Abstract
Background
The purpose of this study was to examine the utility of remnant uptake on postoperative radioiodine scans as an oncologic indicator after thyroidectomy for differentiated thyroid cancer (DTC).
Methods
We conducted a retrospective review of patients undergoing total thyroidectomy for DTC and subsequent radioactive iodine (RAI) treatment. Of the eight surgeons included, three were considered high volume, performing at least 20 thyroidectomies per year. Patients with distant metastases at diagnosis or poorly differentiated variants were excluded. To control for the effect of varying RAI doses, the remnant uptake was analyzed as a ratio of the percentage uptake to the dose received (uptake to dose ratio [UDR]). Multivariate logistic regression was used to determine the influence of UDR on recurrence.
Results
Of the 223 patients who met inclusion criteria, 21 patients (9.42%) experienced a recurrence. Those with a recurrence had a 10-fold higher UDR compared with those who did not (0.030 vs. 0.003, p=0.001). Similarly, patients with increasing postoperative thyroglobulin measurements (0.339 vs. 0.003, p<0.001) also had significantly greater UDRs compared with those with stable thyroglobulin. The UDRs of high-volume surgeons were significantly smaller than low-volume surgeons (0.003 vs. 0.025, p=0.002). When combined with other known predictors for recurrence, UDR (OR 3.71 [95%CI 1.05–13.10], p=0.041) was significantly associated with recurrence. High-volume surgeons maintained a low level of permanent complications across all UDRs, whereas low-volume surgeons had greater permanent complications associated with higher uptake.
Conclusions
Remnant uptake is a useful postoperative oncologic quality indicator that can predict a patient's risk of disease recurrence and indicate the completeness of resection.
Introduction
The overall mortality rates for differentiated thyroid cancer (DTC) depend on age and sex. For most patients, survival is 97% or better (1). Since mortality from DTC is relatively low, disease recurrence becomes another relevant oncologic outcome. Large series report recurrence rates between 2% and 14% (2–5). Both surgical and nonsurgical treatments influence both the recurrence and mortality in DTC (4,6–8).
Treatment guidelines for DTC recommend near-total or total thyroidectomy for patients with tumors greater than 1 cm in size or for patients with other high-risk features such as multifocal tumors, regional or distant metastases, a history of radiation exposure, or family history of thyroid cancer (9). These recommendations stem from improved survival and recurrence rates associated with total or near-total thyroidectomy when performed by surgeons with low complication rates (2,3,7,10,11). Although the appropriate extent of thyroidectomy is still debated for low-risk tumors, removal of the entire gland facilitates adjuvant therapy with radioactive iodine (RAI) ablation and the ability to detect recurrence with thyroglobulin levels (12–15). Lifelong complications after total thyroidectomy include recurrent laryngeal nerve injury and hypoparathyroidism, since both recurrent laryngeal nerves and all four parathyroids become at risk during removal of the entire gland. These risks diminish when a high-volume, or experienced, surgeon performs the thyroidectomy (16–21).
The importance of the completeness or extent of thyroidectomy is underscored by its inclusion in some prognostic scoring systems. In the MACIS prognostic scoring system, incomplete resection significantly contributed to thyroid cancer–specific mortality (7). Similarly, Bilimoria et al. (2) found that for tumors ≥1 cm, total thyroidectomy resulted in lower recurrence rates and improved survival. Extent of resection also affects recurrence. Hay et al. (8) found that patients treated with lobectomy experienced significantly higher recurrence rates (14%) compared with patients treated with total thyroidectomy (2%).
Studies analyzing the extent of thyroidectomy only compare lobectomy to total thyroidectomy. It is difficult to quantify the amount of tissue left behind, and the definitions of subtotal versus near-total versus total thyroidectomy are not exact (22,23). Often surgeons will leave some thyroid tissue near the upper parathyroid and the insertion of the recurrent laryngeal nerve to protect these structures, and this is termed “near-total thyroidectomy.” In this scenario, the remnant is often ablated with RAI (24). Total thyroidectomy, on the other hand, eliminates the need to administer an ablative RAI dose to destroy the thyroid remnant, and any RAI that is given is more effective in destroying metastatic disease (22,25).
As most thyroid surgeons know, variation in the extent of thyroid tissue excised exists even for procedures labeled as “total thyroidectomy.” Consensus guidelines recommend RAI for tumors greater than 4 cm in size, gross extrathyroidal extension, or when distant metastases are present (high-risk patients). RAI is not recommended for the lowest risk patients (well-differentiated tumors <1 cm, confined to the thyroid gland, and without lymph node or distant metastases). Selective use is recommended for patients who do not fall into either the high- or low-risk categories (9). A whole-body scan is obtained 3–7 days after administration of the radioisotope to evaluate the remnant uptake within the neck and distant metastases (26–28). The remnant uptake is the percentage of the total dose administered detected within the thyroid bed after adjusting for decay (29,30). This provides a quantitative assessment of how much thyroid tissue remains.
Erbil and colleagues (31) found that remnant thyroid volume assessed by ultrasound correlated with remnant uptake in benign diseases, but the evidence for remnant uptake as an oncologic indicator after thyroidectomy for DTC is lacking. This becomes relevant in evaluating new endoscopic and robotic techniques for performing thyroidectomy because a larger remnant of normal thyroid may be left behind. The purpose of this study was to examine the utility of remnant uptake as an oncologic indicator after thyroidectomy for DTC with a primary outcome of disease recurrence. Furthermore, we compared remnant uptake between high- and low-volume thyroid surgeons.
Methods
We conducted a retrospective review of our prospectively collected thyroid database. We selected patients with DTC undergoing total thyroidectomy followed by RAI therapy between 2004 and 2010. Excluded were patients with more aggressive or poorly differentiated variants (anaplastic cancers, insular, tall cell, columnar cell, sclerosing, and large cell variants). Also excluded were patients with distant metastases at the time of diagnosis or patients with gross disease left behind as indicated by the operative note.
Data on demographics, number of positive lymph nodes, lymph node yield, histologic tumor features, and recurrence were extracted from our database. The RAI dose and remnant uptake were also abstracted from patient charts. To control for the effect of varying RAI doses, the remnant uptake was analyzed as a ratio of the percentage uptake to the dose received (uptake to dose ratio [UDR]). Recurrence was defined as any pathologically or cytologically confirmed evidence of disease that was not present at the time of initial surgery. Postoperative thyroglobulin levels (unstimulated) were recorded for the entire follow-up period, and increasing thyroglobulin was defined as an increase greater than the laboratory error (1.3 ng/mL). Staged neck dissections were not considered recurrent disease.
Eight different surgeons operated on patients included in this cohort. Sosa et al. (21) found that surgeons who performed over 100 thyroidectomies over a 5-year period (100 divided over 5 years=20 per year) had the lowest complication rates. Therefore, we designated surgeons as high volume that performed more than 20 thyroid operations per year, and the others were considered low volume. Complications (hypocalcemia and hoarseness) were classified as either temporary if they resolved within 6 months from surgery or permanent if they persisted beyond 6 months postoperatively.
Kaplan–Meier analysis was used to plot disease-free survival curves. Multivariate regression with the Cox proportional hazards model was used to determine the influence of UDR on disease recurrence while accounting for other known clinical and pathologic predictors of outcome. Binary comparisons were made using the Student's t test, Wilcoxon rank sum test, or chi-squared test where appropriate; p<0.05 was considered significant.
Results
Preoperative characteristics
There were 223 patients who met our inclusion criteria and presented for the initial surgical treatment of DTC followed by RAI at our institution between 2004 and 2011. The preoperative patient characteristics are shown in Table 1. The mean age of patients included in this cohort was 46.5±4.0 years and 66.8% were women. Fourteen (6.3%) had a history of head and neck radiation exposure and 16 (7.2%) had a family history of thyroid cancer. DTC was discovered in the setting of a goiter in 34.9% of patients, and the mean preoperative thyrotropin (TSH) was 1.8±1.3 μIU/mL.
Table 1.
Preoperative Patient Characteristics (n=223)
| Variable | n (%)a |
|---|---|
| Age (years) | 46.5±4.0 |
| Female | 149 (66.8) |
| Family history | 16 (7.2) |
| Radiation exposure | 14 (6.3) |
| Goiter | 78 (34.9) |
| TSH (μIU/mL) | 1.8±1.3 |
The number of patients with each characteristic is given and the corresponding percentage is indicated in parentheses. Continuous variables are represented as the mean±standard error of the mean unless otherwise indicated.
TSH, thyrotropin.
Tumor characteristics
Greater than 90% of the patients in this series were treated for papillary thyroid cancer while 11 (4.9%) had follicular thyroid cancer and 8 (3.6%) had Hürthle cell carcinoma (Table 2). The mean tumor size was 2.0±1.2 cm and 53.6% of tumors were multifocal. Forty-one (18.5%) tumors exhibited extrathyroidal extension, and 19 (8.6%) had lymphovascular invasion on final pathology (Table 2). Sixty-seven patients (30.0%) were found to have central compartment lymph node metastases, while 13 patients (16.6%) had lateral compartment lymph node metastases (Table 2).
Table 2.
Tumor Characteristics
| Variable | n (%)a |
|---|---|
| Histology | |
| PTC | 204 (91.5) |
| FTC | 11 (4.9) |
| HCC | 8 (3.6) |
| Tumor size, cm | 2.0±1.2 |
| Multifocal | 119 (53.6) |
| Extrathyroidal extension | 41 (18.5) |
| Lymphovascular invasion | 19 (8.6) |
| Central neck lymph node metastases | 67 (30.0) |
| Lateral neck lymph node metastases | 13 (16.6) |
The number of patients with each characteristic is given and the corresponding percentage is indicated in parentheses. Continuous variables are represented as the mean±standard error of the mean.
PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; HCC, Hürthle cell carcinoma.
RAI treatment
At our institution, RAI is administered to patients with DTC≥1 cm and/or when lymph node metastases, lymphovascular invasion, multifocality, or extrathyroidal extension are present. Details of the radioiodine doses received by patients in this cohort are shown in Table 3. Although the doses ranged from 17 to 177.5 mCi (Table 3), most of the doses clustered around 30, 50, and 100 mCi. To standardize the remnant uptake according to the dose received, a UDR was calculated for each patient. The median UDR was 0.006 (Table 3), and these data were normally distributed.
Table 3.
Radioactive Iodine Therapy
| Variable | Median | Range | SEM |
|---|---|---|---|
| Dose, mCi | 83 | 17–177.5 | 6.90 |
| Remnant uptake, % | 0.03 | 0.001–0.90 | 0.59 |
| Uptake to dose ratioa | 0.006 | 6.7×10–7–0.053 | 0.19 |
Uptake to dose ratio is the percentage uptake divided by the radioiodine dose administered.
SEM, standard error of the mean.
Uptake and oncologic outcomes
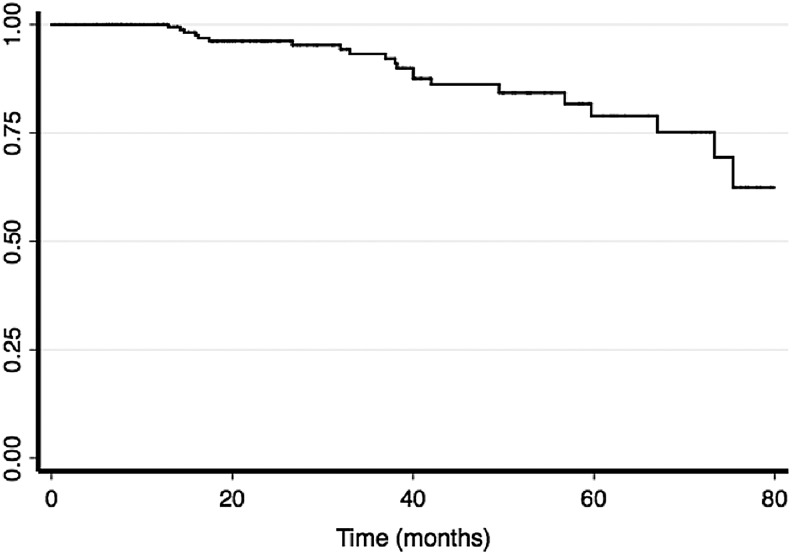
Since there were no known deaths in this series of patients with DTC, we evaluated the influence of UDR on disease recurrence. We defined recurrence as any cytologically or pathologically proven disease found after the initial treatment (surgery and RAI). In this cohort, 21 patients (9.42%) experienced a recurrence. The Kaplan–Meier estimates for disease-free survival are depicted in Figure 1. The median follow-up time was 25 months (interquartile range 17 months). Over 90% of patients had at least 2 years follow-up time. Six of the 21 patients (28.57%) who had a recurrence were found to have disease in a previously operated field. The locations and frequencies of recurrence were lateral neck (57.14%), central neck (28.57%), mediastinum (9.52%), and sacrum (4.76%).
FIG. 1.
Kaplan–Meier disease-free survival estimates. Recurrence was defined as any pathologically or cytologically proven disease found after the initial operation, and disease free survival estimates are plotted.
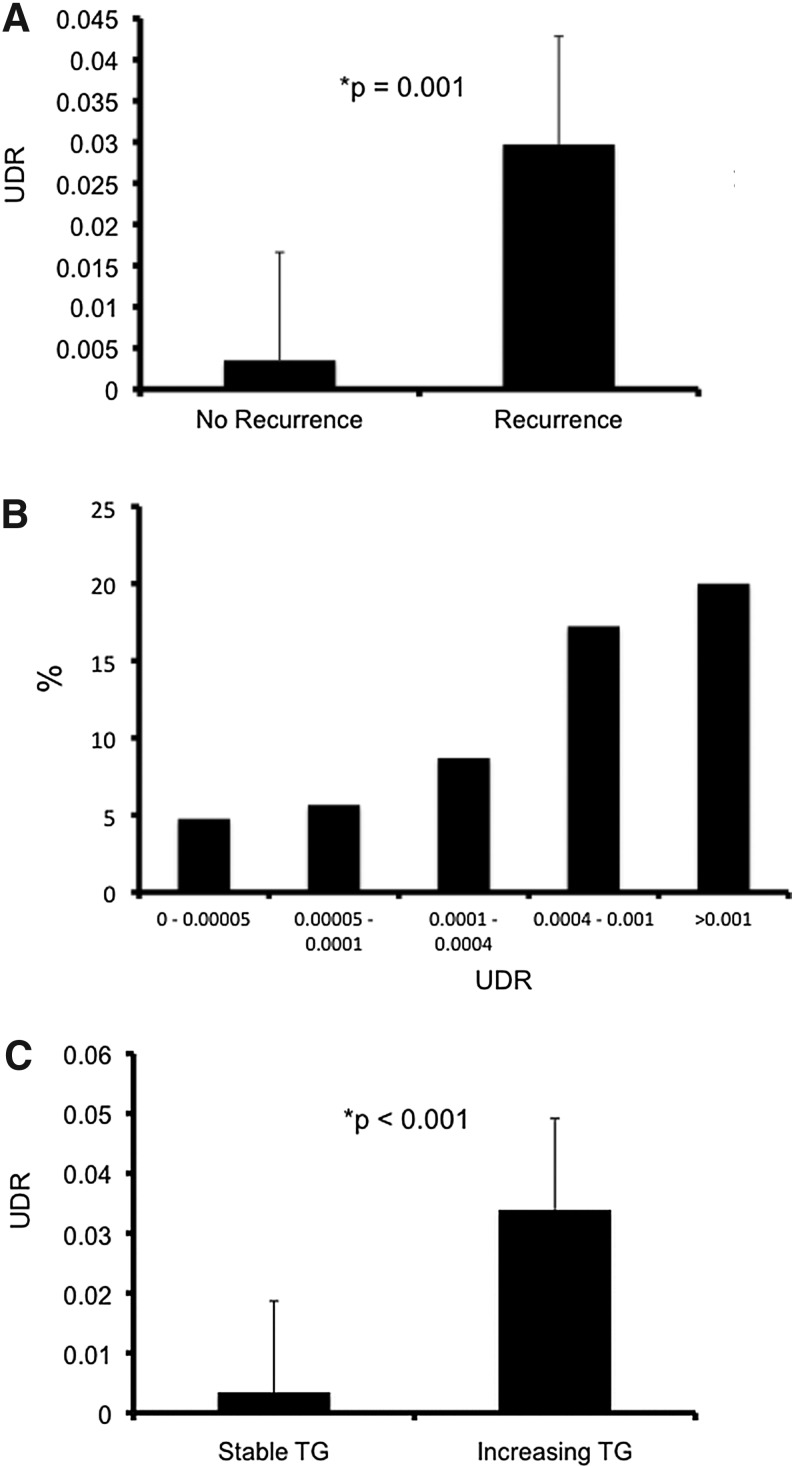
Those who had a recurrence had a 10-fold higher UDR compared with those who did not (0.030 vs. 0.003, p=0.001, Fig. 2A). Only five patients (2.24%) had uptake outside of the neck on their post ablation scan. When we examined the percentage of patients who had a recurrence over five successive divisions in the UDR, the proportion of patients demonstrated a stepwise increase with increasing UDR (Fig. 2B). Patients who required additional neck surgery for recurrent disease also had significantly higher UDRs compared with patients who did not require additional surgery (0.028 vs. 0.003, p<0.001).
FIG. 2.
Remnant uptake and recurrence. Remnant uptake data are represented as the mean uptake to dose ratio (UDR), and error bars indicate the standard error of the means. UDRs are compared between patients who recurred and those who did not recur (A) as well as patients with increasing postoperative thyroglobulin to those with stable or decreasing thyroglobulin (C). The recurrence rates for five different divisions in UDR are also shown (B).
Since this definition of recurrence may not capture all patients who truly have evidence of recurrent disease, we also examined the UDRs in patients with increasing postoperative unstimulated thyroglobulin levels. Patients with increasing thyroglobulin had a mean UDR of 0.034 compared with 0.003 in patients with stable or decreasing thyroglobulins (p<0.001; Fig. 2C).
Multivariate analysis
To evaluate the relative importance of the remnant uptake in determining disease recurrence, we performed a multivariate analysis including other well-known disease and patient factors associated with recurrence in DTC. In this model, UDR was significantly associated with recurrence (OR 3.71 [95% CI 1.05–13.10], p=0.04; Table 4). Lymph node metastases (OR 10.45 [95% CI 2.88–37.97], p<0.001; Table 4) were also significantly associated with recurrence. Age, sex, tumor size, extrathyroidal extension, positive margins, lymphovascular invasion, and multifocality were included in the model, but these factors did not independently predict recurrence (Table 4).
Table 4.
Multivariate Regression for Disease Recurrence
| Variablea | HR | 95% CI | p |
|---|---|---|---|
| Age | 1.00 | [0.20–1.65] | 0.69 |
| Female sex | 0.58 | [0.98–1.04] | 0.31 |
| Lymph node metastases | 10.45 | [2.88–37.97] | <0.01 |
| Tumor size | 1.26 | [0.93–1.70] | 0.14 |
| Extrathyroidal extension | 0.60 | [0.15–2.41] | 0.47 |
| % Uptake (UDR) | 3.71 | [1.05–13.10] | 0.04 |
| Positive margins | 2.58 | [0.56–11.95] | 0.23 |
| Lymphovascular invasion | 1.36 | [0.26–7.02] | 0.71 |
| Multifocality | 3.12 | [0.91–10.67] | 0.07 |
Statistically significant variables are in bold print.
OR, odds ratio; CI, confidence interval.
Uptake and surgeon volume
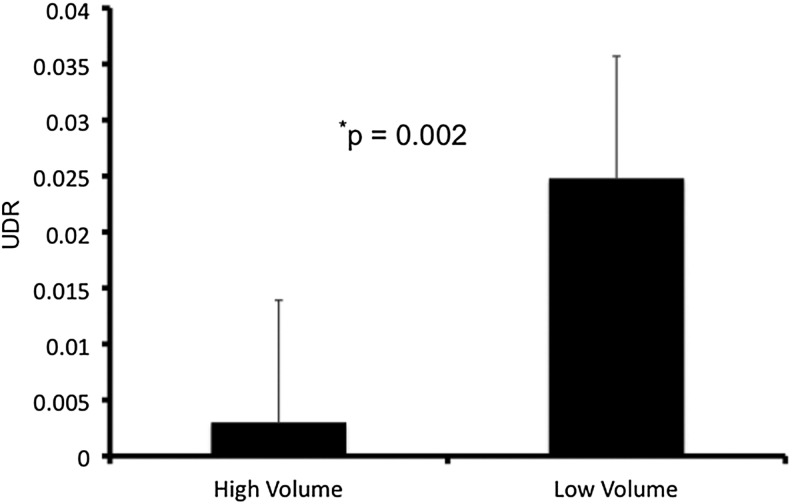
Surgeons who operated on patients in this study were classified as either high volume or low volume based on a threshold of 20 thyroid operations per year (21). In this study, three surgeons met the volume threshold to be considered high volume, and the remaining five were low volume. The UDRs of high-volume surgeons were significantly smaller than low-volume surgeons (0.003 vs. 0.025, p=0.002, Fig. 3).
FIG. 3.
Remnant uptake by surgeon volume. Data are represented as the mean UDR, and error bars indicate the standard error of the means. UDRs are compared between high-volume and low-volume surgeons.
Uptake and complications
Thirty-three patients (14.80%) in this cohort experienced any type of complication. Of these, 24 (10.76%) were temporary complications and nine (4.04%) were permanent. Temporary hypocalcemia occurred in 16 patients (7.17%) and four patients (1.79%) experienced permanent hypocalcemia. Eight patients (3.59%) had temporary hoarseness and five patients (2.24%) suffered permanent hoarseness.
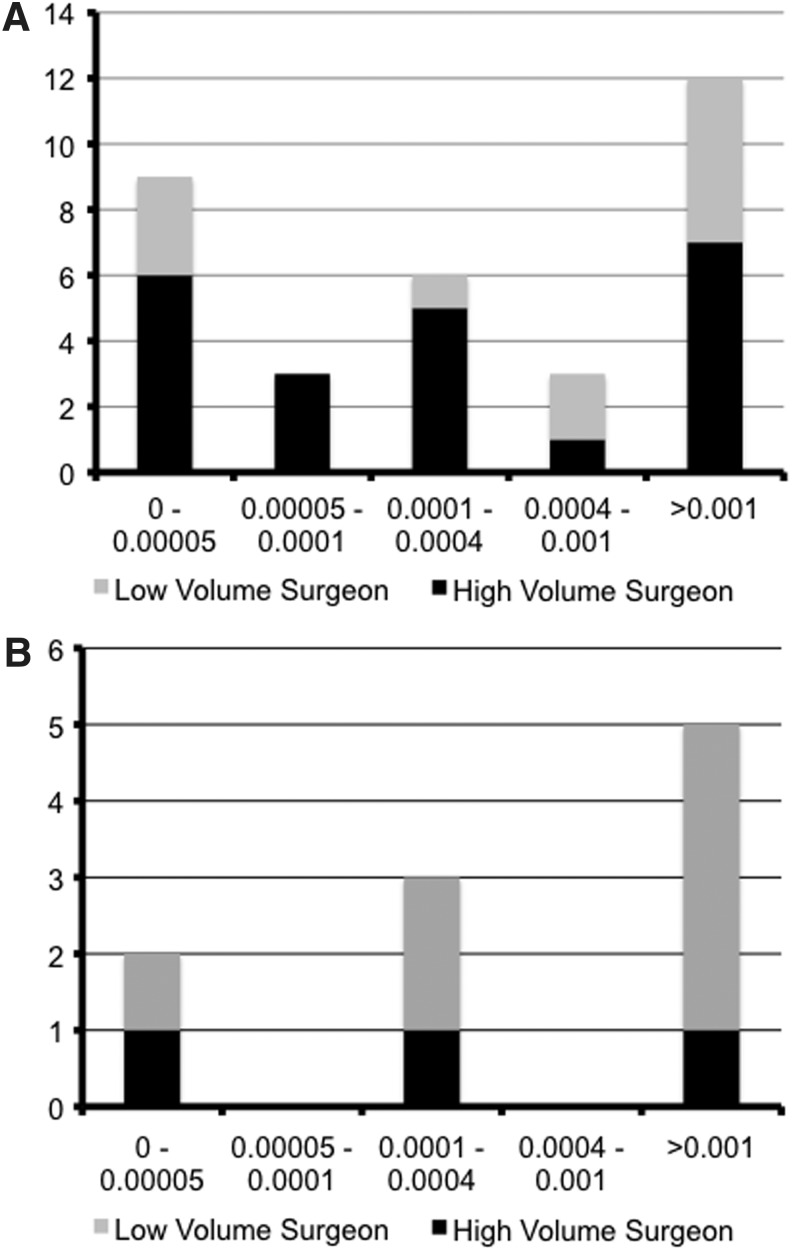
Figure 4 shows the distribution of complications by UDR. These complications exhibited a bimodal distribution with peaks at the lowest and highest UDRs (Fig. 4A). However, there were more complications in the highest UDR category with a greater proportion of these complications from low-volume surgeons (Fig. 4A). There was a stepwise increase in permanent complications as UDR increased (Fig. 4B). While the number of complications from high-volume surgeons remained low across all UDRs, low-volume surgeons accounted for the incremental increase in permanent complications as UDRs increased (Fig. 4B).
FIG. 4.
Remnant uptake and complications. The number of complications across successive UDR categories is shown for both the total number of complications (A) and permanent complications alone (B). The proportion of complications from high-volume surgeons is black while the proportion from low-volume surgeons is gray.
Discussion
In this study we demonstrate that the remnant uptake serves as a useful postoperative oncologic indicator because it correlates with a patient's chance of disease recurrence. Patients who recurred had a 10-fold higher UDR compared to those who did not recur (Fig. 2). When combined with other known clinical and pathologic determinants of DTC outcomes, the remnant uptake independently predicted disease recurrence (Table 4). This quality measure was also related to surgeon volume since high-volume surgeons had significantly lower remnant uptake with fewer permanent complications compared to low-volume surgeons (Fig. 3).
Unlike more aggressive cancers such as breast, melanoma, or colon cancer, relatively few quality indicators exist for the surgical treatment of thyroid cancer (32–34). Recently, several studies have evaluated how the adequacy of lymph node dissection in DTC impacts both recurrence and disease-specific mortality (35–38), but oncologic quality indicators in the performance of total thyroidectomy for malignancy are lacking. Typically cited performance measures for thyroidectomy include hypocalcemia, vocal cord paresis (hoarseness), and hematoma rates (39,40). Tracking accurate oncologic outcomes for DTC remains challenging since mortality is rare and recurrence can take years or even decades to manifest (28,41,42). Remnant uptake becomes a more immediate indicator, providing readily available data at 6–8 weeks postoperatively. Whereas disease recurrence, mortality, or the lymph node ratio reflects both the nature of disease and adequacy of surgical treatment, the remnant uptake is a more direct indication of the surgeon's ability to perform a complete resection. While this may not apply to cases of locally advanced disease, such patients were excluded from this study.
The volume–quality relationship has been well described for many surgical procedures, and many of these studies focus on oncologic outcomes (43–47). In thyroid surgery, several authors have found that surgeon volume correlates inversely with operative complications (16,21), but there are few data on the relationship between surgeon volume and DTC outcomes. Here, we demonstrate a relationship between volume and the remnant uptake, indicating that higher volume surgeons had smaller remnant uptake percentages, or more complete resections (Fig. 3). Thus, surgeon volume also influences a marker for oncologic outcome in DTC.
A more complete resection (lower uptake) did come with increased total complications, but an even greater number was seen in the highest UDR category in which low-volume surgeons accounted for a greater proportion of complications (Fig. 4). The data did not exhibit a stepwise decrease in total complications across the remaining UDR categories, making it difficult to recommend an optimal uptake or amount of thyroid tissue remnant that would minimize risk of recurrence and complications. Instead, thyroid surgeons can select certain patients for a subtotal or near-total thyroidectomy based on individualized recurrence risk, preoperative laryngeal function, and comorbidities. These factors may include the socioeconomic status of the patient because this may affect his or her ability to take calcium and/or vitamin D supplements multiple times per day in the event of permanent hypoparathyroidism. Importantly, high-volume surgeons maintained a constant number of permanent complications over the full range of UDRs, while low-volume surgeons had a much greater proportion of permanent complications at the highest uptake levels (Fig. 4). In terms of quality, this is the worst scenario: more permanent complications and a poor oncologic indicator. High-volume surgeons, on the other hand, maintained a low level of permanent complications with a more complete resection—a much better combination in terms of quality.
Aside from prognosticating cancer outcomes for the patient, the remnant uptake can also help practitioners who care for patients with DTC. For example, if a patient moves or travels to receive care at a different center from where he or she underwent surgery, the new caregivers may not be familiar with the surgeon who performed the thyroidectomy. Using the UDR, these new caregivers can readily assess the adequacy of resection, and thereby the risk of recurrence. Remnant uptake adds to the other known clinical and pathologic determinants of recurrence or thyroid cancer specific mortality such as tumor size, extrathyroidal extension, multifocality, lymph node metastases, and distant metastases. That is, remnant uptake is another piece of information the clinician can use to determine a patient's risk of recurrence and the appropriate surveillance schedule.
Remnant uptake is useful for the surgeon in assessing his or her own performance. The remnant uptake provides more objective feedback on the completeness of resection relatively soon (6–8 weeks) after the operation itself. This type of information can assist young surgeons or lower volume surgeons with self-improvement when they are on the steeper portion of the learning curve. Alternatively, it may prompt lower volume surgeons to refer thyroid cancer cases if their remnant uptakes are too high. A growing body of evidence suggests that providing this type of feedback, or audit, improves surgical quality measures for various types of cancer (48–50). Furthermore, feedback is recognized as an integral part of surgical education and training (51).
Recently new techniques such as robotic transaxillary thyroidectomy, endoscopic transaxillary thyroidectomy, bilateral axillo-breast approach robotic thyroidectomy, and direct or cervical endoscopic thyroidectomies have emerged (52,53). As newer techniques become available for performing a thyroidectomy, there is a need to evaluate whether they maintain oncologic quality. As mentioned, outcomes for DTC require decades to assess, but the remnant uptake becomes a quality measure available within a few months of surgery.
This study is limited by its retrospective nature. Even though our median follow-up time was 25 months with a maximum of 80 months, this may underestimate the true recurrence rate in this cohort. The number of patients who suffered a recurrence is relatively small. Furthermore, some patients may have sought care elsewhere for their recurrence. Although this is a single institution study, there were a large number of providers included. In addition to the surgeons, there were many endocrinologists and nuclear medicine specialists who cared for this cohort of patients. We attempted to control for variation in treatment by limiting inclusion to fairly well differentiated tumors limited to the neck, and by normalizing the remnant uptake to the radioiodine dose administered (the UDR). Nevertheless, we cannot account for all the variation in surgical treatment or RAI therapy. At our institution, we do not routinely measure pre-ablation thyroglobulin levels, so we cannot assess how remnant uptake compares to thyroglobulin levels as a quality indicator. The scan, however, provides anatomic information about the location of any remaining thyroid tissue, whereas serum thyroglobulin levels simply indicates if thyroid tissue is present anywhere within the body. Another limitation is that we only obtain pre-ablation scans for selected patients to help clarify the proper dose of RAI. It makes intuitive sense that a greater pre-ablation remnant would also correlate with recurrence based on the data presented here and other studies examining how extent of surgery impacts disease outcomes (2,11,22,54).
Despite these limitations, this study demonstrates how the remnant uptake serves as a readily available quality indicator for the treatment of DTC. This information can contribute to hospital and surgeon quality improvement efforts. Furthermore, it provides another piece of information to risk stratify DTC patients for their risk of recurrence.
Acknowledgement
This study was supported by NIH T32 CA009614-23.
Disclosure Statement
No competing financial interests exist.
References
- 1.SEER cancer statistics review. National Cancer Institute; Bethesda, MD: 1975–2005. National Cancer Institute. [Google Scholar]
- 2.Bilimoria KY. Bentrem DJ. Ko CY. Stewart AK. Winchester DP. Talamonti MS. Sturgeon C. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381. doi: 10.1097/SLA.0b013e31814697d9. ; discussion 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay ID. Hutchinson ME. Gonzalez-Losada T. McIver B. Reinalda ME. Grant CS. Thompson GB. Sebo TJ. Goellner JR. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987. doi: 10.1016/j.surg.2008.08.035. ; discussion 987–988. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–518. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL. Young RL. Oertel JE. Kemmerer WT. Page CP. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine. 1977;56:171–196. [PubMed] [Google Scholar]
- 6.Mazzaferri EL. Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 7.Hay ID. Bergstralh EJ. Goellner JR. Ebersold JR. Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. ; discussion 1057–1058. [PubMed] [Google Scholar]
- 8.Hay ID. Grant CS. Bergstralh EJ. Thompson GB. van Heerden JA. Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958–964. discussion 964–966. [PubMed] [Google Scholar]
- 9.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 10.Jonklaas J. Sarlis NJ. Litofsky D. Ain KB. Bigos ST. Brierley JD. Cooper DS. Haugen BR. Ladenson PW. Magner J. Robbins J. Ross DS. Skarulis M. Maxon HR. Sherman SI. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 11.Barney BM. Hitchcock YJ. Sharma P. Shrieve DC. Tward JD. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011;33:645–649. doi: 10.1002/hed.21504. [DOI] [PubMed] [Google Scholar]
- 12.Nixon IJ. Ganly I. Patel SG. Palmer FL. Whitcher MM. Tuttle RM. Shaha A. Shah JP. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151:571–579. doi: 10.1016/j.surg.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 2009;23:579–588. [PubMed] [Google Scholar]
- 14.Shah JP. Loree TR. Dharker D. Strong EW. Lobectomy versus total thyroidectomy for differentiated carcinoma of the thyroid: a matched-pair analysis. Am J Surg. 1993;166:331–335. doi: 10.1016/s0002-9610(05)80326-5. [DOI] [PubMed] [Google Scholar]
- 15.Shaha AR. Shah JP. Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4:328–333. doi: 10.1007/BF02303583. [DOI] [PubMed] [Google Scholar]
- 16.Stavrakis AI. Ituarte PH. Ko CY. Yeh MW. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007;142:887–899. doi: 10.1016/j.surg.2007.09.003. ; discussion 887–899. [DOI] [PubMed] [Google Scholar]
- 17.Gourin CG. Tufano RP. Forastiere AA. Koch WM. Pawlik TM. Bristow RE. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010;136:1191–1198. doi: 10.1001/archoto.2010.212. [DOI] [PubMed] [Google Scholar]
- 18.Youngwirth L. Benavidez J. Sippel R. Chen H. Parathyroid hormone deficiency after total thyroidectomy: incidence and time. J Surg Res. 2010;163:69–71. doi: 10.1016/j.jss.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 19.Duclos A. Peix JL. Colin C. Kraimps JL. Menegaux F. Pattou F. Sebag F. Touzet S. Bourdy S. Voirin N. Lifante JC; CATHY Study Group. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012;344:d8041. doi: 10.1136/bmj.d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders BD. Wainess RM. Dimick JB. Doherty GM. Upchurch GR. Gauger PG. Who performs endocrine operations in the United States? Surgery. 2003;134:924–931. doi: 10.1016/s0039-6060(03)00420-3. discussion 931. [DOI] [PubMed] [Google Scholar]
- 21.Sosa JA. Bowman HM. Tielsch JM. Powe NR. Gordon TA. Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320–330. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark OH. Total thyroidectomy: the treatment of choice for patients with differentiated thyroid cancer. Ann Surg. 1982;196:361–370. doi: 10.1097/00000658-198209000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roher HD. Goretzki PE. Management of goiter and thyroid nodules in an area of endemic goiter. Surg Clin North Am. 1987;67:233–249. doi: 10.1016/s0039-6109(16)44181-2. [DOI] [PubMed] [Google Scholar]
- 24.Leung SF. Law MW. Ho SK. Efficacy of low-dose iodine-131 ablation of post-operative thyroid remnants: a study of 69 cases. Br J Radiol. 1992;65:905–909. doi: 10.1259/0007-1285-65-778-905. [DOI] [PubMed] [Google Scholar]
- 25.Attie JN. Moskowitz GW. Margouleff D. Levy LM. Feasibility of total thyroidectomy in the treatment of thyroid carcinoma: postoperative radioactive iodine evaluation of 140 cases. Am J Surg. 1979;138:555–560. doi: 10.1016/0002-9610(79)90418-5. [DOI] [PubMed] [Google Scholar]
- 26.Sawka AM. Thephamongkhol K. Brouwers M. Thabane L. Browman G. Gerstein HC. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668–3676. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 27.Gharib H. Papini E. Valcavi R. Baskin HJ. Crescenzi A. Dottorini ME. Duick DS. Guglielmi R. Hamilton CR., Jr Zeiger MA. Zini M AACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 28.Sippel RS. Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19:1373–80. doi: 10.1089/thy.2009.1606. [DOI] [PubMed] [Google Scholar]
- 29.Schlumberger M. Mancusi F. Baudin E. Pacini F. 131I therapy for elevated thyroglobulin levels. Thyroid. 1997;7:273–276. doi: 10.1089/thy.1997.7.273. [DOI] [PubMed] [Google Scholar]
- 30.Van Nostrand D. Wartofsky L. Radioiodine in the treatment of thyroid cancer. Endocrinol Metab Clin North Am. 2007;36:807–822. doi: 10.1016/j.ecl.2007.04.006. , vii–viii. [DOI] [PubMed] [Google Scholar]
- 31.Erbil Y. Barbaros U. Salmaslioglu A. Issever H. Tukenmez M. Adalet I. Bozbora A. Ozarmagan S. Tezelman S. Determination of remnant thyroid volume: comparison of ultrasonography, radioactive iodine uptake and serum thyroid-stimulating hormone level. J Laryngol Otol. 2008;122:615–622. doi: 10.1017/S0022215107008997. [DOI] [PubMed] [Google Scholar]
- 32.Mocellin S. Pasquali S. Rossi CR. Nitti D. Validation of the prognostic value of lymph node ratio in patients with cutaneous melanoma: a population-based study of 8,177 cases. Surgery. 2011;150:83–90. doi: 10.1016/j.surg.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Chagpar AB. Scoggins CR. Martin RC., 2nd Sahoo S. Carlson DJ. Laidley AL. El-Eid SE. McGlothin TQ. McMasters KM; University of Louisville Breast Sentinel Lymph Node Study 2007 Factors determining adequacy of axillary node dissection in breast cancer patients. Breast J. 13:233–237. doi: 10.1111/j.1524-4741.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 34.Engstrom PF. Benson AB., 3rd Chen YJ. Choti MA. Dilawari RA. Enke CA. Fakih MG. Fuchs C. Kiel K. Knol JA. Leong LA. Ludwig KA. Martin EW., Jr Rao S. Saif MW. Saltz L. Skibber JM. Venook AP. Yeatman TJ; National Comprehensive Cancer Network. Colon cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:468–491. doi: 10.6004/jnccn.2005.0024. [DOI] [PubMed] [Google Scholar]
- 35.Schneider DF. Chen H Sippel RS. The impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2012;20:1906–1911. doi: 10.1245/s10434-012-2802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beal SH. Chen SL. Schneider PD. Martinez SR. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76:28–32. [PubMed] [Google Scholar]
- 37.Lang BH. Wong KP. Wan KY. Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19:1257–1263. doi: 10.1245/s10434-011-2105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider DF. Mazeh H. Chen H. Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013;18:157–162. doi: 10.1634/theoncologist.2012-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connolly TM. Watters DAK. Monitoring performance in thyroidectomy: cumulative sum analysis of outcomes. Thyroid. 2010;20:407–412. doi: 10.1089/thy.2009.0259. [DOI] [PubMed] [Google Scholar]
- 40.Adler JT. Sippel RS. Schaefer S. Chen H. Preserving function and quality of life after thyroid and parathyroid surgery. Lancet Oncol. 2008;9:1069–1075. doi: 10.1016/S1470-2045(08)70276-6. [DOI] [PubMed] [Google Scholar]
- 41.Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010;95:5241–5248. doi: 10.1210/jc.2010-1500. [DOI] [PubMed] [Google Scholar]
- 42.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 43.Nattinger AB. Laud PW. Sparapani RA. Zhang X. Neuner JM. Gilligan MA. Exploring the surgeon volume outcome relationship among women with breast cancer. Arch Intern Med. 2007;167:1958–1963. doi: 10.1001/archinte.167.18.1958. [DOI] [PubMed] [Google Scholar]
- 44.Sosa JA. Bowman HM. Gordon TA. Bass EB. Yeo CJ. Lillemoe KD. Pitt HA. Tielsch JM. Cameron JL. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman MD. Kilburn H. Lindsey M. Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borowski DW. Bradburn DM. Mills SJ. Bharathan B. Wilson RG. Ratcliffe AA. Kelly SB Northern Region Colorectal Cancer Audit Group (NORCCAG) Volume-outcome analysis of colorectal cancer-related outcomes. Br J Surg. 2010;97:1416–1430. doi: 10.1002/bjs.7111. [DOI] [PubMed] [Google Scholar]
- 47.Dillman RO. Aaron K. Heinemann FS. McClure SE. Identification of 12 or more lymph nodes in resected colon cancer specimens as an indicator of quality performance. Cancer. 2009;115:1840–1848. doi: 10.1002/cncr.24185. [DOI] [PubMed] [Google Scholar]
- 48.Wright FC. Fitch M. Coates AJ. Simunovic M. A qualitative assessment of a provincial quality improvement strategy for pancreatic cancer surgery. Ann Surg Oncol. 2011;18:629–635. doi: 10.1245/s10434-010-1337-0. [DOI] [PubMed] [Google Scholar]
- 49.Habib MR. Solomon MJ. Young JM. Armstrong BK. O'Connell D. Armstrong K. Evidence-based and clinical outcome scores to facilitate audit and feedback for colorectal cancer care. Dis Colon Rectum. 2009;52:616–622. doi: 10.1007/DCR.0b013e31819edb7d. ; discussion 622–623. [DOI] [PubMed] [Google Scholar]
- 50.Ugolini G. Rosati G. Montroni I. Zanotti S. Manaresi A. Giampaolo L. Taffurelli M. Pricolo V. An easy-to-use solution for clinical audit in colorectal cancer surgery. Surgery. 2009;145:86–92. doi: 10.1016/j.surg.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Sadideen H. Kneebone R. Practical skills teaching in contemporary surgical education: how can educational theory be applied to promote effective learning? Am J Surg. 2012;204:396–401. doi: 10.1016/j.amjsurg.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Mazeh H. Chen H. Advances in surgical therapy for thyroid cancer. Nat Rev Endocrinol. 2011;7:581–588. doi: 10.1038/nrendo.2011.140. [DOI] [PubMed] [Google Scholar]
- 53.Lang BH. Lo CY. Technological innovations in surgical approach for thyroid cancer. J Oncol. 2010;2010;pii:490719. doi: 10.1155/2010/490719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hundahl SA. Fleming ID. Fremgen AM. Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]