, and current guidelines from the American Thyroid Association do not address the extent of thyroid surgery for patients with aggressive variants (36). Overall, studies of DSV and TCV show that prognosis is worse, with increased recurrence and decreased survival (14,17,28–30,37). Some authors have suggested that this difference should be attributed to higher rates of aggressive pathologic features rather than variant histology, and they do not recommend aggressive treatment based on histology alone (27,37,38). Contrary studies that control for these aggressive features show that histology remains an independent risk factor for adverse outcomes, and many advocate total thyroidectomy with prophylactic central lymphadenectomy in aggressive variants regardless of tumor size (8,16,28,30,39). None of these studies has examined mDSV and mTCV specifically, and it is unclear whether histology alone warrants more extensive surgery in tumors ≤1 cm. In our study, no differences in survival were observed between the different histologies, suggesting histology alone may not warrant extensive surgery. However, because mDSV and mTCV appear to exhibit aggressive characteristics with increased rates of multifocality, extrathyroidal extension, and nodal metastasis, we postulate that it is more likely that patients may experience higher rates of tumor recurrence. In patients diagnosed pre- or intraoperatively with micro-aggressive variants of PTC or who exhibit extrathyroidal extension, multifocality, or evidence of lymph-node metastases, we recommend total thyroidectomy, central lymphadenectomy, and postoperative RAI, if indicated. For those diagnosed postoperatively after initial lobectomy, completion thyroidectomy should be considered. Performing a redo prophylactic ipsilateral central lymphadenectomy at the time of completion thyroidectomy is not common practice, even though it has been shown to be safe if performed in a high-volume center with experienced surgeons (40).
Preoperative diagnosis of DSV and TCV is difficult (8,39). TCV and DSV have unique cytopathologic characteristics, and although diagnostic criteria have not been rigorously evaluated, several reports indicate that finding tadpole-shaped cells or inflammation and squamous metaplasia on FNA can raise preoperative suspicion of TCV or DSV (19,20,22,41,42). Nevertheless, preoperative diagnosis of variant histologies on fine needle aspiration (FNA) is limited, and it is more common for patients to be diagnosed postoperatively after histopathologic examination. Our study suggests that aggressive features found in DSV and TCV persist in tumors ≤1 cm in size, and that therefore mDSV and mTCV are disproportionately represented in microcarcinomas presenting with size >7 mm, multifocality, extrathyroidal extension, and nodal metastases (9–17,23–30). Therefore, in patients with aggressive pre- or intraoperative findings, such as a high degree of sclerosis, obvious extrathyroidal extension or evidence of positive lymph nodes, clinicians should have a high degree of suspicion for an aggressive variant and be prepared to perform a more aggressive operation with respect to the extent of thyroid surgery and possible lymphadenectomy.
Use of molecular tests is becoming more common, and may aid in the preoperative diagnosis of more aggressive variants of PTC. BRAF V600E has been shown in mPTC to be associated with tumor size, extrathyroidal extension, multifocality, nodal metastases, and advanced stage (43–46). Furthermore, the mutation is highly prevalent in TCV, with reports ranging from 66% to 100% (43,46–50). While the molecular pathogenesis of DSV is less well studied, RET/PTC rearrangements appear to predominate (51). More work needs to be done on the molecular testing of these variants in order to provide valuable diagnostic, prognostic, and therapeutic information (52).
Limitations of this study include those inherent to the SEER database, such as coding errors, limited data for variables where collection began only recently (e.g., multifocality and location of cervical lymph-node metastases), and lack of data on variables not collected by SEER (e.g., BRAF/RET gene status, central vs. lateral lymphadenectomy, reoperation, and recurrence). mTCV was first captured in the SEER database in 2001 and a mean follow-up of 3.8 years limits our analysis of survival. Because mDSV and mTCV are rare tumors, our study may still be underpowered to document more subtle differences between mDSV, mTCV, and mPTC. Despite these limitations, it is the largest study to date.
Overall, mDSV and mTCV are rare tumors that share many characteristics with histologically identical tumors >1 cm in size. They tend to be more aggressive compared to classic mPTC, and while they do not appear to differ with respect to survival, given our findings of higher nodal involvement and extrathyroidal extension, we postulate that they may have higher recurrence rates. Treatment with total thyroidectomy and possible central lymphadenectomy may be indicated. Long-term data on recurrence and more highly powered studies of survival will elucidate the prognosis of patients with mDSV and mTCV. Further understanding of the molecular pathogenesis of mDSV and mTCV will improve future diagnostic and prognostic power.
Acknowledgments
This publication was made possible by Grant Number TL 1 RR024137 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent office views of NCRR or NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Hughes DT. Haymart MR. Miller BS. Gauger PG. Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 4.Sugitani I. Toda K. Yamada K. Yamamoto N. Ikenaga M. Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–1231. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 5.Yu XM. Wan Y. Sippel RS. Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 6.Vickery AL., Jr Carcangiu ML. Johannessen JV. Sobrinho-Simoes M. Papillary carcinoma. Semin Diagn Pathol. 1985;2:90–100. [PubMed] [Google Scholar]
- 7.Carcangiu ML. Bianchi S. Diffuse sclerosing variant of papillary thyroid carcinoma. Clinicopathologic study of 15 cases. Am J Surg Pathol. 1989;13:1041–1049. doi: 10.1097/00000478-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Carling T. Ocal IT. Udelsman R. Special variants of differentiated thyroid cancer: does it alter the extent of surgery versus well-differentiated thyroid cancer? World J Surg. 2007;31:916–923. doi: 10.1007/s00268-006-0837-3. [DOI] [PubMed] [Google Scholar]
- 9.Soares J. Limbert E. Sobrinho-Simoes M. Diffuse sclerosing variant of papillary thyroid carcinoma. A clinicopathologic study of 10 cases. Pathol Res Pract. 1989;185:200–206. doi: 10.1016/S0344-0338(89)80252-3. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto Y. Obara T. Ito Y. Kodama T. Aiba M. Yamaguchi K. Diffuse sclerosing variant of papillary carcinoma of the thyroid. Clinical importance, surgical treatment, and follow-up study. Cancer. 1990;66:2306–2312. doi: 10.1002/1097-0142(19901201)66:11<2306::aid-cncr2820661109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Albareda M. Puig-Domingo M. Wengrowicz S. Soldevila J. Matias-Guiu X. Caballero A. Chico A. De Leiva A. Clinical forms of presentation and evolution of diffuse sclerosing variant of papillary carcinoma and insular variant of follicular carcinoma of the thyroid. Thyroid. 1998;8:385–391. doi: 10.1089/thy.1998.8.385. [DOI] [PubMed] [Google Scholar]
- 12.Sywak M. Pasieka JL. Ogilvie T. A review of thyroid cancer with intermediate differentiation. J Surg Oncol. 2004;86:44–54. doi: 10.1002/jso.20044. [DOI] [PubMed] [Google Scholar]
- 13.Lam AK. Lo CY. Diffuse sclerosing variant of papillary carcinoma of the thyroid: a 35-year comparative study at a single institution. Ann Surg Oncol. 2006;13:176–181. doi: 10.1245/ASO.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 14.Falvo L. Giacomelli L. D'Andrea V. Marzullo A. Guerriero G. de Antoni E. Prognostic importance of sclerosing variant in papillary thyroid carcinoma. Am Surg. 2006;72:438–444. [PubMed] [Google Scholar]
- 15.Lee JY. Shin JH. Han BK. Ko EY. Kang SS. Kim JY. Oh YL. Chung JH. Diffuse sclerosing variant of papillary carcinoma of the thyroid: imaging and cytologic findings. Thyroid. 2007;17:567–573. doi: 10.1089/thy.2006.0321. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima M. Ito Y. Hirokawa M. Akasu H. Shimizu K. Miyauchi A. Clinicopathologic characteristics and prognosis of diffuse sclerosing variant of papillary thyroid carcinoma in Japan: an 18-year experience at a single institution. World J Surg. 2009;33:958–962. doi: 10.1007/s00268-009-9940-6. [DOI] [PubMed] [Google Scholar]
- 17.Kazaure HS. Roman SA. Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Annals Surg Oncol. 2012;19:1874–1880. doi: 10.1245/s10434-011-2129-x. [DOI] [PubMed] [Google Scholar]
- 18.Hawk WA. Hazard JB. The many appearances of papillary carcinoma of the thyroid. Cleve Clin Q. 1976;43:207–215. doi: 10.3949/ccjm.43.4.207. [DOI] [PubMed] [Google Scholar]
- 19.Gamboa-Dominguez A. Candanedo-Gonzalez F. Uribe-Uribe NO. Angeles-Angeles A. Tall cell variant of papillary thyroid carcinoma. A cytohistologic correlation. Acta Cytol. 1997;41:672–676. doi: 10.1159/000332682. [DOI] [PubMed] [Google Scholar]
- 20.Bocklage T. DiTomasso JP. Ramzy I. Ostrowski ML. Tall cell variant of papillary thyroid carcinoma: cytologic features and differential diagnostic considerations. Diagn Cytopathol. 1997;17:25–29. doi: 10.1002/(sici)1097-0339(199707)17:1<25::aid-dc5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Ghossein R. Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid. 2008;18:1179–1181. doi: 10.1089/thy.2008.0164. [DOI] [PubMed] [Google Scholar]
- 22.Urano M. Kiriyama Y. Takakuwa Y. Kuroda M. Tall cell variant of papillary thyroid carcinoma: Its characteristic features demonstrated by fine-needle aspiration cytology and immunohistochemical study. Diagn Cytopathol. 2009;37:732–737. doi: 10.1002/dc.21086. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TL. Lloyd RV. Thompson NW. Beierwaltes WH. Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1988;12:22–27. doi: 10.1097/00000478-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski ML. Merino MJ. Tall cell variant of papillary thyroid carcinoma: a reassessment and immunohistochemical study with comparison to the usual type of papillary carcinoma of the thyroid. Am J Surg Pathol. 1996;20:964–974. doi: 10.1097/00000478-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Prendiville S. Burman KD. Ringel MD. Shmookler BM. Deeb ZE. Wolfe K. Azumi N. Wartofsky L. Sessions RB. Tall cell variant: an aggressive form of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2000;122:352–357. doi: 10.1016/S0194-5998(00)70047-7. [DOI] [PubMed] [Google Scholar]
- 26.Machens A. Holzhausen HJ. Lautenschlager C. Dralle H. The tall-cell variant of papillary thyroid carcinoma: a multivariate analysis of clinical risk factors. Langenbecks Arch Surg. 2004;389:278–282. doi: 10.1007/s00423-004-0485-8. [DOI] [PubMed] [Google Scholar]
- 27.Michels JJ. Jacques M. Henry-Amar M. Bardet S. Prevalence and prognostic significance of tall cell variant of papillary thyroid carcinoma. Human Pathol. 2007;38:212–219. doi: 10.1016/j.humpath.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Ghossein RA. Leboeuf R. Patel KN. Rivera M. Katabi N. Carlson DL. Tallini G. Shaha A. Singh B. Tuttle RM. Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: biologic behavior and clinical implications. Thyroid. 2007;17:655–661. doi: 10.1089/thy.2007.0061. [DOI] [PubMed] [Google Scholar]
- 29.Leung AK. Chow SM. Law SC. Clinical features and outcome of the tall cell variant of papillary thyroid carcinoma. Laryngoscope. 2008;118:32–38. doi: 10.1097/MLG.0b013e318156f6c3. [DOI] [PubMed] [Google Scholar]
- 30.Morris LG. Shaha AR. Tuttle RM. Sikora AG. Ganly I. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid. 2010;20:153–158. doi: 10.1089/thy.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay ID. Hutchinson ME. Gonzalez-Losada T. McIver B. Reinalda ME. Grant CS. Thompson GB. Sebo TJ. Goellner JR. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987. doi: 10.1016/j.surg.2008.08.035. discussion 987–988. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Overview of the SEER program. http://seer.cancer.gov/about/overview.html. [Aug 28;2012 ]. http://seer.cancer.gov/about/overview.html
- 33.Akslen LA. Varhaug JE. Thyroid carcinoma with mixed tall-cell and columnar-cell features. Am J Clin Pathol. 1990;94:442–445. doi: 10.1093/ajcp/94.4.442. [DOI] [PubMed] [Google Scholar]
- 34.Pilotti S. Collini P. Manzari A. Marubini E. Rilke F. Poorly differentiated forms of papillary thyroid carcinoma: distinctive entities or morphological patterns? Semin Diagn Pathol. 1995;12:249–255. [PubMed] [Google Scholar]
- 35.Ceresini G. Corcione L. Michiara M. Sgargi P. Teresi G. Gilli A. Usberti E. Silini E. Ceda GP. Thyroid cancer incidence by histological type and related variants in a mildly iodine-deficient area of Northern Italy, 1998 to 2009. Cancer. 2012;118:5473–5480. doi: 10.1002/cncr.27591. [DOI] [PubMed] [Google Scholar]
- 36.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 37.Regalbuto C. Malandrino P. Frasca F. Pellegriti G. Le Moli R. Vigneri R. Pezzino V. The tall cell variant of papillary thyroid carcinoma: clinical and pathological features and outcomes. J Endocrinol Investigat. 2013;36:249–254. doi: 10.3275/8515. [DOI] [PubMed] [Google Scholar]
- 38.Regalbuto C. Malandrino P. Tumminia A. Le Moli R. Vigneri R. Pezzino V. A diffuse sclerosing variant of papillary thyroid carcinoma: clinical and pathologic features and outcomes of 34 consecutive cases. Thyroid. 2011;21:383–389. doi: 10.1089/thy.2010.0331. [DOI] [PubMed] [Google Scholar]
- 39.Silver CE. Owen RP. Rodrigo JP. Rinaldo A. Devaney KO. Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011;33:1052–1059. doi: 10.1002/hed.21494. [DOI] [PubMed] [Google Scholar]
- 40.Alvarado R. Sywak MS. Delbridge L. Sidhu SB. Central lymph node dissection as a secondary procedure for papillary thyroid cancer: Is there added morbidity? Surgery. 2009;145:514–518. doi: 10.1016/j.surg.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Ohori NP. Schoedel KE. Cytopathology of high-grade papillary thyroid carcinomas: tall-cell variant, diffuse sclerosing variant, and poorly differentiated papillary carcinoma. Diagn Cytopathol. 1999;20:19–23. doi: 10.1002/(sici)1097-0339(199901)20:1<19::aid-dc5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Caruso G. Tabarri B. Lucchi I. Tison V. Fine needle aspiration cytology in a case of diffuse sclerosing carcinoma of the thyroid. Acta Cytol. 1990;34:352–354. [PubMed] [Google Scholar]
- 43.Lee X. Gao M. Ji Y. Yu Y. Feng Y. Li Y. Zhang Y. Cheng W. Zhao W. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16:240–245. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 44.Kwak JY. Kim EK. Chung WY. Moon HJ. Kim MJ. Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253:854–860. doi: 10.1148/radiol.2533090471. [DOI] [PubMed] [Google Scholar]
- 45.Lin KL. Wang OC. Zhang XH. Dai XX. Hu XQ. Qu JM. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:3294–3300. doi: 10.1245/s10434-010-1129-6. [DOI] [PubMed] [Google Scholar]
- 46.Virk RK. Van Dyke AL. Finkelstein A. Prasad A. Gibson J. Hui P. Theoharis CG. Carling T. Roman SA. Sosa JA. Udelsman R. Prasad ML. BRAF(V600E) mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol. 2013;26:62–70. doi: 10.1038/modpathol.2012.152. [DOI] [PubMed] [Google Scholar]
- 47.Adeniran AJ. Zhu Z. Gandhi M. Steward DL. Fidler JP. Giordano TJ. Biddinger PW. Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 48.Sheu SY. Grabellus F. Schwertheim S. Handke S. Worm K. Schmid KW. Lack of correlation between BRAF V600E mutational status and the expression profile of a distinct set of miRNAs in papillary thyroid carcinoma. Horm Metab Res. 2009;41:482–487. doi: 10.1055/s-0029-1215558. [DOI] [PubMed] [Google Scholar]
- 49.Basolo F. Torregrossa L. Giannini R. Miccoli M. Lupi C. Sensi E. Berti P. Elisei R. Vitti P. Baggiani A. Miccoli P. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. doi: 10.1210/jc.2010-0337. [DOI] [PubMed] [Google Scholar]
- 50.Finkelstein A. Levy GH. Hui P. Prasad A. Virk R. Chhieng DC. Carling T. Roman SA. Sosa JA. Udelsman R. Theoharis CG. Prasad ML. Papillary thyroid carcinomas with and without BRAF V600E mutations are morphologically distinct. Histopathology. 2012;60:1052–1059. doi: 10.1111/j.1365-2559.2011.04149.x. [DOI] [PubMed] [Google Scholar]
- 51.Sheu SY. Schwertheim S. Worm K. Grabellus F. Schmid KW. Diffuse sclerosing variant of papillary thyroid carcinoma: lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod Pathol. 2007;20:779–787. doi: 10.1038/modpathol.3800797. [DOI] [PubMed] [Google Scholar]
- 52.Theoharis C. Roman S. Sosa JA. The molecular diagnosis and management of thyroid neoplasms. Curr Opin Oncol. 2012;24:35–41. doi: 10.1097/CCO.0b013e32834dcfca. [DOI] [PubMed] [Google Scholar]