Abstract
Background
The aims of this study were (i) to obtain insight into the prevalence of fatigue among short- and long-term thyroid cancer (TC) survivors, by comparing a sample of TC survivors with an age- and sex-matched normative population, and (ii) to investigate which demographic, clinical, and TC-specific health-related quality of life (HRQoL) characteristics were associated with fatigue.
Methods
All patients found to have TC between 1990 and 2008, as registered in the Eindhoven Cancer Registry, received a cross-sectional survey on fatigue (Fatigue Assessment Scale), TC-specific HRQoL (THYCA-QoL), and psychological distress (Hospital Anxiety and Depression Scale). The fatigue scores were compared with those of an age- and sex-matched normative population (n=530). Multiple logistic regression analyses were conducted to investigate the independent associations between clinical and demographic characteristics, TC-specific HRQoL, and psychological distress with fatigue.
Results
Eighty-six percent (n=306) responded. TC survivors were more often classified as fatigued or very fatigued (short-term <5 years: 43%; long-term 5–10 years: 44%; long-term 10–15 years: 47%; long-term >15 years: 39%) compared to the normative population (25%; p<0.001). Anxiety (odds ratio (OR) 1.15, 95% confidence interval [CI] 1.03–1.28) and depression (OR 1.43 [CI 1.22–1.68]) were associated with fatigue, as was also the case for TC-specific neuromuscular (OR 1.03 [CI 1.01–1.06]), concentration (OR 1.03 [CI 1.01–1.06]), and psychological TC-specific HRQoL (OR 1.06 [CI 1.02–1.10]).
Conclusion
Short- and long-term TC survivors report higher levels of fatigue than an age- and sex-matched normative population do. Both TC-specific HRQoL and psychological distress were associated with fatigue.
Introduction
Worldwide, the incidence of thyroid cancer (TC) is rising (1). As a result of the very good prognosis of papillary and follicular TC (exceeding >90% 5-year survival), the number of TC survivors is also rising (2,3). Treatment of TC involves surgery, predominantly (near-)total thyroidectomy, followed by radioactive iodine (131I) therapy to ablate the remaining thyroid tissue. Depending on type and size of the tumor, hemithyroidectomy can suffice. The removal of the thyroid gland is accompanied by a lifelong dependence on substitution therapy with levothyroxine and in the first two years with dosing regimens suppressing thyrotropin (TSH) production (4,5), causing subclinical hyperthyroidism. Despite the efficacy of these primary treatments and the high long-term survival rates, the disease can recur even decades later. Therefore, long-term follow-up is necessary. Given the longevity of TC patients, the possible long-term effects of cancer and its treatment on patients' well-being are of increasing importance.
Fatigue is a common problem among different groups of cancer survivors, with prevalence rates ranging between 17% and 90% (6–8). Decreased health-related quality of life (HRQoL) (9) and high levels of psychological distress (10–12) are associated with high levels of fatigue in cancer survivors. There are a limited number of studies reporting on the levels of fatigue among TC patients (13–20); nevertheless, exact prevalence rates of fatigue among long-term TC cancer survivors (≥5 years after diagnosis) are lacking. Since short-term TC survivors receive suppressive doses of levothyroxine, causing subclinical hyperthyroidism, which is frequently accompanied by high levels of fatigue (21), it can be expected that these survivors experience higher levels of fatigue than long-term TC survivors who have returned to a euthyroid state.
The primary objective of our study was to obtain insight into the prevalence of fatigue among short- and long-term TC survivors, by comparing a sample of TC survivors with an age- and sex-matched normative population. Second, our objective was to investigate the associations between demographic and clinical characteristics, TC-specific HRQoL, and psychological distress with levels of fatigue in TC survivors. Our hypotheses were as follows: (i) short-term TC survivors report higher levels of fatigue than long-term survivors and the normative population do; (ii) long-term survivors return to normal levels of fatigue and therefore report similar fatigue levels compared to the normative population; (iii) clinical characteristics, TC-specific HRQoL, and psychological distress are significantly associated with fatigue.
Materials and Methods
Setting and population
This study was a population-based survey among TC survivors registered within the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre South (CCCS). The ECR compiles data of all individuals newly found to have cancer in the southern part of the Netherlands, an area with 10 hospitals serving 2.3 million inhabitants (22). All individuals found to have TC between 1990 and 2008 as registered in the ECR were eligible for participation (n=568). We excluded patients who had cognitive impairment or were too ill at the time of the study (medical records and advice from the attending specialist; n=31), had unverifiable addresses (n=90), or died prior to the start of the study (according to the ECR, the Central Bureau for Genealogy, which collects information on all deceased Dutch citizens via the civil municipal registries, and hospital records; n=6). One hospital declined to participate (n=86). Questionnaires were sent to the remaining 355 patients. This study was approved by the certified Medical Ethics Committee of the Maxima Medical Centre in Eindhoven.
Data collection
Data collection started in November 2010 and was done within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship) (23). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short- and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR. Details of the PROFILES data collection method have been previously described (23). In summary, survivors were informed of the study via a letter from their (ex-)attending specialist. The letter included a link to a secure website, a login name, and a password, so that interested patients could provide informed consent and complete questionnaires online. If the patient did not have access to the Internet, or preferred written rather than digital communication, (s)he could return our postcard by mail after which (s)he received our paper-and-pencil version of the informed consent form and questionnaire. Data from the PROFILES registry are available for noncommercial scientific research, subject to study question, privacy and confidentiality restrictions, and registration.
Study measures
Sociodemographic and clinical characteristics
Survivors' sociodemographic and clinical characteristics at the time of cancer diagnosis were available from the ECR. The ECR routinely collects data on tumor characteristics, including date of diagnosis, tumor grade, and stage according to the Tumor-Node-Metastasis clinical classification (24), treatment, and patient background characteristics, including date of birth and comorbidity at the time of diagnosis. Self-reported comorbidity at the time of survey was categorized according to the adapted Self-administered Comorbidity Questionnaire (25), including heart disease, stroke, high blood pressure, lung disease, diabetes, ulcer or stomach disease, kidney disease, anemia or other blood disease, depression, osteoarthritis, back pain, rheumatoid arthritis, and a question on other medical problems. Questions on marital status, educational level, and current occupation were added to the questionnaire.
Psychological distress
Psychological distress was assessed with the Hospital Anxiety and Depression Scale, with seven items each assessing anxiety and depression (26). Clinical level of anxiety or depressive symptoms was indicated with a score of ≥8 on each subscale (26,27).
TC-specific HRQoL
TC-specific HRQoL was measured by the THYCA-QoL, which was developed to assess side effects caused by TC or its treatment (28). The questionnaire consists of 24 items, with a time frame of the previous week, except for the sexual interest item, which is 4 weeks. Each item is scored on a 4-point response scale ranging from 1, “not at all,” to 4, “very much.” The THYCA-QoL consists of seven scales (neuromuscular, voice, concentration, sympathetic, throat/mouth, psychological, and sensory problems) and six single items. Scores were linear-transformed to a 0–100 scale. A higher score on this scale means more complaints.
Fatigue
Fatigue was assessed with the Fatigue Assessment Scale (FAS), a questionnaire consisting of 10 items: five questions reflecting physical fatigue and five questions for mental fatigue. The response scale is a 5-point scale (1, never, to 5, always) and total scores can range from 10 to 50. Participants can be divided into three groups based on total FAS scores (29): not fatigued (as defined by a score of 10 to 21), fatigued (22 to 34), and very fatigued (35 to 50). The psychometric properties are good (30,31), and the questionnaire has been used with cancer patients before (32).
Normative population
Normative population data were obtained from CentERpanel, an online household panel that is representative of the Dutch population. The process of the annual data collection, which started in 2009 by our study group, is described elsewhere (33). The most recent data wave in 2011 also included an assessment of fatigue with the FAS. From the 2040 (82%) members of 18 years and older, an age- and sex-matched normative sample (n=530) was selected for this study to reflect the age and sex distribution of the TC sample. Sociodemographic data such as marital status, and comorbidity with the Self-administered Comorbidity Questionnaire were also collected for this group. TC-specific HRQoL was not assessed in the normative population.
Statistical analyses
All statistical analyses were performed using SPSS version 17.0 (Statistical Package for Social Sciences, Chicago, IL) and p-values <0.05 were considered statistically significant. Missing items from the FAS total scale were mean imputed if at least eight of the items from the scale were answered.
Demographic and clinical data of respondents, nonrespondents, and patients with unverifiable addresses were compared using chi-square statistics for categorical variables and analysis of variance for continuous variables. The nonparametric Wilcoxon test was applied when normality and homogeneity assumptions of continuous variables were violated.
Analysis of variance was furthermore used to compare the mean scores of the TC sample (stratified by years since diagnosis: short-term <5 years; long-term 5–10 years; long-term 10–15 years; long-term >15 years) on the FAS items and total score with those of the Dutch normative population. Chi-square analyses were performed to compare the categorized FAS scores (not fatigued, fatigued, very fatigued) between short- and long-term survivors and the normative population. Clinically meaningful differences were determined with Norman's “rule of thumb,” whereby a difference of≈0.5 SD indicates a threshold of discriminant change in scores of a chronic illness (34).
Logistic regression models were conducted to identify associations of demographic and clinical characteristics, TC-specific HRQoL, and psychological distress with fatigue, since all these factors were associated with fatigue among other groups of cancer survivors (9,11,35,36). The models were composed as follows: (i) demographics; (ii) demographics+clinical variables; (iii) demographics+clinical variables+psychological distress; (iv) demographics+clinical variables+TC-specific HRQoL. Patients with missing items concerning the selected variables were excluded from the analyses.
The total FAS score was divided into two groups, 10–21 (not fatigued) and 22–50 (fatigued), as previously done (29).
Results
Patient and tumor characteristics
Three hundred six patients returned a completed questionnaire (response 86%). A comparison of respondents, nonrespondents, and patients with unverifiable addresses indicated that patients with unverifiable addresses were younger than nonrespondents and respondents (mean 52, 56, and 54 years, respectively; p=0.04) (37). No differences between groups were seen regarding sex, type of TC, stage of the disease, or primary treatment.
The mean age at the time of the survey was 56 (SD 15) years. The median time since diagnoses was 9.0 years. More than half of the patients were found to have stage I disease (57%). Surgery (99%) was followed by 131I ablation therapy in 69% and by 131I therapy in 3% of the cases. More than three quarters (78%) of the patients had one or more comorbid conditions. The most common comorbidities were backache (36%), high blood pressure (29%), osteoarthritis (28%), and cardiovascular problems (12%).
Comparisons on clinical and sociodemographic characteristics, TC-specific HRQoL, and psychological distress of respondents stratified by years since diagnosis showed that short-term survivors were more likely to be found to have a higher stage disease than long-term survivors (Table 1). Furthermore, survivors <5 years since diagnosis were more likely to report voice, sympathetic, and throat/mouth problems than survivors >15 years since diagnosis and higher levels of anxiety than survivors ≥10 years since diagnosis (Table 2). There was a trend toward diminishing TC-specific problems over time.
Table 1.
Clinical and Sociodemographic Characteristics of Thyroid Cancer Survivors Stratified by Time Since Diagnosis and the Normative Population [n (%)]
| Short-term survivors, <5 years (n=81) | Long-term survivors, 5–10 years (n=86) | Long-term survivors, 10–15 years (n=75) | Long-term survivors, >15 years (n=64) | Normative population (n=530) | pa | |
|---|---|---|---|---|---|---|
| Mean age at time of survey (±SD) | 55.6±16.0 | 54.5±14.8 | 56.8±13.1 | 59.6±13.4 | 55.8±14.6 | 0.26 |
| Mean years since initial diagnosis (±SD) | 3.3±0.9 | 7.3±1.5 | 12.1±1.5 | 17.8±1.8 | n.a. | <0.001 |
| Sex | 0.95 | |||||
| Male | 20 (25) | 24 (28) | 17 (23) | 15 (23) | 132 (25) | |
| Female | 61 (75) | 62 (72) | 58 (77) | 49 (77) | 397 (75) | |
| Type of thyroid cancer | 0.15 | |||||
| Papillary | 63 (80) | 58 (67) | 55 (73) | 42 (66) | n.a. | |
| Follicular (including Hürthle cell) | 13 (17) | 23 (27) | 20 (27) | 17 (27) | ||
| Medullary | 3 (4) | 5 (6) | — | 5 (8) | ||
| Primary treatment | 0.24 | |||||
| Surgery alone | 19 (24) | 26 (30) | 22 (29) | 16 (25) | n.a. | |
| Surgery+131I ablation | 59 (73) | 59 (69) | 51 (68) | 43 (67) | ||
| Surgery+131I therapy | 3 (4) | — | 1 (1) | 5 (8) | ||
| Other (chemotherapy/131I therapy or no oncological treatment) | — | 1 (1) | 1 (1) | — | ||
| Stage | <0.001 | |||||
| 1 | 34 (43) | 54 (64) | 41 (57) | 43 (68) | n.a. | |
| 2 | 11 (14) | 14 (17) | 18 (25) | 16 (25) | ||
| 3 | 22 (28) | 12 (14) | 11 (15) | 3 (5) | ||
| 4 | 12 (15) | 5 (6) | 2 (3) | 1 (2) | ||
| Comorbidity at time of study | 0.01 | |||||
| None | 19 (24) | 29 (34) | 17 (23) | 16 (25) | 215 (41) | |
| 1 | 26 (32) | 24 (28) | 24 (32) | 18 (28) | 128 (24) | |
| ≥2 | 36 (44) | 33 (38) | 34 (45) | 30 (47) | 188 (35) | |
| Partner | 0.28 | |||||
| Yes | 58 (72) | 69 (80) | 62 (83) | 49 (77) | 389 (73) | |
| No | 23 (28) | 17 (20) | 13 (17) | 15 (23) | 142 (27) | |
| Educational levelb | <0.001 | |||||
| Low | 5 (6) | 11 (13) | 9 (12) | 8 (13) | 20 (4) | |
| Medium | 50 (62) | 49 (58) | 49 (65) | 44 (69) | 315 (60) | |
| High | 26 (32) | 25 (29) | 17 (23) | 12 (19) | 194 (37) | |
| Employment status | 0.29 | |||||
| Yes | 40 (50) | 48 (57) | 40 (54) | 26 (42) | 250 (47) | |
| No | 40 (50) | 36 (43) | 34 (46) | 36 (58) | 281 (53) |
Some variables exceed 100% because of rounding off; some variables do not add up to 100% because of missing data.
p-Value for comparison of all available groups; Bold=p<0.05.
Low (no or primary school); medium (lower general secondary education or vocational training); high (preuniversity education, high vocational training, university.
n.a., these items were not assessed in the normative population; SD, standard deviation.
Table 2.
Mean Scores (±SD) of Thyroid Cancer–Specific Health-Related Quality of Life and Psychological Distress Stratified by Time Since Diagnosis and the Normative Population
| Short-term survivors, <5 years (n=81) | Long-term survivors, 5–10 years (n=86) | Long-term survivors, 10–15 years (n=75) | Long-term survivors, >15 years, (n=64) | Normative population (n=530) | pa | |
|---|---|---|---|---|---|---|
| Thyroid cancer–specific HRQoL | ||||||
| Neuromuscular problems | 27.7±22.1 | 23.8±23.3 | 22.5±20.4 | 21.6±21.3 | n.a. | 0.35 |
| Voice problems | 16.0±22.7 | 12.1±20.6 | 9.0±15.7 | 5.2±11.7 | 0.006 | |
| Concentration problems | 19.6±21.5 | 18.5±25.2 | 12.1±19.3 | 12.1±18.5 | 0.06 | |
| Sympathetic problems | 23.3±29.2 | 21.8±28.3 | 14.4±20.5 | 13.6±19.7 | 0.04 | |
| Throat/mouth problems | 19.3±20.1 | 13.2±18.1 | 12.3±15.3 | 4.8±8.0 | <0.001 | |
| Psychological problems | 16.9±17.1 | 15.8±18.7 | 12.9±16.1 | 10.7±12.4 | 0.12 | |
| Sensory problems | 17.5±19.2 | 14.3±19.8 | 14.1±18.9 | 14.3±21.2 | 0.66 | |
| Psychological distress | ||||||
| HADS anxiety | 5.2±3.6 | 5.1±4.4 | 4.1±3.2 | 4.2±3.7 | 3.7±3.3 | 0.001 |
| HADS depression | 3.6±2.9 | 3.7±3.5 | 3.0±2.6 | 3.4±3.2 | 3.6±3.3 | 0.66 |
| % above the ≥8 clinical cutoff (26,27) | ||||||
| HADS anxiety | 19 (24) | 20 (24) | 8 (12) | 8 (14) | 64 (12) | 0.004 |
| HADS depression | 11 (14) | 13 (16) | 5 (7) | 7 (12) | 7 (12) | 0.60 |
p-Value for comparison of all available groups. Bold=p<0.05.
HADS, Hospital Anxiety and Depression Scale; HRQoL, health-related quality of life; n.a., these items were not assessed in the normative population.
The normative population was more likely to be higher educated, reported less comorbidities, and was less likely to have anxiety than short- and long-term TC survivors (Tables 1 and 2).
Fatigue of TC survivors compared with a normative population
In general, short-term survivors reported the highest levels of mean fatigue scores, whereas the normative population reported the lowest scores (Table 3).
Table 3.
Mean Fatigue Scores of Thyroid Cancer Survivors by Years Since Diagnosis and the Normative Population
| |
|
|
|
|
|
p |
|
|---|---|---|---|---|---|---|---|
| FAS items (range 1–5) | Short-term survivors, <5 years (n=80) | Long-term survivors, 5–10 years (n=84) | Long-term survivors, 10–15 years (n=72) | Long-term survivors, >15 years (n=60) | Normative population (n=530) | All survivors | Normative population+all survivors |
| 1. I am bothered by fatigue. | 2.7±1.1 | 2.8±1.2 | 2.7±1.2 | 2.5±1.0 | 2.1±0.9 | 0.45 | <0.001 |
| 2. I get tired very quickly. | 2.6±1.2a | 2.6±1.3a | 2.4±1.1a | 2.3±1.1 | 1.9±0.9 | 0.46 | <0.001 |
| 3. I do not do much during the day. | 2.2±1.1 | 2.3±1.3 | 1.9±1.0 | 2.0±1.1 | 1.8±0.9 | 0.14 | <0.001 |
| 4. I have enough energy for everyday life.b | 2.9±1.3 | 2.7±1.3 | 2.7±1.3 | 2.5±1.3 | 2.6±1.3 | 0.51 | 0.43 |
| 5. Physically, I feel exhausted. | 2.1±1.0a | 2.0±1.1 | 1.9±0.9 | 1.8±0.9 | 1.6±0.8 | 0.49 | <0.001 |
| 6. I have problems starting things. | 2.0±0.9 | 2.0±1.1 | 1.8±0.7 | 1.9±0.9 | 1.7±0.8 | 0.43 | 0.01 |
| 7. I have problems thinking clearly. | 1.9±0.9a | 1.8±1.1 | 1.6±0.6 | 1.7±0.7 | 1.4±0.7 | 0.08 | <0.001 |
| 8. I feel no desire to do anything. | 2.0±0.8 | 2.0±1.0 | 1.8±0.7 | 2.0±0.8 | 1.7±0.7 | 0.47 | <0.001 |
| 9. Mentally, I feel exhausted. | 1.8±0.9 | 1.8±1.1 | 1.6±0.7 | 1.6±0.8 | 1.5±0.7 | 0.19 | <0.001 |
| 10. When I am doing something, I can concentrate quite well.b | 2.5±1.2 | 2.6±1.3 | 2.5±1.3 | 2.3±1.2 | 2.4±1.3 | 0.62 | 0.52 |
| FAS mean total score | 22.6±7.3a | 22.6±8.5 | 20.9±6.6 | 20.5±6.7 | 18.7±5.9 | 0.20 | <0.001 |
Higher scores indicate higher levels of fatigue. Bold=p<0.05.
Clinically meaningful difference based on Norman's rule of≈0.5 SD (34) detected between indicated group and the normative population.
Reversed items.
FAS, Fatigue Assessment Scale.
The normative population was significantly less likely than the survivors group to report being bothered by fatigue, getting tired quickly, not being able to do much, feeling exhausted physically and mentally, problems with starting things and thinking clearly, and no desire to do anything. Also, the FAS total score was significantly lower for the normative population than that for the TC survivors. No significant differences were found between short- and long-term survivors.
Clinically meaningful differences between the normative population and the short-term survivors were found for the items getting tired very quickly, feeling physically exhausted, problems with thinking clearly, and the FAS total score. Furthermore, a clinically meaningful difference was found between long-term survivors (5–15 years after diagnosis) and the normative population for the item getting tired very quickly. Comparison between short- and long-term survivor groups showed no clinically meaningful differences in fatigue scores (Table 3).
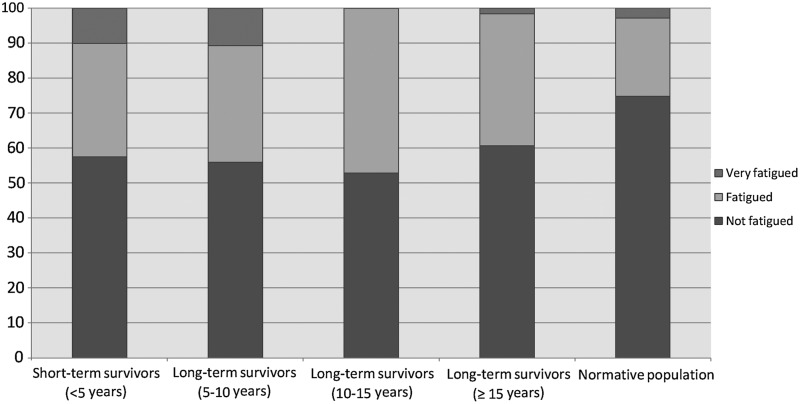
When stratified by time since diagnosis and divided into three fatigue groups, significant differences were noted between all the survivors (Fig. 1) and the normative population (p<0.001). Survivors were more often classified as fatigued or very fatigued (short-term: 43%; long-term 5–10 years: 44%; long-term 10–15 years: 47%; long-term >15 years: 39%) than the normative population (25%). Furthermore long-term survivors >15 years after diagnoses reported less fatigue compared to the other three survivor groups, and survivors <10 years after diagnosis reported more often to be very fatigued compared to survivors ≥10 years after diagnosis (p=0.03).
FIG. 1.
Fatigue levels of TC survivors compared to an age- and sex-matched normative population. % TC survivors stratified by years since diagnosis (short-term: <5 years; long-term: ≥5 years), and the normative population, which is fatigued. Category cutoffs were based on FAS total scores: not fatigued (10–21), fatigued (22–34), very fatigued (35–50). Significant differences were noted between the survivors and the normative population (p<0.001).
Logistic regression
Logistic regression models were conducted to identify associations of demographic and clinical characteristics, TC-specific HRQoL, and psychological distress with fatigue. Model 1 consisting of only demographic variables showed that lower education (odds ratio (OR) 2.79, [95% confidence interval (CI) 1.55–5.04], p<0.01) was associated with a higher risk for fatigue (Table 4).
Table 4.
Logistic Models of Factors Associated with Fatigue
| Model 1 (demographics) OR [CI] | Model 2 (model 1+clinical) OR [CI] | Model 3 (model 2+psychological distress) OR [CI] | Model 4 (model 2+thyroid cancer–specific HRQoL) OR [CI] | |
|---|---|---|---|---|
| Block 1 (demographic variables) | ||||
| Age at survey | 0.99 [0.97–1.01] | 0.99 [0.97–1.01] | 0.98 [0.95–1.01] | 0.97 [0.93–1.00]* |
| Sex | 1.21 [0.69–2.12] | 0.99 [0.55–1.18] | 1.54 [0.68–3.49] | 0.60 [0.23–1.56] |
| Educational level | 2.79 [1.55–5.04]** | 2.68 [1.46–4.93]** | 2.09 [0.99–4.38] | 2.57 [1.06–6.62]* |
| Marital status | 1.77 [0.98–3.18] | 1.94 [1.05–3.58]* | 1.38 [0.62–3.07] | 1.00 [0.39–2.56] |
| Block 2 (clinical variables) | ||||
| Time since diagnosis | 0.99 [0.94–1.04] | 1.03 [0.97–1.09] | 1.01 [0.93–1.09] | |
| Comorbidity | — | 2.60 [1.42–4.74]** | 2.39 [1.14–5.00]* | 1.47 [0.58–3.73] |
| Stage of cancer | — | 1.01 [0.51–2.0] | 0.90 [0.38–2.15] | 0.55 [0.18–1.75] |
| Treatment | 1.23 [0.70–2.17] | 1.12 [0.57–2.24] | 0.95 [0.42–2.18] | |
| Block 3 (psychological distress) | ||||
| HADS anxiety | — | — | 1.15 [1.03–1.28]* | — |
| HADS depression | — | — | 1.43 [1.22–1.68]** | — |
| Block 4 (thyroid cancer–specific HRQoL) | ||||
| Neuromuscular problems | — | — | — | 1.03 [1.01–1.06]** |
| Voice problems | — | — | — | 1.01 [0.99–1.03] |
| Concentration problems | — | — | — | 1.03 [1.01–1.06]* |
| Sympathetic problems | — | — | — | 1.02 [0.99–1.04] |
| Throat/mouth problems | — | — | — | 1.00 [0.97–1.02] |
| Psychological problems | — | — | — | 1.06 [1.02–1.10]** |
| Sensory problems | — | — | — | 1.00 [0.98–1.02] |
Fatigue: not fatigued (10–21) vs. fatigued (22–50). Continuous variables: time since diagnosis, age at survey, anxiety, depression, and thyroid cancer–specific HRQoL. Sex=male (reference) vs. female; educational status=high (reference) vs. medium/low; marital status=partner (reference) vs. no partner; comorbidity=no comorbidity (reference) vs. one or more comorbidities; stage=1 and 2 (reference) vs. 3 and 4; treatment=surgery (reference) vs. surgery+additional therapy.
p<0.05; **p<0.01.
CI, 95% confidence interval; OR, odds ratio.
With the inclusion of clinical variables in model 2, education remained significantly associated with fatigue, while partnership became significant in this model, whereby absence of a partner was associated with more fatigue (OR 1.94 [CI 1.05–3.58], p=0.04). The presence of comorbid conditions (OR 2.60 [CI 1.42–4.74], p<0.01) was also significantly associated with fatigue.
In model 3, anxiety (OR 1.15 [CI 1.03–1.28], p=0.01) and depression (OR 1.43 [CI 1.22–1.68], p<0.01) were significantly associated with fatigue. Following the inclusion of these psychological factors, only comorbidity (OR 2.39 [CI 1.14–5.00], p=0.02) remained significant in this model.
In model 4, neuromuscular (OR 1.04 [CI 1.02–1.06], p<0.01), concentration (OR 1.03 [CI 1.01–1.06], p=0.01), and psychological problems (OR 1.05 [CI 1.02–1.28], p<0.01) were significantly associated with fatigue, whereby more problems indicated higher levels of fatigue. All clinical variables were not significantly associated with fatigue anymore, while age at survey (OR 0.97 [CI 0.94–1.00], p=0.05) and educational level (OR 2.57 [CI 1.06–6.62], p=0.04) were also significant, whereby an older age and higher educational level were associated with less fatigue. The single items of the TC-specific HRQoL questionnaire (problems with scar, feeling chilly, tingling hands and/or feet, headaches, weight gain) were not related to fatigue, except for sexual function (OR 0.98 [CI 0.97–1.00], p=0.05), whereby less interest in sex was related to higher levels of fatigue.
The logistic regression models were also conducted for patients with stage I or II only, which resulted in the same significant associations as found for the total group (data not shown).
Discussion
This large population-based study among survivors of TC showed that fatigue remains a problem long after diagnosis and initial therapy. In general, regardless of time since diagnosis, TC survivors reported statistically significant higher levels of fatigue than an age- and sex-matched normative population did, although clinically meaningful differences were only found between short-term survivors and the normative population. Levels of fatigue decreased with increasing time since diagnosis, but these differences were not significant. TC-specific HRQoL (neuromuscular, concentration, and psychological) and psychological distress were most strongly associated with fatigue.
Our results are consistent with the findings of a recent German study showing that TC patients at the beginning of inpatient rehabilitation do suffer from higher levels of fatigue than the general population (13), as was also found in a Korean study among short-term TC survivors (median 2.7 years after diagnosis) (14). A Dutch study among cured differentiated TC patients (median duration of cure of 6.3 years) showed that a longer duration of cure was correlated with better scores on general, mental, and physical fatigue (15). Our results also show decreasing levels of fatigue with increasing years since diagnoses; however, levels of fatigue of long-term survivors were higher than those of the normative population although not clinically relevant.
The high doses of replacement therapy (TSH <0.1 mU/L) in the first years of follow-up for patients who underwent ablation therapy or had high risk of recurrence, and the subsequent TSH suppression, may cause symptoms of (subclinical) hyperthyroidism. Subclinical hyperthyroidism is a reversible cause of fatigue (21). However, after the first years, the doses of replacement therapy are lowered for low-risk survivors (TSH level around median, 1 mU/L) to restore euthyroidism, and fatigue levels are therefore expected to decrease. This might explain the decrease in fatigue levels for long-term survivors compared to short-term survivors in our study. However, the long-term survivors still had statistically significant higher fatigue levels compared to the normative population. This is in contrast with another Dutch study showing that TC patients (n=24) under long-term subclinical hyperthyroidism (>10 years) do not differ in the levels of general, mental, and physical fatigue compared to an age- and sex-matched reference group (16). Our finding that TC-specific HRQoL was associated with fatigue could indicate that the TSH level of around 1 mU/L might not be the optimal (preoperative) level for the specific patient (38), and that the patient therefore does not feel well. It could also be that substitution with levothyroxine alone may not replicate the physiological situation of the patient, and that a subset of patients may benefit from a combination therapy with liothyronine. However, studies on the efficacy of this combined levothyroxine/liothyronine therapy for hypothyroid patients show contradictory results (39).
Another explanation of our findings could be the presence of cancer-related fatigue (CRF). Fatigue levels of TC survivors (39–47%) are comparable to those observed in other cancer survivor populations (17–90%) (6,8,40). Results of our own research group showed that 39% of the colorectal cancer survivors could be classified as (very) fatigued (35) and 40% of the non-Hodgkin survivors reported constant levels of fatigue over time according to the FAS (36). According to the guidelines of the National Comprehensive Cancer Network, CRF is defined as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or its treatment that is not proportional to recent activity and interferes with usual functioning (41). The underlying mechanisms involved in CRF are not well understood. Possible mechanisms include effects of cancer or its treatment on the central nervous system, muscle energy metabolism, sleep rhythms (42), mediators of inflammation and stress (43), immune activation (44), and hormonal changes related to effects on the hypothalamic–pituitary axis (7). A Norwegian study showed that type or intensity of cancer treatment is unrelated to the level of CRF and more related to mental and somatic health problems (45). This finding is in line with the results of a Dutch study showing that levels of fatigue of TC survivors (median duration of cure of 6.3 years) were not affected by initial tumor stage, total activity of 131I, tumor recurrence, and replacement therapy dose (15). In addition to this, our own results show no associations between clinical factors and fatigue (except for comorbidity), while TC-specific HRQoL and psychological distress were most strongly associated with fatigue.
Because of the high prevalence of fatigue and the profound effect that it can have on a patient's daily life, healthcare providers should be encouraged to inquire about the presence of this symptom (46). Both nonpharmacologic and pharmacologic treatments are available for the treatment of CRF. Currently, the best treatments are psychosocial interventions and exercise (46). Other nonpharmacologic and pharmacologic interventions have less supporting evidence but still may be effective for some patients. Recently, an Internet-based education program based on National Comprehensive Cancer Network guidelines showed good results to help patients manage CRF (47). Further research is needed to determine if an intervention for fatigue can reduce the high levels of fatigue among long-term TC survivors.
The present study has some limitations that should be mentioned. Although demographic and clinical information of nonrespondents and survivors with unverified addresses were available for comparison, the health status of these survivors and its possible effects on the current results remain unknown. Some selection bias might have occurred since short- and long-term TC survivors did not differ significantly in age at the time of the survey. It could be that long-term survivors more often had unverifiable addresses or died prior to the study. Furthermore, the cross-sectional study design limits the determination of causal association between cancer-related factors and fatigue. Also, information about levothyroxine dose and serum TSH levels was lacking in our study, which made it difficult to draw definitive conclusions about the mechanisms leading to the increased levels of fatigue. In addition, a logistic regression model including psychological distress and TC-specific HRQoL could not be performed, since there is a high correlation between the TC-specific psychological HRQoL scale and the anxiety scale of the Hospital Anxiety and Depression Scale. Therefore, it was not possible to determine which of the factors was most strongly associated with fatigue.
Nevertheless, the present study provides an important contribution to the limited data available on fatigue of short- and long-term TC survivors. Since our study has a high response rate, extrapolating these results to the larger population of TC survivors seems justified. Furthermore, we were able to compare fatigue levels with an age- and sex-matched normative sample. Future research with longitudinal designs should make use of additional reference groups, for example, a group of patients with subnormal TSH caused by nodular autonomous goiter or another chronic disease, to see whether it is the history of TC accompanied by CRF or (subclinical) hyperthyroidism that causes the higher levels of fatigue compared to the normative population.
In conclusion, about 40% of the TC survivors report a high level of fatigue up till 20 years after diagnosis. Short- and long-term TC survivors report higher levels of fatigue compared with the normative population. TC-specific HRQoL and psychological distress were highly associated with fatigue.
Acknowledgments
The data collection of this study was funded by the Comprehensive Cancer Centre South, Eindhoven, The Netherlands, and a Medium Investment Grant from the Netherlands Organisation for Scientific Research (NWO#480-08-009). F.M. was supported by a VENI grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, The Netherlands). L.v.d.P.-F. was supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349). These funding agencies had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the article; or in the decision to submit the article for publication.
Author Disclosure Statement
The authors have nothing to disclose. This article has been prepared in accordance with the style of the journal, and all authors have approved of its contents. This article is not being considered for publication elsewhere, and the findings of this study have not been previously published. There is no conflict of interest.
References
- 1.Colonna M. Bossard N. Guizard AV. Remontet L. Grosclaude P. Descriptive epidemiology of thyroid cancer in France: incidence, mortality and survival. Ann Endocrinol (Paris) 2010;71:95–101. doi: 10.1016/j.ando.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N. Noone AM. Krapcho M. Neyman N. Aminou R. Waldron W. Altekruse SF. Kosary CL. Ruhl J. Tatalovich Z. Cho H. Mariotto A. Eisner MP. Lewis DR. Chen HS. Feuer EJ. Cronin KA. Edwards BKe. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 2010. 1975–2008. [Google Scholar]
- 3.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Pacini F. Castagna MG. Brilli L. Pentheroudakis G. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v214–v219. doi: 10.1093/annonc/mdq190. [DOI] [PubMed] [Google Scholar]
- 5.Links TP. Huysmans DA. Smit JW. de Heide LJ. Hamming JF. Kievit J. van Leeuwen M. van Pel R. de Klerk JM. van der Wel Y. Guideline “Differentiated thyroid carcinoma,” including diagnosis of thyroid nodules. Ned Tijdschr Geneeskd. 2007;151:1777–1782. [PubMed] [Google Scholar]
- 6.Cella D. Davis K. Breitbart W. Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 7.Campos MP. Hassan BJ. Riechelmann R. Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22:1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 8.Servaes P. Verhagen C. Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta D. Lis CG. Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Barsevick A. Frost M. Zwinderman A. Hall P. Halyard M. I'm so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19:1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fossa SD. Dahl AA. Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–1254. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Reinertsen KV. Cvancarova M. Loge JH. Edvardsen H. Wist E. Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer S. Lincke T. Gamper E. Bhaskaran K. Schreiber S. Hinz A. Schulte T. Quality of life in patients with thyroid cancer compared with the general population. Thyroid. 2012;22:117–124. doi: 10.1089/thy.2011.0139. [DOI] [PubMed] [Google Scholar]
- 14.Lee JI. Kim SH. Tan AH. Kim HK. Jang HW. Hur KY. Kim JH. Kim KW. Chung JH. Kim SW. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes. 2010;8:101. doi: 10.1186/1477-7525-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoftijzer HC. Heemstra KA. Corssmit EP. van der Klaauw AA. Romijn JA. Smit JW. Quality of life in cured patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:200–203. doi: 10.1210/jc.2007-1203. [DOI] [PubMed] [Google Scholar]
- 16.Eustatia-Rutten CF. Corssmit EP. Pereira AM. Frolich M. Bax JJ. Romijn JA. Smit JW. Quality of life in longterm exogenous subclinical hyperthyroidism and the effects of restoration of euthyroidism, a randomized controlled trial. Clin Endocrinol (Oxf) 2006;64:284–291. doi: 10.1111/j.1365-2265.2006.02458.x. [DOI] [PubMed] [Google Scholar]
- 17.Gning I. Trask PC. Mendoza TR. Harle MT. Gutierrez KA. Kitaka SA. Sherman SI. Cleeland CS. Development and initial validation of the thyroid cancer module of the M. D. Anderson Symptom Inventory. Oncology. 2009;76:59–68. doi: 10.1159/000178809. [DOI] [PubMed] [Google Scholar]
- 18.Taieb D. Sebag F. Cherenko M. Baumstarck-Barrau K. Fortanier C. Farman-Ara B. De Micco C. Vaillant J. Thomas S. Conte-Devolx B. Loundou A. Auquier P. Henry JF. Mundler O. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin Endocrinol (Oxf) 2009;71:115–123. doi: 10.1111/j.1365-2265.2008.03424.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang SM. Lee CH. Chien LY. Liu HE. Tai CJ. Postoperative quality of life among patients with thyroid cancer. J Adv Nurs. 2004;47:492–499. doi: 10.1111/j.1365-2648.2004.03128.x. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza A. Shaffer B. Karakla D. Mason ME. Elkins D. Goffman TE. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid. 2004;14:133–140. doi: 10.1089/105072504322880373. [DOI] [PubMed] [Google Scholar]
- 21.Kaltsas G. Vgontzas A. Chrousos G. Fatigue, endocrinopathies, and metabolic disorders. PM R. 2010;2:393–398. doi: 10.1016/j.pmrj.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Janssen-Heijnen MLG. Louwman WJ. Van de Poll-Franse LV. Coebergh JWW. Eindhoven Cancer Registry, Eindhoven; The Netherlands: 2005. Results of 50 Years Cancer Registry in the South of The Netherlands: 1955–2004 (in Dutch) [Google Scholar]
- 23.van de Poll-Franse LV. Horevoorts N. Eenbergen MV. Denollet J. Roukema JA. Aaronson NK. Vingerhoets A. Coebergh JW. de Vries J. Essink-Bot ML. Mols F. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–2194. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 24.International Union Against Cancer (UICC) 6th. John Wiley and Sons; New Jersey: 2002. TNM Classification of Malignant Tumors. [Google Scholar]
- 25.Sangha O. Stucki G. Liang MH. Fossel AH. Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS. Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsson I. Mykletun A. Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husson O. Haak HR. Mols F. Nieuwenhuijzen G. Nieuwlaat W. Reemst PH. Huysmans D. Toorians A. Van de Poll-Franse LV. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52:447–454. doi: 10.3109/0284186X.2012.718445. [DOI] [PubMed] [Google Scholar]
- 29.Michielsen HJ. Drent M. Peros-Golubicic T. De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006;130:989–994. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- 30.Michielsen HJ. De Vries J. Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 31.Michielsen HJ. De Vries J. Drent M. Peros-Golubicic T. Psychometric qualities of the Fatigue Assessment Scale in Croatian sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:133–138. [PubMed] [Google Scholar]
- 32.Michielsen HJ. Van der Steeg AF. Roukema JA. De Vries J. Personality and fatigue in patients with benign or malignant breast disease. Support Care Cancer. 2007;15:1067–1073. doi: 10.1007/s00520-007-0222-2. [DOI] [PubMed] [Google Scholar]
- 33.van de Poll-Franse LV. Mols F. Gundy CM. Creutzberg CL. Nout RA. Verdonck-de Leeuw IM. Taphoorn MJ. Aaronson NK. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47:667–675. doi: 10.1016/j.ejca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Norman GR. Sloan JA. Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 35.Thong MS. Mols F. Wang XS. Lemmens VE. Smilde TJ. van de Poll-Franse LV. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry. Eur J Cancer. 2013;49:1957–1966. doi: 10.1016/j.ejca.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oerlemans S. Mols F. Issa DE. Pruijt JH. Peters WG. Lybeert M. Zijlstra W. Coebergh JW. van de Poll-Franse LV. A high level of fatigue among long-term survivors of non-Hodgkin's lymphoma: results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica. 2013;98:479–486. doi: 10.3324/haematol.2012.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husson O. Haak HR. Buffart LM. Nieuwlaat W. Oranje WA. Mols F. Kuijpens JL. Coebergh JW. Van de Poll-Franse LV. Health-related quality of life and disease specific symptoms among (long-term) thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52:249–258. doi: 10.3109/0284186X.2012.741326. [DOI] [PubMed] [Google Scholar]
- 38.Andersen S. Pedersen KM. Bruun NH. Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 39.Regalbuto C. Maiorana R. Alagona C. Paola RD. Cianci M. Alagona G. Sapienza S. Squatrito S. Pezzino V. Effects of either LT4 monotherapy or LT4/LT3 combined therapy in patients totally thyroidectomized for thyroid cancer. Thyroid. 2007;17:323–331. doi: 10.1089/thy.2006.0084. [DOI] [PubMed] [Google Scholar]
- 40.Campos MP. Hassan BJ. Riechelmann R. Del Giglio A. Cancer-related fatigue: a review. Rev Assoc Med Bras. 2011;57:211–219. doi: 10.1590/s0104-42302011000200021. [DOI] [PubMed] [Google Scholar]
- 41.Mock V. Atkinson A. Barsevick A. Cella D. Cimprich B. Cleeland C. Donnelly J. Eisenberger MA. Escalante C. Hinds P. Jacobsen PB. Kaldor P. Knight SJ. Peterman A. Piper BF. Rugo H. Sabbatini P. Stahl C. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Williston Park) 2000;14:151–161. [PubMed] [Google Scholar]
- 42.Parker KP. Bliwise DL. Ribeiro M. Jain SR. Vena CI. Kohles-Baker MK. Rogatko A. Xu Z. Harris WB. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26:2464–2472. doi: 10.1200/JCO.2007.12.2135. [DOI] [PubMed] [Google Scholar]
- 43.Cleeland CS. Bennett GJ. Dantzer R. Dougherty PM. Dunn AJ. Meyers CA. Miller AH. Payne R. Reuben JM. Wang XS. Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 44.Collado-Hidalgo A. Bower JE. Ganz PA. Cole SW. Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 45.Orre IJ. Fossa SD. Murison R. Bremnes R. Dahl O. Klepp O. Loge JH. Wist E. Dahl AA. Chronic cancer-related fatigue in long-term survivors of testicular cancer. J Psychosom Res. 2008;64:363–371. doi: 10.1016/j.jpsychores.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Escalante CP. Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(Suppl 2):S412–S416. doi: 10.1007/s11606-009-1056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun YH. Lee KS. Kim YW. Park SY. Lee ES. Noh DY. Kim S. Oh JH. Jung SY. Chung KW. Lee YJ. Jeong SY. Park KJ. Shim YM. Zo JI. Park JW. Kim YA. Shon EJ. Park S. Web-based tailored education program for disease-free cancer survivors with cancer-related fatigue: a randomized controlled trial. J Clin Oncol. 2012;30:1296–1303. doi: 10.1200/JCO.2011.37.2979. [DOI] [PubMed] [Google Scholar]