Abstract
Background/Aim
Thoracic irradiation results in an acute inflammatory response, latent period, and late fibrosis. Little is known about the mechanisms involved in triggering late radiation fibrosis.
Materials and Methods
Thoracic irradiated fibrosis prone C57BL/6NTac mice were followed for detectable mRNA transcripts in isolated lung cells and micro-RNA in whole tissue, and the effect of administration of water-soluble oxetanyl sulfoxide MMS350 was studied. Marrow stromal cell motility in medium from fibrotic phase explanted pulmonary endothelial and alveolar type II cells was measured.
Results
RNA and micro-RNA expression in lung correlated with fibrosis. MMS350 reduced pro-fibrotic gene expression in both endothelial and alveolar type II cells in irradiated mice. Conditioned medium from irradiated cells did not alter cell motility in vitro.
Conclusion
These findings should allow potential new drug targets for ameliorating irradiation-induced pulmonary fibrosis to be identified.
Keywords: Ionizing irradiation, endothelial cells, alveolar type II cells, motility
Radiotherapy is effective in the treatment of lung and esophageal cancer (1). Complications of thoracic radiotherapy include acute pneumonitis and late radiation fibrosis (2). Recent studies have shown that the development of fibrosis involves both resident lung fibroblasts, and recruitment to the lungs of bone marrow origin progenitor cells (3, 4). The biological, molecular, and cellular changes involved in stimulating the onset of the late fibrotic response are unknown.
Endothelial cell apoptosis has been shown to be a critical mechanism in irradiation tissue damage to several organs, including the lung (5, 6). Previous studies have shown that irradiation of endothelial cells results in the release of growth factors including fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) (7). FGF release from endothelial cells has been shown to protect against irradiation-induced apoptotic programmed cell death (8). In addition, persistent elevation in gene expression within irradiated cells has been shown throughout the acute and late stages of endothelial cell apoptosis (9). Persistent ionizing irradiation-induced pulmonary endothelial cell responses have been shown to disrupt endothelial mesenchymal interactions, and promote both fibroblast proliferation and collagen deposition (10). We recently demonstrated that the water soluble oxetanyl sulfoxide, MMS350, reduces the level of luciferase+ (luc +) bone marrow stromal cell migration to the irradiated lungs of C57BL/6NTac mice and reduces late-stage fibrosis (11).
We now report the results of studies comparing the effect of lung irradiation on endothelial compared to alveolar type II cells, the effect of MMS350 on expression of pro-fibrotic gene transcripts in separated endothelial and alveolar II cell populations, and the effect of thoracic radiation on modulation of micro-RNA profibrotic gene expression. We also investigated whether growth factors produced by irradiated, explanted, lung epithelial or endothelial cells stimulated the motility of bone marrow origin fibroblast progenitor cells in vitro.
Materials and Methods
C57BL/6NTac mouse thoracic radiation
C57BL/6NTac mice were obtained from Taconic Farms (Hudson, NY, USA) and C57BL6-Luc + GFP + mice were obtained from Steve Thorne, University of Pittsburgh Cancer Institute, and housed 5 per cage according to Institutional (IACUC) protocols. Mice were irradiated to the thoracic cavity with shielding of the head and neck, abdomen, and lower body according to published methods (12). Animals received 20 Gy single fraction thoracic irradiation and were then maintained according to IACUC directed laboratory conditions. Mice were sacrificed at serial time points after thoracic irradiation including pre-irradiation, days 2, 7, 14, 28, 50, 75, 100, 110, 150 and 200 post-irradiation. Transplantation of luciferase-positive (Luc +) marrow was performed as published (13).
Separation of mouse lung cell populations
To isolate different cellular components of the lung, irradiated mice were sacrificed, the pulmonary cavity was opened, and lungs were perfused by injecting 5 ml of phosphate-buffered saline (PBS) into the right ventricle of the heart (18). To isolate pulmonary endothelial cells, the lungs were filled with 1 ml of dispase (39.65 mg/ml), allowed to collapse, and then expanded with 0.5 ml of a 1% low-melt agarose, which had been stored at 45°C in a water-bath. The lungs were immediately covered with ice and incubated for 2 min. The lungs were then removed, placed in 4 ml of digestion buffer [trypsin (10 ml), HBSS (10 mL), dispase (62.4 mg), and collagenase I (40 mg)], incubated for 45 min at 37°C, and placed on ice. The lungs were then transferred to 7 ml of Dulbecco’s modified Eagle’s medium (DMEM) containing 0.01% DNA, then teased away from the airways and swirled for 5–10 min at room temperature. The resulting suspension was filtered through a 40 μm cell strainer, centrifuged at 12 × g for 10 min at 4°C, and resuspended in 10 mL of DMEM. A PE-conjugated monoclonal antibody to platelet endothelial cell adhesion molecule (PECAM-1) and an APC-Cy7-conjugated monoclonal antibody to CD45 (Santa Cruz Biotechnology, Santa Cruz, Ca, USA) were added to the cells which were then incubated for 30 min at 4°C. The cells were then washed in DMEM and DAPI was added to identify live cells. Flow cytometric cell sorting was used to isolate PECAM positive endothelial cells and PECAM negative and CD45 negative alveolar cells. This procedure resulted in 11% endothelial cells and 70% alveolar II cells, other cells including macrophages comprised 19% (18).
Administration of radiation mitigator MMS350 drug
Mice were administered MMS350 by adding the drug to the water bottle in each mouse cage. The dose of drug per bottle was 400 μM of MMS350, and it was available continuously starting on day 88 post irradiation. Water bottles were changed every seven days. The estimated total amount consumed by each animal daily was 436 μg (19 mg/kg/day for mice weighing 23 g) based on an estimation of each mouse drinking 5 ml of water per day.
Luc+ bone marrow (BM) stromal cell motility
Real-time tracking/imaging of Luc+ BM stromal cell motility in response to endothelial cell and type II alveolar cell conditioned medium (CM) was performed using a CytoWorks imaging system (Kairos Instruments Pittsburgh, PA, USA). Endothelial and type II alveolar cells were isolated from lungs of nonirradiated control mice, and from treated mice 150 days post irradiation using flow cytometry. The isolated cells were cultured and the CM was harvested. The motility of cells from a Luc+ BM stromal cell line (11) grown in CM from the above experimental groups was tracked. BioImageXD was used for analysis of tracks from sequences of selected cytes (view fields). Results from two experiments are presented as the mean rate of motility in (μm/min) for individual cell tracks within the time interval between 3 and 10.5 hours after the addition of conditioned medium. The number of individual cell tracks measured per condition ranged from 244 to 273.
Measurement of mRNA and mi-RNA expression by real time polymerase chain reaction
RNA was extracted from mouse lung samples using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions, quantified using a spectrophotometer, and stored at −80°C. Reverse transcription of 33.3 ng of total RNA to complementary DNA (cDNA) was accomplished using 5X mi-RNA Primer and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol.
Representative lung lobes were tested by RT-PCR for levels of detectable expression of specific RNA moieties including: (Gapdh; Gen-Bank: NM_008084.2) (Gusb; Gen-Bank: NM_010368.1), (Nfkb; Gen-Bank: NM_199267.2), (Nrf2; Gen-bank: NM_010902.3), (Tgfb1; Gen-Bank: NM_011577.1), (Vegfa; Gen-Bank: NM_001025250.3), (Vwf; Gen-Bank: NM_011708.3), (Tlr4; Gen-Bank: NM_021297.2), (Sod2; Gen-Bank: NM_013671.3), (Igfbp7; Gen-Bank: NM_001159518.1), and mi-RNAs MiR-107, MiR-126, MiR-155, and MiR-511 (Applied Biosystems, Foster City, CA, USA)
RNA expression was quantitated by RT-PCR as previously described (11, 12). Micro-RNA expression was assessed using 96 well plates prepared with 5 μl of Taqman Gene Expression Master mix, 3.84 μl of RNase-free water, 0.5 μl of the corresponding Taqman 20X Gene Expression primer, and 0.66 μl of cDNA using the Eppendorf epMotion 5070 automated pipetting system (Eppendorf, Westbury, NY, USA). The cDNA was amplified with 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf Realplex2 Mastercycler.
Data for each gene transcript were normalized by calculating the differences ΔCt-Gusb and Ct-Target mRNA, or Ct- Gapdh and Ct-Target mi-RNA. The relative increase or decrease in expression was calculated by comparing the reference gene with the target gene (ΔΔCt) and using the formula for relative expression (=2ΔΔCt). Subsequently, ΔΔCt levels were compared and P-values were calculated using one-way ANOVA followed by Tukey’s multiple comparison tests. The results are presented as the percentage increase in RNA above baseline levels which were adjusted to that for C57BL/6NTac control mice. The pre-irradiation baseline levels were used to determine the magnitude of decrease or elevation in mRNA/mi-RNA detectable by RT-PCR.
Statistical methods
Gene transcription was analyzed by comparing the reference gene with the target gene and calculating the relative increase or decrease in expression. Data for gene transcription, gene expression, and cell motility were summarized by mean ± standard deviation (SD) in each subgroup, and compared between groups using ANOVA followed by pairwise Student’s T-tests (4, 11). In all these tests, a p-value of less than 0.05 was regarded as significant. In these explanatory analyses, we did not adjust p-values for multiple comparisons.
Results
Pulmonary endothelial and alveolar cell-specific up-regulation of gene transcripts following pulmonary irradiation
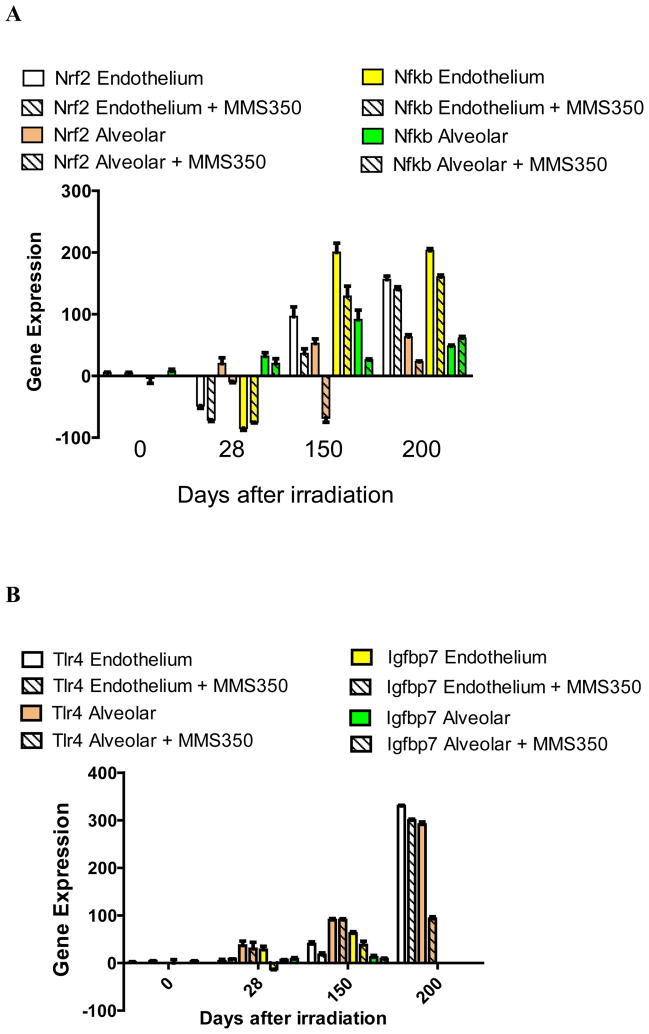
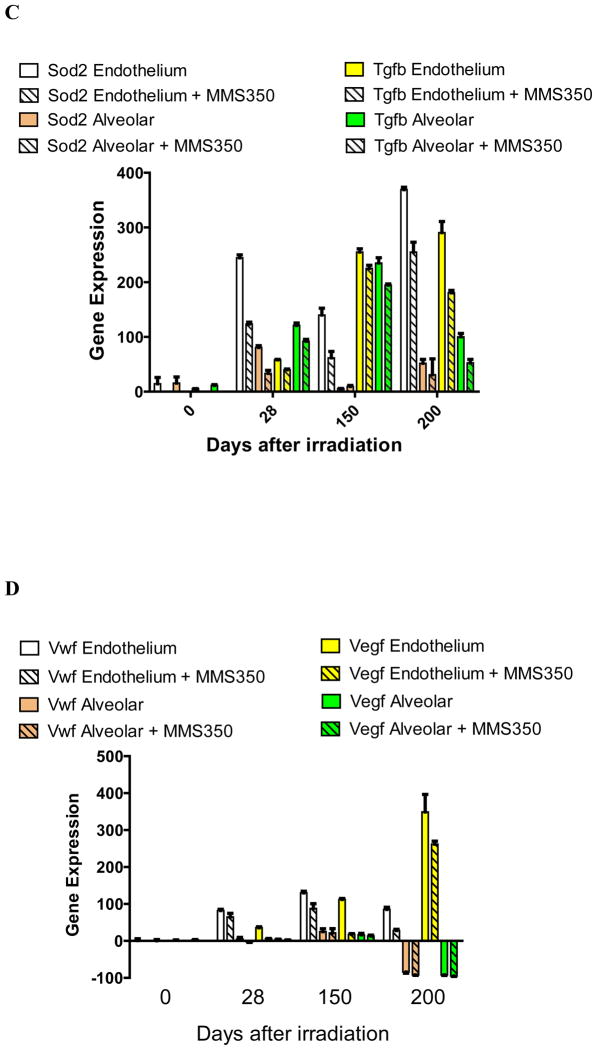
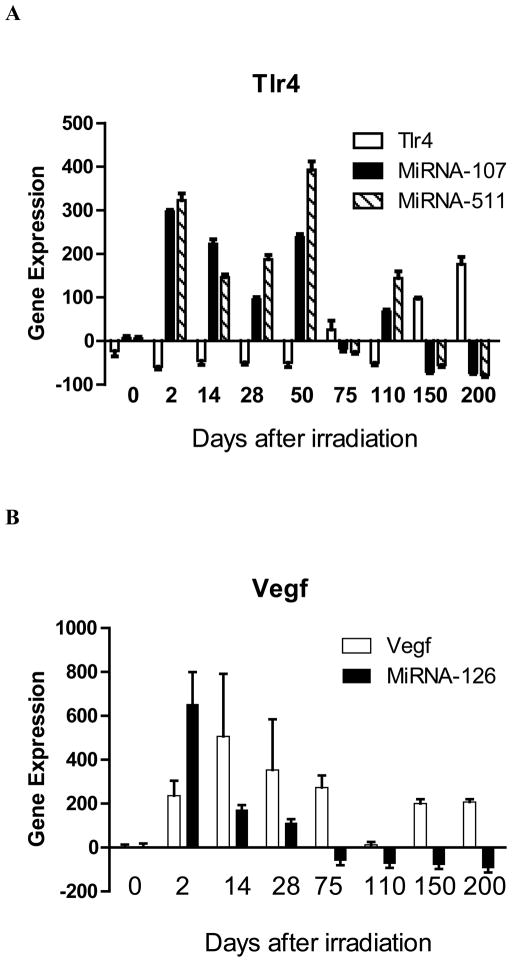
We sought to determine if there were distinct patterns of transcription of specific groups of genes in lung endothelial cells compared to alveolar II cells during the latent period that may correlate with the onset of fibrosis. Endothelial and alveolar II cells were separated from explanted irradiated lungs at serial time points and were compared for irradiation induced transcript levels. The results shown in Figure 1 and Table I demonstrate that there was an elevation of RNA transcripts in both alveolar and endothelial cells of several genes including: Nfkb, Nrf2 (Figure 1A); Tlr4, Igfbp7 (Figure 1B); Sod2, Tgfb (Figure 1C); and endothelial cell-specific elevation of Vwf, and Vegfa (Figure 1D). These results establish that elevations in specific pulmonary RNA transcripts include genes that are endothelial cell-specific (Vwf and Vegfa) and genes elevated in both alveolar and endothelial cells (Sod2 and Tgfb) (Table I).
Figure 1. MMS350 reduces levels of irradiation-induced endothelial and alveolar cell specific RNA levels.
Endothelial and alveolar cells were isolated from the lungs of irradiated control or MMS350-treated mice at days 0, 28, 150, and 200 after 20 Gy thoracic irradiation. Subgroups of mice had received MMS350 continuously in the drinking water beginning 88 days post irradiation. RNA was isolated and RT-PCR was performed for gene expression of A: oxidative stress induced promoters, B: endothelial and alveolar cell markers, C: fibrosis associated genes.
Table I.
Endothelial compared to alveolar cell specific time course of induction and elevated gene transcription in irradiated lung.
| Gene | Cell type | Days after irradiation | |||
|---|---|---|---|---|---|
| 0 | 28 | 150 | 200 | ||
| Vegf | Endothelial | 1.7 ± 2.2 | 35.5 ± 3.5 | 112.4 ± 5.3 | 348.5 ± 67.2 |
| Alveolar | 3.1 ± 1.0 p = 0.2891 |
4.4 ± 0.7 p = 0.0005 |
16.5 ± 8.0 p < 0.0001 |
−91.5 ± 2.1 p = 0.0682 |
|
| Vwf | Endothelial | 1.0 ± 9.4 | 82.0 ± 7.2 | 129.8 ± 8.9 | 85.0 ± 8.5 |
| Alveolar | 0.5 ± 5.9 p = 0.9313 |
5.2 ± 7.9 p < 0.0001 |
25.1 ± 15.7 p < 0.0001 |
−83.5 ± 4.9 p = 0.0017 |
|
| Nfkb | Endothelial | −3.4 ± 12.2 | −84.5 ± 4.9 | 200.3 ± 30.3 | 202.5 ± 4.9 |
| Alveolar | 7.5 ± 4.9 p = 0.3623 |
31.1 ± 8.6 p = 0.0036 |
91.1 ± 31.2 p = 0.0236 |
48.1 ± 2.7 p = 0.0007 |
|
| Nrf2 | Endothelial | 4.3 ± 1.3 | −48.0 ± 8.0 | 96.0 ± 22.6 | 156.4 ± 8.0 |
| Alveolar | 3.2 ± 1.9 p = 0.5738 |
19.5 ± 13.4 p = 0.0053 |
−52.5 ± 10.6 p = 0.0139 |
63.3 ± 5.2 p = 0.0052 |
|
| Sod2 | Endothelial | 14.5 ± 16.3 | 244.5 ± 7.8 | 140.1 ± 25.0 | 368.5 ± 6.4 |
| Alveolar | 15.8 ± 15.9 p = 0.9451 |
80.5 ± 4.9 p = 0.0016 |
5.4 ± 1.2 p < 0.0001 |
51.8 ± 10.3 p = 0.0007 |
|
| Tgfb | Endothelial | 5.7 ± 0.5 | 59.0 ± 1.4 | 255.2 ± 51.0 | 290.5 ± 29.0 |
| Alveolar | 9.5 ± 2.1 p = 0.1297 |
125.5 ± 6.4 p = 0.0048 |
234.9 ± 16.9 P = 0.5527 | 99.5 ± 9.2 p = 0.0124 |
|
| Tlr4 | Endothelial | 1.6 ± 2.3 | 4.0 ± 5.7 | 36.3 ± 7.8 | 329.5 ± 2.1 |
| Alveolar | 3.9 ± 1.7 p = 0.2407 |
36.7 ± 40.0 p = 0.3710 |
92.4 ± 4.8 p = 0.0004 |
290.5 ± 7.8 p = 0.0207 |
|
| Igfbp7 | Endothelial | 7.2 ± 1.2 | −7.3 ± 1.5 | 62.5 ± 3.5 | ND |
| Alveolar | 4.0 ± 0.8 p = 0.0916 |
6.5 ± 0.8 p = 0.0011 |
12.0 ± 4.0 p = 0.0007 |
ND | |
C57BL/6NTac mice were irradiated with 20 Gy to the pulmonary cavity. The mice were sacrificed at day 28, 150, or 200 after irradiation at which time the lungs were removed. Endothelial and alveolar cells were isolated, mRNA was extracted and RT-PCR was performed. Percentage difference in mRNA levels compared to baseline level between endothelial and alveolar cells were performed and significant p values are shown in bold font. ND, Not Determined.
Mice given MMS350 in the drinking water beginning on day 88 after 20 Gy thoracic irradiation demonstrated decreases in both alveolar and endothelial cell gene transcript elevation compared to levels observed in cells from irradiated control mice (Figure 1, Table III). Nfkb was not reduced by MMS350 at day 200 in endothelial or epithelial cells. The data establish that MMS350 administration in vivo results in significant reductions in expression of Sod2, Tgfb, Tlr4, and Igfbp7 in both explanted pulmonary endothelial and alveolar type II cells.
Table III.
Effect of MMS350 treatment on modulation of alveolar cell gene transcription in irradiated lung.
| Gene | MMS350 Treatment | Days After 20 Gy | ||
|---|---|---|---|---|
| 28 | 150 | 200 | ||
| VEGF | No | 4.4 ± 0.7 | 16.5 ± 8.0 | −91.5 ± 2.1 |
| Yes | 2.8 ± 0.5 p= 0.0256 |
13.5 ± 4.4 p = 0.5368 |
−95.0 ± 4.2 p = 0.4063 |
|
| vWF | No | 5.2 ± 7.9 | 25.1 ± 15.7 | −83.5 ± 4.9 |
| Yes | −2.6 ± 2.3 p = 0.1737 |
21.5 ± 23.6 p = 0.8078 |
−92.0 ± 1.4 p = 0.1446 |
|
| NFkβ | No | 31.1 ± 8.6 | 91.1 ± 31.2 | 48.1 ± 2.7 |
| Yes | 20.0 ± 11.3 p = 0.3855 |
25.7 ± 4.0 p = 0.0237 |
61.0 ± 4.3 p = 0.0701 |
|
| Nrf2 | No | 19.5 ± 13.4 | −52.5 ± 10.6 | 63.3 ± 5.2 |
| Yes | −9.0 ± 2.8 p = 0.0991 |
−68.0 ± 9.9 p = 0.2699 |
23.1 ± 1.5 p = 0.0090 |
|
| MnSOD | No | 80.5 ± 4.9 | 5.4 ± 1.2 | 51.8 ± 10.3 |
| Yes | 33.5 ± 7.8 p = 0.0187 |
9.5 ± 3.5 p = 0.0732 |
31.2 ± 4.0 p = 0.1182 |
|
| TFGβ | No | 125.5 ± 6.4 | 234.9 ± 16.9 | 99.5 ± 9.2 |
| Yes | 95.5 ± 4.9 p = 0.0343 |
194.7 ± 3.7 p = 0.0159 |
52.4 ± 9.4 p = 0.0368 |
|
| TLR4 | No | 36.7 ± 40.0 | 92.4 ± 4.8 | 290.5 ± 7.8 |
| Yes | 30.0 ± 38.2 p = 0.8798 |
36.0 ± 11.3 p = 0.0039 |
92.8 ± 5.9 p = 0.0012 |
|
| IGFbp7 | No | 6.5 ± 0.8 | 12.0 ± 4.0 | ND |
| Yes | 8.2 ± 3.2 p = 0.6319 |
9.3 ± 2.7 p = 0.6706 |
ND | |
C57BL/6NTac mice treated with MMS350 in the drinking water along with control mice which had been irradiated to 20 Gy to the pulmonary cavity were sacrificed on day 28, 150 or 200 after irradiation. Lungs were removed, the alveolar cells isolated, mRNA was extracted and RT-PCR performed. Results represent percentage increase in level of each transcript relate to baseline level. p-Values are for comparisons between mice treated with MMS350 and control irradiated mice. Significant p-values are shown in bold. ND, not determined.
mi-RNA expression levels correlate with respective RNA expression
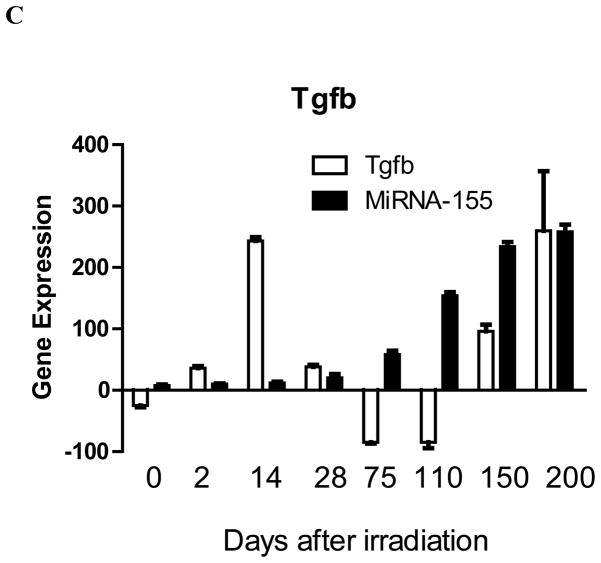
To determine whether irradiation effects on increasing pulmonary RNA transcripts and the observed MMS350 modulation of these effects correlated with established mi-RNA levels, we measured levels of mi-RNA known to correlate with levels of RNA transcripts for Tlr4, Vegfa, and Tgfb. We tested explanted whole lung segments at serial time points after irradiation. Previous publications established that Tlr4 down-regulates expression of MiR-107 (14) and that Tlr4 is reciprocally down-regulated by increased levels of MiR-511 (15). The data from the present experiments (Figure 2A) were consistent with these prior findings. The data include levels of MiR-107 and MiR-511, which also correlated with levels of Tlr4 gene expression in whole lung following irradiation.
Figure 2. MicroRNA expression during the acute phase, latent period, and late radiation fibrotic phase in lungs of mice irradiated with 20 Gy.
To determine if irradiation affected regulation of gene expression by miRNA, C57BL/6NTac mice were irradiated with 20 Gy to the pulmonary cavity. At various time points after irradiation, the mice were sacrificed, the lungs removed, mRNA was extracted, and RT-PCR was performed for: A: Tlr4, Tlr4-associated miRNA-107 and miRNA-511; B: Vegfa, Vegfa-associated miRNA-126; and C: Tgfb and Tgfb-associated mi-RNA-155.
As a second test correlation of mi-RNA with RNA, we measured MiR-126 levels, which have been shown to directly increase with increased Vegfa and Fgf1 RNA (16). As shown in Figure 2B, Mi-R-126 was elevated during the early post-irradiation inflammatory phase, but decreased during both the latent period and late fibrotic phase; however, Vegfa expression remained elevated at both times, including at the times when Mi-R-126 decreased (Fig. 2B). Thus, unlike the relationship of MiR-511 with Tlr4, MiR-126 levels relative to Vegfa were inconsistent with previously reported findings (Figure 2B).
As a third example of correlation between miRNA and a RNA transcript, we measured MiR-155 levels which have been shown to be increased by Tnfa, and reduced by Tgfb (17). In the present studies, MiR-155 levels were decreased in expression during the acute phase correlating with increased Tgfb at day 14 and, therefore, were consistent with previous work (17). However, as with the discordance seen with MiR-126 and Vegfa, MiR-155 levels increased in the late fibrotic phase days 110, 150, and 200, while that for Tgfb remained elevated (Figure 2C). These data establish that miRNA-155 levels did not correlate with RNA transcript levels in the late pro-fibrotic irradiated mouse lung.
Lack of detectable migration-stimulating factors released by irradiated lung endothelial or alveolar II cells
Endothelial and alveolar II cells from late fibrotic phase irradiated lung may release humoral factor(s) that stimulate proliferation of fibroblasts and/or migration of stromal cells to the lungs through the circulation. We performed real time tracking/imaging of a pro-fibrotic Luc+ BM stromal cell line (11) for motility in response to endothelial cell and type II alveolar cell CM using an established protocol (4). Endothelial and type II alveolar cells were isolated from mouse lung on day 150 post irradiation and from nonirradiated age matched control lungs using flow cytometry. The isolated cells were cultured in vitro and CM was harvested after 24 h. As shown in Table 4, there was no significant effect of CM from explanted late-phase irradiated pulmonary endothelial or alveolar cells on Luc+ BM cell motility in vitro.
Table 4.
Migration of Luc+ stromal cells in vitro is not altered by conditioned medium from pulmonary or endothelial cells explanted from irradiated mice.
| Conditioned Medium Source | Average Motility (μm/mm) | n | p-Value |
|---|---|---|---|
| Control | 7.95 ± 0.19 | 273 | |
| Endothelial cells | 7.97 ± 0.67 | 244 | 0.975 |
| Type II alveolar cells | 7.25 ± 0.13 | 244 | 0.093 |
| Irradiated endothelial cells | 7.84 ± 0.51 | 270 | 0.864 |
| Irradiated type II alveolar cells | 7.55 ± 0.43 | 248 | 0.476 |
BioImageXD analysis of tracks from sequences of selected cytes (view fields).
Cells were removed from C57BL/6NTac irradiated lungs at 150 days after 20 Gy thoracic irradiation. Motility was measured as described in the methods.
Discussion
Cell signaling mechanisms are involved in both acute and late tissue responses to ionizing irradiation (4, 8–11). The present studies were designed to identify potential endothelial and alveolar II cell signaling processes that may influence late irradiation pulmonary fibrosis.
Explanted, then separated, populations of pulmonary endothelial and alveolar II cells were obtained from irradiated mice and also from a subgroup of irradiated mice that had been treated starting at day 88 after irradiation with MMS350. Both sets of cells showed irradiation-induced increase in mRNA transcripts, including those for genes specific to pulmonary endothelial cells (Vegfa, Vwf) and others found in both endothelial and alveolar type-II cells (Sod2 (MnSOD), Tgfb, Tlr4, and Igfbp7). In prior studies, MMS350 delivered in daily drinking water reduced the levels of fibrosis detected after day 150 in irradiated C57BL/6NTac mouse lungs (11). The present results show that MMS350 reduced the levels of damage-associated gene transcripts in both endothelial and alveolar II cells. MMS350 reduced the expression of transcripts for genes involved in both acute radiation damage and late fibrosis pathways in both endothelial and alveolar cells. The data demonstrate that MMS350 has action on both endothelial and epithelial cell populations in the lung. The antioxidant and hydroxyl radical scavenging properties of MMS350 may explain its radioprotective effects (11, 12).
Several assays for quantitation of Mi-RNA levels showed both consistencies and inconsistencies with the published literature. A consistent relationship was found between elevated expression of mRNA for Tlr4 and the published down-regulation of miR-107. Furthermore, the published increase in Tlr4 following a decrease in MiR-511 was also confirmed.
In contrast, MiR-126 stimulation of Vegfa was detected only during the acute phase but not during the late phase of the lung irradiation response. The early increase in MIR-126 has been shown to facilitate the acute inflammatory response to irradiation (16). The decrease (or absence of a late increase) of MiR-126 may reflect an ineffective or neutralized negative feedback signal, which left Vegfa levels elevated at days 120 and 200.
As a third example of miRNA interaction with RNA, MiR-155 (15) was down-regulated during the acute phase and correlated with Tgfb elevation at day 14; however, the level of MiR-155 was elevated during the late phase and did not down-regulate Tgfb, which stayed elevated at days 150 and 200. MiR-155 elevation during the late phase may have been attributable to a simultaneous up-regulation of TNFa, which is a positive regulator of MiR-155 and may have over-ridden the down-regulatory effect of Tgfb (17).
The present studies found no significant change in BM stromal cell migration in vitro by growth in CM obtained from explanted late phase irradiated lung endothelial or alveolar II cells. No changes in stromal cell fibroblast progenitor cell motility were observed following growth in CM from control lung nor irradiated lung explanted endothelial or alveolar type II cells. Our experimental design may have removed a potential volatile or adherent motility stimulating factor from the irradiated explanted cell culture medium. Alternatively, absent pulmonary cell adhesion molecules may have been necessary in culture to stimulate the motility of BM stromal cell progenitors of pulmonary fibrosis and these could only have been present in situ. Further studies will be required to elucidate the mechanism of BM stromal cell homing to the irradiated lung during the late fibrotic phase (3, 11).
Investigation of drugs that modify each of the cell signaling pathways described here may allow further identification of the mechanism of the late irradiation pulmonary fibrotic response. A comparison of the current data with that obtained in fibrosis-resistant C3Hf/Kam mice may also help identify potential novel targets for ameliorating irradiation-induced pulmonary fibrosis. The anti-fibrosis effect of MMS350 in addition to its radiation mitigation (11) potential should emphasize the value of this drug relative to other previously reported (13), prominently its lack of dose-related toxicity and water solubility.
Table II.
Effect of MMS350 treatment on modulation of endothelial cell gene transcription in irradiated lung.
| Gene | MMS350 Treatment | Days after 20 Gy | ||
|---|---|---|---|---|
| 28 | 150 | 200 | ||
| Vegf | No | 35.5 ± 3.5 | 112.4 ± 5.3 | 348.5 ± 67.2 |
| Yes | 24.3 ± 4.7 p = 0.1138 |
17.9 ± 3.6 p < 0.0001 |
262.0 ± 11.3 p = 0.2144 |
|
| Vwf | No | 82.0 ± 7.2 | 129.8 ± 8.9 | 85.0 ± 8.5 |
| Yes | 64.5 ± 9.5 p = 0.0257 |
87.6 ± 26.4 p = 0.0230 |
27.5 ± 4.9 p = 0.0143 |
|
| Nfkb | No | −84.5 ± 4.9 | 200.3 ± 30.3 | 202.5 ± 4.9 |
| Yes | −75.0 ± 1.4 p = 0.1208 |
128.8 ± 33.2 p = 0.0190 |
159.5 ± 4.9 p = 0.0130 |
|
| Nrf2 | No | −48.0 ± 8.0 | 96.0 ± 22.6 | 156.4 ± 8.0 |
| Yes | −71.7 ± 5.0 p = 0.0123 |
36.0 ± 11.3 p = 0.0786 |
139.6 ± 6.2 p = 0.1443 |
|
| Sod2 | No | 244.5 ± 7.8 | 140.1 ± 25.0 | 368.5 ± 6.4 |
| Yes | 123.5 ± 4.9 p = 0.0029 |
61.7 ± 23.7 p = 0.0039 |
255.0 ± 25.5 p = 0.0257 |
|
| Tgfb | No | 59.0 ± 1.4 | 255.2 ± 51.0 | 290.5 ± 29.0 |
| Yes | 41.5 ± 2.1 p = 0.0104 |
225.1 ± 10.3 p = 0.3732 |
180.5 ± 6.4 p = 0.0345 |
|
| Tlr4 | No | 4.0 ± 5.7 | 36.3 ± 7.8 | 329.5 ± 2.1 |
| Yes | 7.0 ± 1.3 p = 0.5475 |
18.7 ± 5.0 p = 0.0298 |
299.5 ± 3.5 p = 0.0093 |
|
| Igfbp7 | No | −7.3 ± 1.5 | 62.5 ± 3.5 | ND |
| Yes | −12.7 ± 1.2 p = 0.0474 |
38.2 ± 3.5 p = 0.0469 |
ND | |
C57BL/6NTac mice treated with MMS350 in the drinking water along with control mice were irradiated with 20 Gy to the pulmonary cavity and were sacrificed on day 28, 150 or 200 after irradiation. Lungs were removed, the endothelial cells isolated, mRNA was extracted, and RT-PCR was performed. Results represent percent induction of each gene transcript relative to baseline level. p-Values are for comparisons between mice treated with MMS350 and control irradiated mice. Significant p values are shown in bold. ND, not determined.
Acknowledgments
Supported by grants from the National Institute of Health, U19-A1068021 and R01-CA119927. This project used the UPCI animal facility and cytometry facility that are supported in part by award P30CA047904.
References
- 1.Rengan R, Maity AM, Stevenson JP, Hahn SM. New strategies in non-small cell lung cancer: Improving outcomes in chemoradiotherapy for locally advanced disease. Clin Cancer Res. 2011;17:4192–4199. doi: 10.1158/1078-0432.CCR-10-2760. [DOI] [PubMed] [Google Scholar]
- 2.Mazeron R, Etienne-Mastroianni B, Perol D, Arpin D, Vincent M, Faichero L, Martel-Lafay I, Carrie C, Claude L. Predictive factors of late radiation fibrosis: A prospective study in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;77:38–43. doi: 10.1016/j.ijrobp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Epperly MW, Sikora CA, Defilippi S, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Mol Cell Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 4.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson A, Shields DS, Goff JP, Shen H, Greenberger JS. Decreased irradiation pulmonary fibrosis in Smad3 −/− marrow chimeric mice correlates to reduced bone marrow stromal cell migration in vitro. In Vivo. 2006;20:573–582. [PubMed] [Google Scholar]
- 5.Paris F, Fuks Z, Kang Z, Capodieci P, Juan G, Ehleiter D, Halmovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 6.Phillips T. An ultrastructural study of the development of radiation injury in the lung. Feb, 1966. USNRDL-TR-973. [DOI] [PubMed] [Google Scholar]
- 7.Witte L, Fuks Z, Haimovitz-Friedman A, Vlodavsky I, Goodman DS, Eldor A. Effects of irradiation on the release of growth factors from cultured bovine, porcine, and human endothelial cells. Cancer Res. 1989;49:5066–5072. [PubMed] [Google Scholar]
- 8.Fuks Z, Persaud R, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, Seddon AP, Cordon-Cardo C, Haimovitz-Friedman A. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 9.Gaugler M, Squiban C, Van Der Meeren A, Bertho JM, Vandamme M, Mouthon MA. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol. 1997;72(2):201–209. doi: 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- 10.Adamson I, Bowden D. Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pathol. 1983;112(2):224–230. [PMC free article] [PubMed] [Google Scholar]
- 11.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, Shields D, Cao S, Franicola D, Wipf P, Berhane H, Greenberger JS. Amelioration of irradiation pulmonary fibrosis by a water-soluble bi-functional sulfoxide radiation mitigator (MMS350) Radiat Res. 2013 doi: 10.1667/RR3233.1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, Franicola D, Dixon T, Cao S, Zhang X, Buchholz BM, Bauer AJ, Choi S, Bakkenist C, Wang H, Greenberger JS. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (Nos−/−) mice. Rad Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz M-C, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennessy E, Sheedy F, Santamaria D, Barbacid M, O’Neill LAJ. Toll-like receptor-4 (TLR4) Down-regulates microRNA107 increasing macrophage adhesion via cyclin-dependent kinase 6. J Biol Chem. 2011;286(29):25531–25539. doi: 10.1074/jbc.M111.256206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tserel L, Runnel T, Kisand K, Pihlap M, Bakhoff L, Kolde R, Peterson H, Vilo J, Peterson P, Rebane A. MicroRNA expression profiles of human blood monocyte derived dendritic cells and macrophages reveal miR-511 as putative positive regulator of toll-like receptor 4. J Biol Chem. 2011;286(30):26487–26495. doi: 10.1074/jbc.M110.213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Aurora A, Johnson B, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pottier N, Maurin T, Chevalier B, Puissegur M-P, Lebrigand K, Robbe-Semesant K, Bertero T, Cardenas CLL, Courcol E, Rios G, Sourre S, Lo-Guidice J-M, Marcel B, Cardinaud B, Barbry P, Mari B. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: Implication in epithelial-mesenchymal interactions. Plos One. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epperly MW, Guo HL, Jefferson M, Wong S, Gretton J, Bernarding M, Bar-Sagi D, Greenberger JS. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Therapy. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]