Abstract
Intracellular transport is vital for the function, survival and architecture of every eukaryotic cell. Long range transport in animal cells is thought to depend exclusively on microtubule tracks. This study reveals an unexpected actin-dependent but microtubule-independent mechanism for long range transport of vesicles. Vesicles organize their own actin tracks by recruiting the actin nucleation factors Spire1, Spire2 and Formin-2, which assemble an extensive actin network from the vesicles’ surfaces. The network connects the vesicles with each other and with the plasma membrane. Vesicles move directionally along these connections in a Myosin 5b-dependent manner to converge and to reach the cell surface. The overall outward directed movement of the vesicle-actin-network is driven by recruitment of vesicles to the plasma membrane in the periphery of the oocyte. Being organized in a dynamic vesicle-actin-network allows vesicles to move in a local random manner and a global directed manner at the same time: they can reach any position in the cytoplasm, but also move directionally to the cell surface as a collective. Thus, collective movement within a network is a powerful and flexible mode of vesicle transport.
According to the text book view of long range vesicle transport in animal cells, vesicles move over long distances along polarized microtubules that originate from the centrosome1-4. In a typical animal cell, microtubule plus ends face the cell surface, and minus ends converge at the centre where the centrosome is located. Accordingly, the minus end-directed motor protein dynein mediates transport to the cell centre, and plus end-directed kinesins mediate transport to the periphery5,6. However, there is evidence for alternative mechanisms of long range transport. Indicative of such mechanisms, secretion in various cell types is hardly affected if microtubules are depolymerized7,8. Also some viruses such as HIV are still able to move through the cell if microtubules are absent9. The only known microtubule-independent motor proteins that could alternatively drive long range transport are myosins, which walk along actin filaments. However, the actin cytoskeleton does not seem to provide suitable tracks for long range transport: actin filaments are randomly oriented in the cytoplasm of typical animal cells10. Only specialized cellular regions such as the actin cortex, microvilli, filopodia or dendritic spines contain polarized actin structures that could potentially be used for myosin-dependent directional transport10. Accordingly, actin filaments have only been implicated in local vesicle transport, for instance in the actin cortex and in dendritic spines1,11-13.
This study reveals a novel mechanism for long range vesicle transport that is entirely actin-dependent. It shows that vesicles organize an extensive actin network that connects them with each other and with the cell surface. Vesicles move along the network in a Myosin 5b-dependent manner which enables them to converge and to collectively move towards the cell surface.
The identity and transport routes of vesicles are defined by members of the large family of Rab GTPases. Different Rab GTPases recruit different effector proteins such as motor proteins, sorting adaptors and tethering factors that guide vesicles to their correct destination14. One of the main pathways that mediates transport to the plasma membrane is defined by the small GTPase Rab11a. Rab11a-positive vesicles are, for instance, involved in transporting the iron-carrying protein transferrin, which is one of the best studied transport cargos15. Whether vesicles use actin filaments for long range transport is hard to investigate in tissue culture cells because the cells are very flat so that prominent cortical actin structures cover intracellular actin assemblies. Thus, mouse oocytes were used to study the transport of Rab11a-positive vesicles. Cortical and intracellular actin structures are well separated in this cell type16, and the large cell volume is ideally suited to analyse quantitatively how vesicles move in three dimensions (3D).
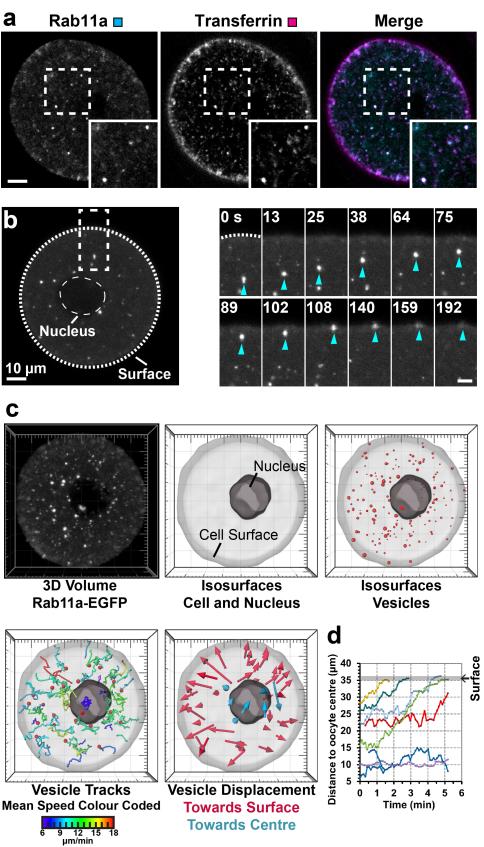
First, it was analysed whether Rab11a-positive vesicles mediate transport to the plasma membrane in this cell type. Indeed, Rab11a colocalised with transferrin (Fig. 1a), and a dominant-negative variant of Rab11a blocked the transport of transferrin in 22/22 cells (Supplementary Movie 1). Moreover, live cell imaging of fluorescently labelled Rab11a revealed that vesicles moved over distances of more than 30 μm from the centre towards the surface of the cell, where they merged with the plasma membrane (Fig. 1b).
Figure 1.
Vesicles move over long distances to the cell surface
(a) Rab11a-EGFP and Transferrin-Cy5 in live cell. Boxed region is magnified in inset. Scale bar: 10 μm.
(b) Live cell expressing Rab11a-EGFP. Boxed region is magnified next to overview. Scale bar in magnification: 5 μm. Time: seconds.
(c) 3D data sets of live cells expressing Rab11a-EGFP were acquired and isosurfaces of cell, nucleus and vesicles reconstructed. Vesicles were tracked in 3D. Only tracks that were longer than 45 s were evaluated to reliably determine the overall directionality of vesicle movements. The tracks’ colour reflects the mean vesicle speed. Displacement of vesicles towards surface (magenta) or centre (cyan) is plotted.
(d) The distance of representative vesicles from the cell’s centre is plotted over time.
To analyse quantitatively how vesicles move, high resolution 3D data sets of vesicles in live cells were recorded, the cell and vesicle surfaces were reconstructed and vesicles were automatically tracked in 3D (Fig. 1c, d and Supplementary Movie 2). Vesicles were very mobile, moving in a zig-zag pattern with a speed of 12.8±3.4 μm/min (Fig. 2a, d). By contrast, lysosomes and fluorescent beads of similar size moved significantly slower (Supplementary Fig. 1a-c and Supplementary Movie 3), demonstrating that neither cytoplasmic flows nor Brownian motion can account for the observed movements. Vesicle movements were also highly directional, with 76.8±8.8% of vesicles moving towards the cell surface over time (Fig. 2a, e). The small fraction of vesicles that did not move towards the cell surface was more stationary than other vesicles and mostly located in proximity of the nucleus of the oocyte (Fig. 1c, d). In contrast to Rab11-positive vesicles, lysosomes and fluorescent beads moved non-directionally, with equal proportions moving towards the centre and the surface of the cell (Supplementary Fig. 1a, b, d). In summary, these data show that Rab11a-positive vesicles move directionally over long distances to the plasma membrane.
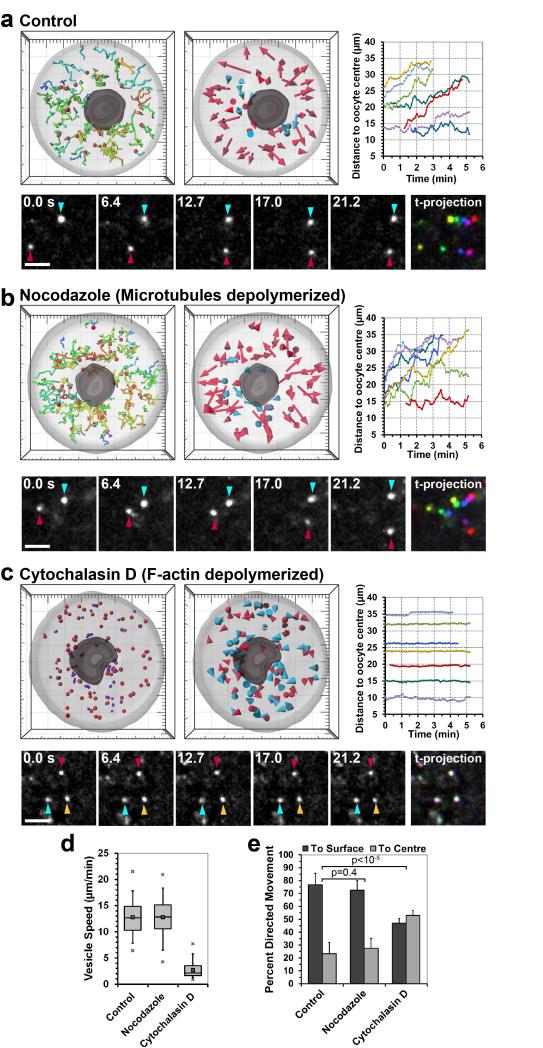
Figure 2.
Vesicle movements depend on actin instead of microtubules
(a-c) 3D data sets of vesicles labelled with Rab11a-EGFP in live control (a), nocodazole (b) and cytochalasin D treated cells (c) were acquired and processed as described for Fig. 1. Vesicle movements in live cells and a time-coloured projection are shown below, with vesicles being coloured if mobile and white if stationary. Time: seconds; Scale bars: 5 μm.
(d) Vesicles were tracked as in (a-c) and their speed in control (599 tracks, 10 oocytes), nocodazole (399 tracks, 8 oocytes) and cytochalasin D treated oocytes (1657 tracks, 8 oocytes) is shown. Box plot displays median (line), mean (small square), 1st, 99th (crosses), 5th, 95th (whiskers) and 25th and 75th percentile (boxes) of vesicle speeds.
(e) Vesicle displacement to the centre or to the surface of the oocyte was scored. Mean from 10 control, 8 nocodazole and 8 cytochalasin D treated oocytes are shown, with error bars displaying s.d.. P-values were calculated with Student’s t-test.
Next, the mechanism by which vesicles were moving was investigated. Long range vesicle transport is generally thought to be microtubule dependent1-4,10,17. To test whether vesicles were transported along microtubules, cells were treated with 1 μM nocodazole, which depolymerized all microtubules (Supplementary Fig. 2)18. Surprisingly, microtubules were dispensable for vesicle movements (Fig. 2b): neither the speed (12.8±3.7 μm/min; p=0.92) nor the directionality of vesicle movements (72.5±7.8% towards cell surface; p=0.39) was significantly different from that in control oocytes (Fig. 2d, e and Supplementary Movie 4). To test whether vesicles moved along actin filaments instead, cells were treated with cytochalasin D which depolymerizes F-actin (Fig. 2c). Without actin filaments, vesicles did not move over long distances anymore. Instead, they only moved locally and their speed was severely decreased to just 2.7±1.5 μm/min (Fig. 2c, d and Supplementary Movie 4). The directionality of vesicle movements was also lost. Instead, equal proportions of vesicles moved to the surface and to the centre of the cell (Fig. 2e). In summary, these data demonstrate that vesicles move over long distances in an actin-dependent but microtubule-independent manner.
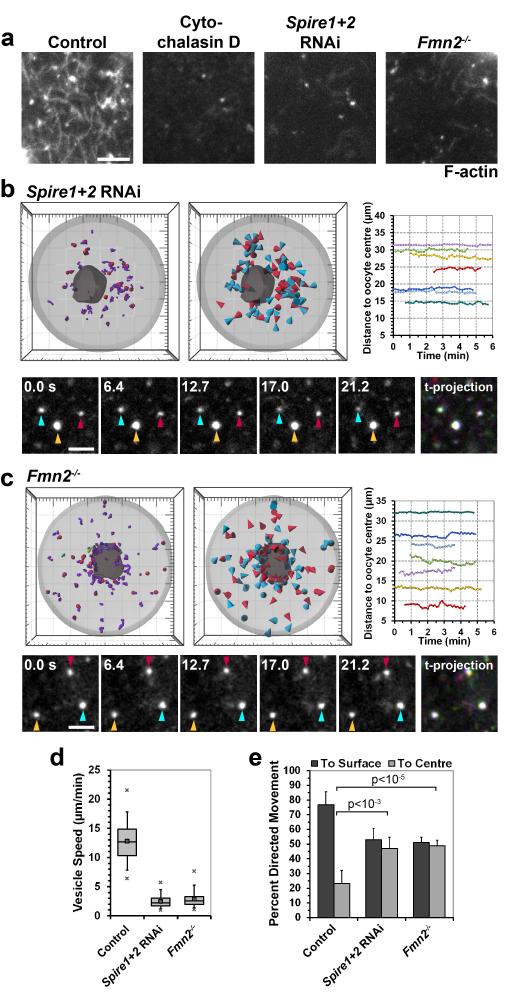
That vesicles moved directionally, even if microtubules were absent, suggested that actin filaments might provide the tracks for vesicle movements. We recently reported that mouse oocytes are filled with a cytoplasmic actin network with an average filament length of 5.7±1.9 μm between branch points, which is required for asymmetric spindle positioning16 (Fig. 3a). The actin network was not affected by depolymerizing microtubules (Supplementary Fig. 2). Thus, it seemed possible that filaments of the actin network mediate vesicle movements. To test this hypothesis, vesicle movements were analysed in oocytes that lack the actin nucleation factors Spire1 and Spire219 (Supplementary Fig. 3a) or Formin-220, which cooperate with each other to assemble the network16,19 (Fig. 3a). When either Fmn2 or Spire1 and Spire2 were absent, the vesicle speed was greatly reduced, down to similar levels as in cytochalasin D treated cells (Fig. 3b-d; RNAi rescue in Supplementary Fig. 3b, c). Also the directionality of vesicle movements was lost (Fig. 3e). By contrast, the actin network was still present and vesicle movements only slightly affected when oocytes were treated with inhibitors of the Arp2/3 complex21 (Supplementary Fig. 3d-f). Together, these data show that vesicle movements require cytoplasmic actin filaments that are nucleated by Spire1, Spire2 and Fmn2.
Figure 3.
Spire1/2 and Fmn2 assemble actin tracks
(a) Control, cytochalasin D treated, Fmn2−/− and Spire1+2 co-depleted cells were fixed and stained with Alexa488-phalloidin to label F-actin.
(b-c) 3D data sets of vesicles labelled with Rab11a-EGFP in live Spire1 and Spire2 co-depleted oocytes (b) and Fmn2−/− oocytes (c) were acquired and processed as described for Fig. 1 and 2.
(d) Vesicles were tracked as in b-c and their speed in control (599 tracks, 10 oocytes), Spire1 and Spire2 co-depleted (936 tracks, 6 oocytes) and Fmn2−/− (1605 tracks, 8 oocytes) oocytes is shown. Box plot as in Fig. 2d.
(e) Vesicle displacement to the centre or to the surface of the oocyte was scored. Mean from 10 control, 6 Spire1 and Spire2 co-depleted (p<10−3) and 8 Fmn2−/− (p<10−5) oocytes are shown, with error bars displaying s.d.. P-values were calculated with Student’s t-test.
(a-c) Scale bars: 5 μm.
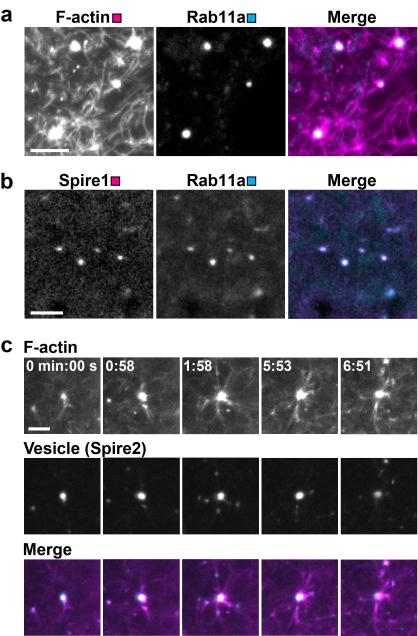
To investigate how vesicles move within the actin network, the behaviour of vesicles and actin filaments was analysed by high resolution microscopy of live and fixed oocytes. Surprisingly, vesicles were located at nodal points in the network, seemingly serving as organizing centres of the network (Fig. 4a and Supplementary Movie 5), with an average of 4.9±1.4 filaments ermerging from each vesicle (n=35). If the actin network is assembled from the vesicle surface, then Spire1, Spire2 and Fmn2 should colocalise with Rab11a-positive vesicles. Indeed, all actin nucleation factors colocalised on the vesicle surface (Fig. 4b and Supplementary Fig. 4a). To test directly whether the actin network was assembled from the vesicle surface, an F-actin regrowth assay was established. Cells were first treated with cytochalasin D to depolymerize F-actin. The drug was then washed out and actin repolymerization was monitored in live cells to determine the sites from where the network was nucleated. This assay confirmed that vesicles served as prominent organizing centres of the network (Fig. 4c and Supplementary Movie 6). In further support of actin nucleation from the vesicle surface, some vesicles were even pushed through the cell upon actin nucleation events (Supplementary Fig. 4b), showing that these nucleation events contribute to local random vesicle movements. Also the movements of vesicles and associated filaments were closely correlated (Supplementary Movie 7), with actin filaments moving with a velocity of 10.6±6.8 μm/min (49 filaments in 5 oocytes).
Figure 4.
Vesicles organize an actin network
(a) Cell expressing Rab11a-mCherry was fixed and stained for F-actin. Scale bar: 5 μm.
(b) Live cell expressing Spire1-mCherry and Rab11a-mEGFP. Scale bar: 5 μm.
(c) Reassembly of F-actin upon washout of cytochalasin D in live cell expressing EGFP-UtrCH (F-actin) and Spire2-mCherry (Vesicle). Time: min:sec; Scale bar: 5 μm.
In summary, these results show that Rab11a-positive vesicles recruit the actin nucleation factors Spire1, Spire2 and Fmn2 to assemble an extensive actin network that fills the cell’s cytoplasm and connects vesicles with each other and with the cell surface.
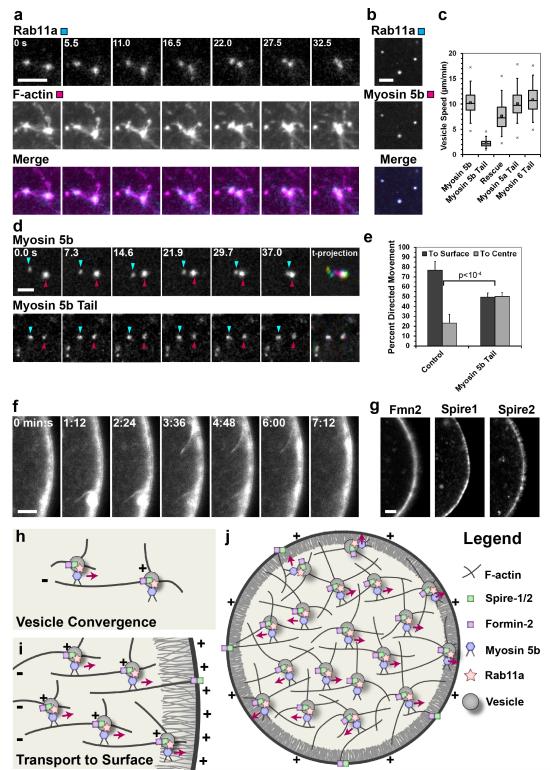
But how are vesicles using the actin network to move directionally? Two types of directional movements were observed: vesicle convergence (Fig. 5a, d) and movement of vesicles towards the plasma membrane (Fig. 1). First, the mechanism of directional movement for vesicle convergence was investigated. High resolution live cell imaging revealed that vesicles moved to neighbouring vesicles along actin filaments connecting them (Fig. 5a and Supplementary Movie 8). When the actin network was absent vesicles could not converge anymore and the average vesicle size was decreased (Supplementary Fig. 5). The lack of convergence and missing transport to the cell surface also significantly increased the number of vesicles per cell (Supplementary Fig. 5). Movements along actin filaments are mediated by motor proteins of the myosin family. Most myosins are plus end-directed. Spire proteins22,23 and formins24,25 associate with the plus ends of actin filaments. This implies that the plus ends of filaments in the network converge on the vesicle surface, where the nucleators were located (Fig. 5h). Thus, a myosin carrying vesicle could attach to an actin filament that is nucleated from a neighbouring vesicle and move towards the neighbouring vesicle along this filament (Fig. 5h). A good candidate for such a myosin was Myosin 5b because it was shown to associate with Rab11a-positive vesicles in other cell types11,26. Indeed, Myosin 5b colocalised with Rab11a-positive vesicles in mouse oocytes (Fig. 5b). To test whether Myosin 5b was required for vesicle convergence, its activity was inhibited by expressing the dominant-negative tail domain of Myosin 5b. This blocked vesicle convergence (Fig. 5d) and decreased the vesicle speed (Fig. 5c). Vesicle movements specifically required Myosin 5b, because first, vesicle movements could be rescued by overexpressing full-length Myosin 5b (Fig. 5c); and because second, vesicle movements were not affected by dominant-negative variants of other myosins, such as Myosin 5a or Myosin 6 (Fig. 5c). Moreover, human Myosin 5b was reported to have a velocity of 13.2±1.8 μm/min in actin gliding assays27, which is remarkably similar to the velocity of Rab11a-positive vesicles of 12.8±3.4 μm/min that is reported in this study (Fig. 2d). In summary, these data suggest that vesicles move towards each other along actin filaments that are nucleated from a neighbouring vesicle in a Myosin 5b dependent manner (Fig. 5h).
Figure 5.
Mechanism of directional vesicle transport
(a) Live cell expressing EGFP-UtrCH (F-actin) and mCherry-Rab11a (Vesicle). Time: seconds; Scale bar: 2 μm.
(b) Live cell expressing Rab11a-mEGFP (Rab11a) and Myosin 5b-mCherry (Myosin 5b).
(c) Vesicle speed in Myosin 5b (229 tracks, 6 oocytes), Myosin 5b tail (446 tracks, 5 oocytes), rescued (1286 tracks, 13 oocytes), Myosin 5a tail (872 tracks, 12 oocytes) and Myosin 6 tail (606 tracks, 12 oocytes) expressing oocytes is shown. Box plot as in Fig. 2d.
(d) Live cell expressing full length Myosin 5b-mCherry (top row) or Myosin 5b tail-mCherry (bottom row) and time-coloured projection, with vesicles being coloured if mobile and white if stationary. Time: seconds.
(e) Vesicle displacement to the centre or to the surface of the oocyte was scored. Mean from 10 control and 5 Myosin 5b tail expressing oocytes are shown, with error bars displaying s.d.. P-values were calculated with Student’s t-test.
(f) Live cell expressing EGFP-UtrCH. Time: min:sec; Scale bar: 5 μm.
(g) Live cells expressing Fmn2-mEGFP, Spire1-mCherry or Spire2-mCherry.
(h-j) Mechanistic model for actin-dependent vesicle movements. The different objects are specified in the legend. For details, see text.
(b, d, f, g) Scale bars: 5 μm.
The same transport principle also explains why vesicles move towards the cell surface over time. The plus ends of actin filaments are not only enriched on vesicles, but also at the cell surface: First, Fmn2, Spire1 and Spire2 all localized to the cell surface (Fig. 5g), from where actin filaments were nucleated (Fig. 5f) and numerously reached into the cytoplasm (Supplementary Movie 9). Second, filaments in the actin cortex are also polarized so that plus ends face the plasma membrane28 (Fig. 5i). Myosin 5b can bind to these polarized filaments and transport vesicles towards the plasma membrane (Fig. 5i). Indeed, vesicles attached to these actin filaments and moved along them towards the cell surface (Supplementary Fig. 6a and Supplementary Movie 10; Fig. 5i). In further support of this model, inhibition of Myosin 5b blocked the outward movement of vesicles (Fig. 5e), and Myosin 5b tail carrying vesicles were frequently located below the cell surface (Supplementary Fig. 6b). But why are vesicles also moving towards the cell surface if they are close to the centre of the cell, far away from polarized filaments in the periphery? Since all vesicles form an interconnected network, short range directional movements in the periphery of the oocyte are sufficient to drag the entire vesicle-actin-network outward (Fig. 5i, j). The most peripheral vesicles, which bind to polarized actin filaments beneath the plasma membrane, will carry neighbouring vesicles with them, and these will carry their neighbours and so on (Fig. 5i). In this way, the entire vesicle-actin-network moves outward over time (Fig. 5j). Thus, the collective movement of vesicles in a network makes long polarized actin filaments or microtubules dispensable for long range transport (Fig. 5i, j).
In summary, this study reveals a novel actin-dependent mechanism for long range vesicle transport. It shows that vesicles recruit the actin nucleation factors Spire1, Spire2 and Fmn2 to organize a cytoplasmic actin network that connects the vesicles with each other and with the cell surface. The filament connections between vesicles are used for Myosin 5b-dependent directional transport which allows vesicles to move towards each other and to reach the cell surface. Directional movements in the cell periphery will automatically drag the network to the surface and thereby determine that vesicles move outward over time. These data uncover a much more flexible transport system than constricted movement along microtubule tracks. Being organized in a dynamic vesicle-actin-network allows vesicles to move in a local random manner and a global directed manner at the same time: they can reach any position in the cytoplasm, but also move directionally to the cell surface as a collective. This new principle of vesicles as organizers of an extensive actin network is surprising, because vesicle associated actin nucleators have so far mostly been implicated in membrane remodelling29-32. Interestingly, morphologically similar actin networks that converge at bright actin nodes, which might correspond to vesicles, have been reported in a range of cell types, including Xenopus epithelial cells33, Drosophila embryos34 and oocytes35 and C. elegans embryos36, and Spire proteins colocalize with vesicles in fibroblasts29, indicating that network mediated long range transport of vesicles might be conserved. Intracellular transport is essential for the existence and function of all eukaryotic cells. This study reveals two new fundamental concepts of how animal cells can achieve this vital task: an unexpected actin-dependent mechanism for long range transport, and a novel principle of intracellular transport, namely the collective movement of vesicles as integral building blocks of a dynamic network.
Supplementary Material
Acknowledgements
The author thanks Philip Leder, Benjamin Leader and Markus Dettenhofer for Fmn2−/− mice; the staff of the LMB’s Animal Facility for expert technical assistance; Matthew Freeman, Sean Munro and Ben Nichols for helpful discussions and critical reading of the manuscript. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 241548.
References
- 1.Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr Opin Cell Biol. 2008;20:41–47. doi: 10.1016/j.ceb.2007.11.006. doi:10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts B, et al. Molecular Biology of the Cell. Fifth edn 2008. [Google Scholar]
- 3.Lodish H, et al. Molecular Cell Biology (Lodish, Molecular Cell Biology) W. H. Freeman; 2007. [Google Scholar]
- 4.Pollard T, Earnshaw W. Cell Biology. (Saunders. 2004 [Google Scholar]
- 5.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. doi:10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 6.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. doi:10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom GS, Goldstein LS. Cruising along microtubule highways: how membranes move through the secretory pathway. The Journal of cell biology. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 9.Fackler OT, Krausslich HG. Interactions of human retroviruses with the host cell cytoskeleton. Curr Opin Microbiol. 2006;9:409–415. doi: 10.1016/j.mib.2006.06.010. doi:10.1016/j.mib.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–252. doi: 10.1016/j.tcb.2009.03.003. doi:10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. doi:S0092-8674(08)01253-1 [pii] 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nature cell biology. 2011;13:40–48. doi: 10.1038/ncb2132. doi:10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. The Journal of cell biology. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. doi:10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 15.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. doi:10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–1992. doi: 10.1016/j.cub.2008.11.022. doi:S0960-9822(08)01502-9 [pii] 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. doi:10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 18.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. doi:S0092-8674(07)00792-1 [pii] 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Current biology : CB. 2011;21:955–960. doi: 10.1016/j.cub.2011.04.029. doi:10.1016/j.cub.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leader B, et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–928. doi: 10.1038/ncb880. [DOI] [PubMed] [Google Scholar]
- 21.Nolen BJ, et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009;460:1031–1034. doi: 10.1038/nature08231. doi:10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, et al. Human spire interacts with the barbed end of the actin filament. J Mol Biol. 2011;408:18–25. doi: 10.1016/j.jmb.2010.12.045. doi:10.1016/j.jmb.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Bosch M, et al. Analysis of the function of Spire in actin assembly and its synergy with formin and profilin. Mol Cell. 2007;28:555–568. doi: 10.1016/j.molcel.2007.09.018. doi:10.1016/j.molcel.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. doi:10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. doi:10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 26.Lapierre LA, et al. Myosin vb is associated with plasma membrane recycling systems. Molecular biology of the cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe S, Mabuchi K, Ikebe R, Ikebe M. Mechanoenzymatic characterization of human myosin Vb. Biochemistry. 2006;45:2729–2738. doi: 10.1021/bi051682b. doi:10.1021/bi051682b. [DOI] [PubMed] [Google Scholar]
- 28.Begg DA, Rebhun LI. pH regulates the polymerization of actin in the sea urchin egg cortex. The Journal of cell biology. 1979;83:241–248. doi: 10.1083/jcb.83.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerkhoff E, et al. The Spir actin organizers are involved in vesicle transport processes. Curr Biol. 2001;11:1963–1968. doi: 10.1016/s0960-9822(01)00602-9. doi:S0960-9822(01)00602-9 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. doi:10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morel E, Parton RG, Gruenberg J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16:445–457. doi: 10.1016/j.devcel.2009.01.007. doi:10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Galletta BJ, Cooper JA. Actin and endocytosis: mechanisms and phylogeny. Curr Opin Cell Biol. 2009;21:20–27. doi: 10.1016/j.ceb.2009.01.006. doi:10.1016/j.ceb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. The Journal of cell biology. 2008;182:77–88. doi: 10.1083/jcb.200804062. doi:10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. doi:10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 35.Dahlgaard K, Raposo AA, Niccoli T, St Johnston D. Capu and Spire assemble a cytoplasmic actin mesh that maintains microtubule organization in the Drosophila oocyte. Dev Cell. 2007;13:539–553. doi: 10.1016/j.devcel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. doi:10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.