Abstract
Ibogaine is a naturally occurring alkaloid that has been reported to decrease various adverse phenotypes associated with exposure to drugs of abuse and alcohol in human and rodent models. Unfortunately, ibogaine cannot be used as a medication to treat addiction because of severe side effects. Previously, we reported that the desirable actions of ibogaine to reduce self-administration of, and relapse to, alcohol consumption are mediated via the upregulation of the expression of the glial cell line-derived neurotrophic factor (GDNF) in the midbrain ventral tegmental area (VTA), and the consequent activation of the GDNF pathway. The ibogaine metabolite, noribogaine, and a synthetic derivative of ibogaine, 18-Methoxycoronaridine (18-MC), possess a similar anti-addictive profile as ibogaine in rodent models, but without some of its adverse side effects. Here, we determined whether noribogaine and/or 18-MC, like ibogaine, increase GDNF expression, and whether their site of action to reduce alcohol consumption is the VTA. We used SH-SY5Y cells as a cell culture model and found that noribogaine, like ibogaine, but not 18-MC, induces a robust increase in GDNF mRNA levels. Next, we tested the effect of intra-VTA infusion of noribogaine and 18-MC on rat operant alcohol self-administration and found that noribogaine, but not 18-MC, in the VTA decreases responding for alcohol. Together, our results suggest that noribogaine and 18-MC have different mechanisms and sites of action.
Keywords: 18-Methoxycoronaridine, addiction, ethanol self-administration, GDNF, ibogaine, noribogaine
INTRODUCTION
Alcohol and drug addiction are chronic relapsing diseases characterized by compulsive drug use, and are major societal issues. However, available medications to treat drug addiction in general, and alcoholism in particular, are very limited. Additionally, the two leading FDA-approved medications to treat alcoholism, Naltrex-one and Acamprosate, have limited efficacy and success rates (Johnson 2008; Garbutt 2009). One drug that initially showed great promise as a possible treatment for multiple types of addiction is ibogaine (Maciulaitis et al. 2008). Ibogaine is a naturally occurring indole alkaloid claimed to reverse addiction to opiates, stimulants, alcohol, and nicotine (Popik, Layer & Skolnick 1995; Mash et al. 1998; Vastag 2002; Alper, Lotsof & Kaplan 2008). Ibogaine is extracted from the root bark of the West African shrub Tabernanthe iboga and is used by indigenous people in low doses to keep hunters awake and motionless, and in higher doses for religious rituals because of its psychostimulant and hallucinogenic properties (Alper et al. 2008; Maciulaitis et al. 2008). The initial discovery that ibogaine eliminates signs and symptoms of opioid withdrawal and diminishes craving for heroin was made in the 1960s by a group of heroin addicts (Mash et al. 1998; Maciulaitis et al. 2008). Since that time, several human anecdotal reports and studies have shown that ibogaine possesses many attractive properties as an anti-addiction agent. First, the compound crosses the blood brain barrier and can be given to addicts orally (Mash et al. 1998). Second, a single dose of ibogaine is claimed to abolish drug craving for up to 6 months, and repeated ibogaine treatment (a series of four oral administrations) was reported to be effective in blocking craving and relapse for up to 3 years in cocaine and opiate addicts (Sheppard 1994; Mash et al. 1998; Alper et al. 2000). Since compliance is a significant problem in medication development to treat addiction, the fact that ibogaine has very long lasting effects (months to years) is very desirable.
Studies in animal models recapitulate human observations. Ibogaine reduces self-administration and withdrawal symptoms for alcohol and various drugs of abuse in mice, rats and monkeys (Glick et al. 2000). For example, single and repeated intraperitoneal (i.p.) injections of a moderate dose of ibogaine (40 mg/kg) can reduce self-administration of cocaine or morphine in rats and cocaine in mice for several days (Cappendijk & Dzoljic 1993; Glick et al. 1994; Sershen, Hashim & Lajtha 1994). Ibogaine decreases heroin self-administration and naloxone/naltrexone-precipitated withdrawal in morphine-dependent rats (Glick et al. 1992; Cappendijk, Fekkes & Dzoljic 1994; but see Sharpe & Jaffe 1990). Finally, ibogaine reduces alcohol (ethanol) intake in several strains of alcohol-preferring rats in a dose-dependent manner, using concentrations ranging from 10 to 60 mg/kg, with no changes in blood ethanol concentration or food intake (Rezvani, Overstreet & Lee 1995). We replicated and extended the findings of Rezvani et al. (1995) on different ethanol-drinking behaviors. We found that an acute injection of ibogaine (40 mg/kg, i.p.) in male Long-Evans rats 3 hours before the test session significantly reduced ethanol intake, but not the concurrent water or sucrose intake, using a two-bottle preference procedure, as well as operant ethanol self-administration (He et al. 2005). We were also interested in testing whether ibogaine would have efficacy in a model of relapse, in which rats are allowed to self-administer ethanol in operant chambers following 3 weeks of extinction training. Ibogaine blocked the excessive intake produced by such forced abstinence period (He et al. 2005), suggesting a potent action of ibogaine to prevent relapse. In summary, human anecdotal reports, as well as several preclinical studies in rodents, suggest that ibogaine is a long-lasting and selective anti-addiction medication for alcohol, opioids, psychostimulants and nicotine.
Unfortunately, despite its attractive properties, ibogaine is not used in the US to treat addiction because of severe side effects, which include hallucination, bradycardia, whole-body tremors and ataxia (Popik et al. 1995; Glick et al. 2000; Maas & Strubelt 2006; Alper et al. 2008; Maciulaitis et al. 2008). In addition, while a moderate dose of ibogaine (40 mg/kg) that is effective to reduce self-administration of several drugs of abuse is not neurotoxic in rodents (Molinari, Maisonneuve & Glick 1996; He et al. 2005), cerebellar Purkinje cell death has been reported in rats after administration of a higher concentration (100 mg/kg, i.p.) (O’Hearn & Molliver 1993, 1997). Moreover, although ibogaine is not addictive, the FDA classified the drug in the schedule I category of narcotics because of its stimulant and hallucinogenic properties.
In an attempt to separate the desirable anti-addictive properties of the drug from the undesirable side effects, we set out to identify the molecular mechanism mediating ibogaine’s effects on voluntary ethanol consumption. We found that systemic administration of ibogaine induced a long-lasting increase in the expression of the glial-derived neurotrophic factor (GDNF) in the dopaminergic ventral tegmental area (VTA) of rodents and that the effect of ibogaine to reduce ethanol self-administration is localized in the VTA (He et al. 2005). Importantly, when the GDNF pathway was inhibited in the VTA, ibogaine was significantly less effective in reducing ethanol self-administration (He et al. 2005). More recently, we observed that upregulation of tyrosine hydroxylase levels resulting from long-term exposure of ethanol in a dopaminergic-like cell line is blocked by ibogaine in a mechanism that requires GDNF (He & Ron 2008). Finally, similarly to ibogaine, GDNF administered into the VTA resulted in a long-lasting inhibition of ethanol-seeking and -drinking behaviors in models of moderate and excessive drinking of ethanol, as well as in a model of relapse (He et al. 2005; Carnicella et al. 2008; Carnicella & Ron 2009; Carnicella, Amamoto & Ron 2009b). Together, our results suggest that the upregulation of the GDNF pathway in the VTA mediates, at least in part, the desirable actions of ibogaine to reduce voluntary ethanol consumption and relapse.
Ibogaine is metabolized by cytochrom P4502D6 into a major metabolite noribogaine (12-hydroxyibogamine) (Obach, Pablo & Mash 1998). Interestingly, while ibogaine levels in the plasma rapidly decline, noribogaine levels remain high for at least 24 hours after oral administration of ibogaine in humans (Mash et al. 1995; Mash et al. 1998; Mash et al. 2000). Similarly, it has been shown in rats that peak blood levels of noribogaine were reported to exceed those of ibogaine, and a small, but significant portion of the administered noribogaine was still detected in the bloodstream 24 hours after an acute i.p. administration (Baumann et al. 2001). Behavioral studies show that noribogaine acts as an active metabolite (Baumann et al. 2001) and possesses a similar anti-addictive profile as ibogaine (Glick et al. 1996b). For example, systemic administration of noribogaine induces a long-lasting decrease of morphine and cocaine self-administration (Glick et al. 1996b). Importantly, noribogaine does not produce tremors and ataxia (Glick et al. 1996b; Baumann et al. 2001), suggesting that it is less neurotoxic than its parent compound. Moreover, noribogaine was recently found to be more than two times less toxic than its parent compound in mice (Kubiliene et al. 2008).
18-MC is a synthetic derivative of ibogaine (Glick et al. 1996a). 18-MC shares ibogaine’s desirable properties but, interestingly, the derivative lacks some of the adverse effects of ibogaine (Glick et al. 2000). Like ibogaine, 18-MC administration results in a long-lasting decrease in ethanol, morphine, cocaine, methamphetamine and nicotine self-administration, and attenuation of opioid withdrawal symptoms (reviewed in Glick et al. 2000). However, unlike ibogaine, 18-MC does not induce bradycardia, whole body tremor and cerebellar toxicity in rats, even at high doses (Glick et al. 2000). Moreover, 18-MC is probably not hallucinogenic as it does not affect serotonin levels (Glick et al. 2000).
Because of similarities in structure and anti-addictive actions of ibogaine, noribogaine and 18-MC, we hypothesized that like ibogaine, the ibogaine derivatives upregulate GDNF expression and that their mechanism of action to reduce ethanol self-administration is localized in the VTA. Therefore, we set out to determine whether noribogaine and 18-MC increase GDNF expression in SH-SY5Y cells and whether the ibogaine derivatives affect operant ethanol self-administration in rats when infused into the VTA.
METHODS
Reagents
Noribogaine hydrochloride was a generous gift from the Addiction Research Institute (Austin, Texas), and 18-MC hydrochloride was obtained from Albany Molecular Research, Inc. (Albany, NY). Ibogaine was purchased from Sigma (St. Louis, MO). Growth medium Dulbecco’s modified Eagle’s medium (DMEM) and Trizol reagent were purchased from Invitrogen (Carlsbad, CA). The Reverse Transcription System kit was purchased from Promega (Madison, WI).
Animals
Male Long-Evans rats (280–300 g at the beginning at the experiment) were obtained from Harlan (Indianapolis, IN). Rats were housed on a 12-hour light/dark cycle, with lights on 7:00 a.m., and food and water available ad libitum. All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Cell culture and drug treatment
SH-SY5Y human neuroblastoma cells were cultured in the growth medium DMEM containing 10% fetal bovine serum (FBS) plus 1 × MEM non-essential amino acid solution. Cells were incubated in a low-serum medium containing 1% FBS for 2 days before experiments. Cells were then treated for 3 hours with different concentrations of ibogaine (5, 10 or 50 μM), noribogaine (5, 10 or 50 μM) or 18-MC (10, 20, 50 or 100 μM) dissolved in the medium. This time point was chosen because we previously observed that 3 hours incubation of SH-SY5Y cells with ibogaine or cabergoline was sufficient time to upregulate GDNF pathway (He et al. 2005; Carnicella et al. 2009b).
Reverse transcription—polymerase chain reaction (RT-PCR)
Total RNAs were isolated using Trizol reagent and reversely transcribed using a Reverse Transcription System kit at 42°C for 30 minutes. GDNF was analyzed by PCR with temperature cycling parameters consisting of initial denaturation at 94°C for 2 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 52°C for 30 seconds, extension at 72°C for 2 minutes, and a final incubation at 72°C for 7 minutes. PCR for actin, an internal control, was run with the same temperature cycling parameters for 30 cycles. The primers used in RT-PCR are as follows: human GDNF, upstream 5′-TGC CAG AGG ATT ATC CTG ATC AGT TCG ATG-3′ and downstream 5′-TTG TCG TAC GTT GTC TCA GCT GCA TCG CAA-3′; human actin, upstream 5′-TCA TGA AGT GTG ACG TTG ACA TC-3′ and downstream 5′-AGA AGC ATT TGC GGT GGA CGA TG-3′. PCR products were separated on 1.8% agarose gels in Tris/acetic acid/EDTA buffer with 0.25 μg/ml ethidium bromide, photographed by Eagle Eye II (Stratagen, La Jolla, CA) and quantified by NIH Image 1.61.
Operant ethanol self-administration after a history of high voluntary ethanol consumption
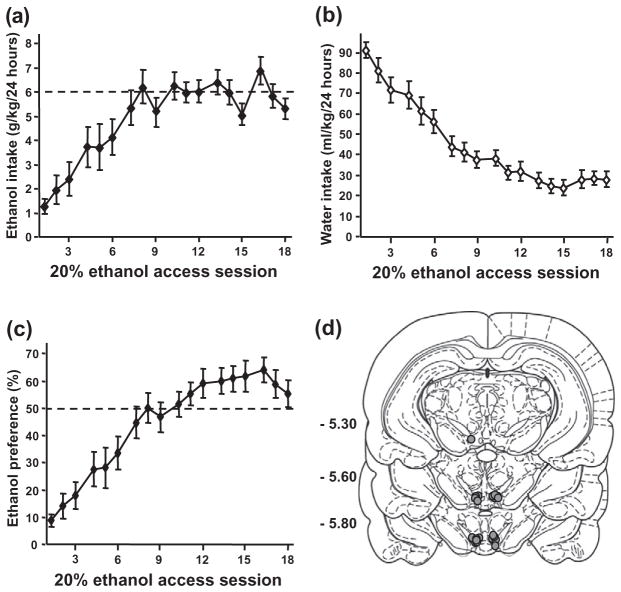
High levels of voluntary ethanol consumption were obtained in an intermittent access two-bottle choice drinking paradigm of 20% ethanol, as previously described (Carnicella et al. 2009b). Briefly, animals were given 24-hour concurrent access to one bottle of 20% (v/v) ethanol in tap water and one bottle of water on Monday, Wednesday and Friday, with 24- or 48-hour ethanol deprivation periods in between the ethanol-drinking sessions. After achieving a stable baseline of ethanol consumption of ~6 g/kg/24 hours and preference for the 20% ethanol solution over water (see Fig 2a–c), rats were trained to self-administer a 20% ethanol solution in operant self-administration chambers. The chambers contained two levers: an active lever, for which presses result in delivery of 0.1 ml of the ethanol solution, and an inactive lever, for which responses are counted but no programmed events occur. After two nights in the chambers to allow acquisition of a lever-press response for ethanol under a fixed ratio 1 (FR1), operant sessions were conducted 5 days per week, with the schedule requirement increased to FR2 and the length of session shortened from 60 to 30 minutes over the first 2 weeks. Because the level of presses on the inactive lever was extremely low after acquisition of the self-administration procedure (< 10 presses), and the activity on this lever was not affected by any of the experimental treatments, this measure was excluded from the figures and the analysis for better clarity. After 1 month of training, surgery to implant cannulae was conducted.
Figure 2.
Escalation of ethanol intake and preference in an intermittent access two-bottle choice paradigm and representation of the cannulae placement. (a–c), Mean ±standard error of the mean (SEM) of ethanol (a), water (b) intake and ethanol preference (c) during acquisition of voluntary consumption of a 20% ethanol solution. (d), Schematic representation of the cannulae placement on coronal section (Paxinos & Watson 2007). The location of the injector tips is represented by gray circles. Numbers on the left side indicate the distance posterior to bregma in millimeters. n = 13
Surgery and intra-VTA infusions
Rats were anesthetized continuously with isoflurane (Baxter Health Care Corporation, Deerfield, IL). Bilateral guide cannulae (C235G-1.5, 26 ga, Plastics One, Roanoke, VA) were aimed dorsal to the VTA (5.6 mm posterior to bregma, 0.75 mm mediolateral, 8.0 mm ventral to the skull surface), according to Paxinos & Watson (2007). The coordinates were chosen according to previous studies (He et al. 2005; Carnicella et al. 2008). After 3 to 5 days of recovery, rats returned to self-administration training and intra-VTA infusions began when operant responding returned to a stable baseline. Noribogaine and 18-MC were dissolved in PBS containing 2% DMSO. Three hours before the beginning of the session, 0.8 μl of noribogaine (1, 10, 100 μM; equivalent to 3, 30, 300 ng/μl), 18-MC (1, 10, 100 μM; equivalent to 3.7, 37, 370 ng/μl) or vehicle were infused over 2 minutes to gently restrained rats via injection cannula extending 0.5 mm beyond the guide cannula tip. The time point of infusion and concentration were chosen according to He et al. (2005), in which we found that intra-VTA infusion of ibogaine 3 hours before the beginning of the session decreased ethanol self-administration. The experiment was performed using a within-subject, Latin Square Design, with an interval of at least 1 week between two infusions, allowing lever responding for ethanol to return to baseline (e.g. at least three sessions with similar operant performance as the pre-infusion period) between treatments. This repeated procedure of infusions does not produce significant physical damage in the VTA (Carnicella et al. 2008) and does not affect the VTA function. Indeed, baseline operant performance stayed the same throughout the experiment without any adverse effects in response to repeated infusion of the vehicle. Moreover, the efficacy of the agents infused into the VTA to reduce operant ethanol self-administration remained similar throughout the experiment. It should be noted that responding for ethanol in the vehicle group decreased somewhat over the 4 days of self-administration. This effect is due to the fact that rats have access to ethanol 5 days a week. Therefore, a small deprivation effect is detected at the beginning of the week (after 2 days of ethanol withdrawal) that disappears over the self-administration session. Importantly, we also observe this effect without manipulations, indicating that it is independent of the intra-brain region infusion.
Histology
Locations of cannulae were verified in 60-μm coronal sections of paraformaldehyde-fixed tissue stained with thionine. Only data from subjects with cannulae located in the region of interest were included in the analysis (see Fig 2d).
Statistical analysis
Data were analyzed using a one-way or two-way ANOVA with repeated measures, followed by Student-Newman-Keuls test or the method of contrasts (Keppel 1991) when indicated.
RESULTS
Noribogaine, but not 18-MC, is a potent inducer of GDNF expression
First, we determined whether exposure of the dopaminergic-like SH-SY5Y cell line to noribogaine or 18-MC results in an increase in the expression of GDNF. We, therefore, treated cells for 3 hours with different concentrations of noribogaine or 18-MC and compared the level of GDNF expression to those obtained upon incubation of cells to ibogaine. As shown in Fig. 1a and b, noribogaine induced a dose-dependent increase in GDNF expression similar to the one observed upon exposure of cells to ibogaine [F(3, 12) = 14.64, P < 0.001 and F(3, 20) = 10.39, P < 0.001, for ibogaine and noribogaine, respectively]. In contrast, incubation of SH-SY5Y cells with 18-MC produced a very small, albeit significant, increase in GDNF expression, even at high concentrations [Fig 1c, F(4 and 34) = 4.02, P < 0.01]. Noribogaine and 18-MC were also tested at other time points (0.5 to 6 hours). Noribogaine increased GDNF expression at 1 hour and 6 hours; however, no significant effects were observed when cells were treated with 18-MC (data not shown), indicating that the absence of effect of 18-MC on GDNF expression is not due to an issue of the time window of action. These results, therefore, suggest that the ibogaine metabolite, noribogaine, but not its synthetic derivative, 18-MC, is a potent inducer of GDNF expression.
Figure 1.
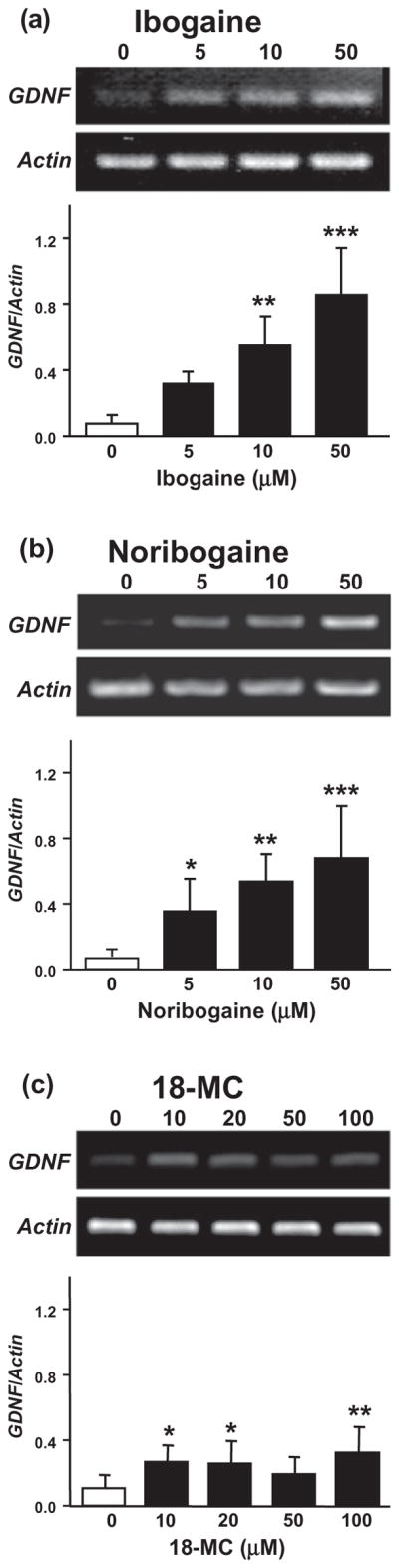
Ibogaine and noribogaine, but not 18-MC, dose-dependently increase GDNF expression in the dopaminergic-like SH-SY5Y cell line. SH-SY5Y cells were treated for 3 hours with ibogaine (5, 10 or 50 μM; a), noribogaine (5, 10 or 50 μM; b) or 18-MC (10, 20, 50 or 100 μM; c), and GDNF expression was analyzed by RT-PCR. Data are expressed as mean ±SD of the GDNF/Actin ratios. n = 6–8. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control
Intra-VTA infusion of noribogaine, but not 18-MC, reduces operant ethanol self-administration in rats
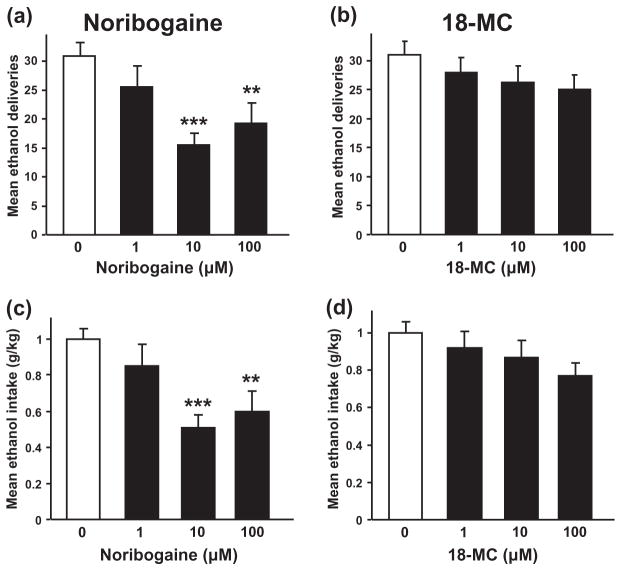
Next, we tested whether infusion of noribogaine or 18-MC into the VTA, the site of action of both ibogaine and GDNF (He et al. 2005; Carnicella et al. 2008; Carnicella et al. 2009b), decreases operant ethanol self-administration in rats with a history of high levels of ethanol consumption. Specifically, intermittent access to a 20% ethanol solution in a two-bottle choice drinking paradigm led to an escalation of ethanol consumption (Fig. 2a), a progressive decrease in the drinking of the water solution (Fig. 2b) and, consequently, to an increase in preference for the 20% ethanol solution over water (Fig. 2c). Rats were then trained to self-administer a 20% ethanol solution in operant self-administration chambers and noribogaine, 18-MC or vehicle was infused into the VTA (Fig. 2d) after acquisition of a stable baseline of responding. As shown in Fig. 3a and b, noribogaine—but not 18-MC—significantly reduced operant responding for ethanol 3 hours after the intra-VTA infusion [F(3, 36) = 6.72, P < 0.001 and F(3, 36) = 1.23, P = 0.31, for noribogaine and 18-MC, respectively], leading to a substantial decrease in ethanol intake only for the rats that received noribogaine [F(3, 36) = 7.66, P < 0.001 and F(3, 36) = 1.85, P = 0.16, for noribogaine and 18-MC, respectively] (Fig. 3c & d).
Figure 3.
Intra-VTA infusion of noribogaine, but not 18-MC, decreases operant ethanol self-administration in rats. Rats were infused into the VTA with noribogaine (1, 10 or 100 μM; a, c), 18-MC (1, 10 or 100 μM; b, d) or vehicle 3 hours before the beginning of the test session. (a&b), Mean ±SEM of the number of ethanol deliveries for noribogaine (a) and 18-MC (b). (c&d), Mean ±SEM of the ethanol intake for noribogaine (c) and 18-MC (d). n = 13. **P < 0.01, ***P < 0.001
To ensure that the absence of behavioral effects in response to intra-VTA administration of 18-MC was not due to the use of too low of a dose, the dose of 10 μg/μl of 18-MC, which has been shown to efficiently reduce morphine and methamphetamine self-administration when infused into the medial habenula or the interpeduncular nucleus (Glick et al. 2006; Glick, Sell & Maisonneuve 2008), was also tested in the VTA and did not affect operant ethanol self-administration (data not shown).
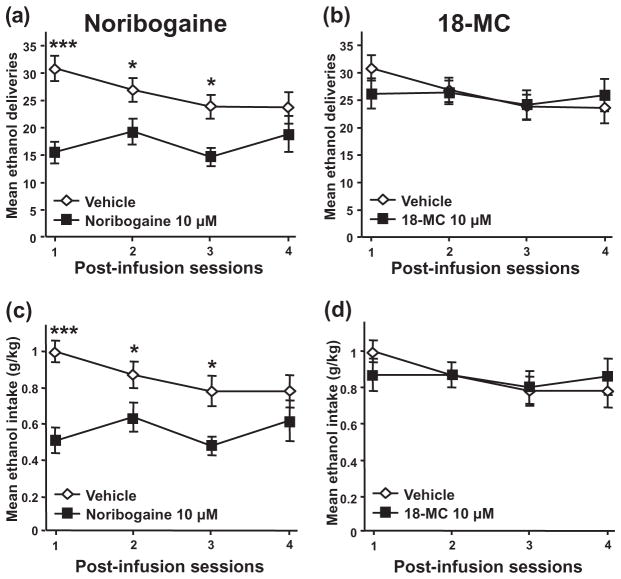
Interestingly, as shown in Fig. 4a and c, noribogaine at a concentration of 10 μM produced a decrease in operant ethanol self-administration that was long-lasting and persisted for more than 48 hours [Treatment, F(1, 36) = 10.63, P < 0.01, Session, F(3, 36) = 2.46, P = 0.08, Treatment–Session interaction, F(3, 36) = 3.26, P < 0.05 and Treatment, F(1, 36) = 11.26, P < 0.01, Session, F(3, 36) = 2.18, P = 0.11, Treatment–Session interaction, F(3, 36) = 3.13, P < 0.05, for ethanol deliveries and ethanol intake, respectively]. Similar results were obtained with the 100 μM concentration of noribogaine (data not shown). In contrast, and consistent with the absence of effect 3 hours post-infusion, no effect of 18-MC on level of response and intake was observed at any of the test sessions [Fig. 4b&d; Treatment, F(1, 36) = 0.07, P = 0.80, Session, F(3, 36) = 2.67, P = 0.06, Treatment-Session interaction, F(3, 36) = 2.26, P = 0.10 and Treatment, F(1, 36) = 0.01, P = 0.92, Session, F(3, 36) = 1.87, P = 0.15, for ethanol deliveries and ethanol intake, respectively]. Taken together, these data suggest that the VTA is an important site of action of noribogaine, but not 18-MC, to reduce operant ethanol self-administration.
Figure 4.
Intra-VTA infusion of noribogaine, but not 18-MC, induces a long-lasting decrease in operant ethanol self-administration in rats. Noribogaine or 18-MC (10 μM) was infused into the VTA and operant ethanol self-administration was monitored over 4 consecutive sessions. The same subjects as in Fig. 2 were tested 24 hours (session 2), 48 hours (session 3) and 72 hours (session 4) after the intra-VTA infusion of the drugs. Session 1 recapitulates the results obtained 3 hours after the infusion and represented in Fig. 2. (a&b), Mean ±SEM of the number of ethanol deliveries for noribogaine (a) and 18-MC (b). (c&d), Mean ±SEM of the ethanol intake for noribogaine (c) and 18-MC (d). n = 13. *P < 0.05, ***P < 0.001
DISCUSSION
Here, we show that noribogaine, like ibogaine, but not 18-MC, potently increases GDNF expression in a dopaminergic-like cell culture model. In addition, we found that administration of noribogaine, but not 18-MC, into the VTA results in a significant and long-lasting decrease in operant self-administration of ethanol.
Different factors can contribute to a decrease in operant performance, including potential suppressant effects of the pharmacological agent, such as sickness, sedation or locomotor deficits. It is, however, unlikely that such factors contribute to the decrease in operant behavior induced by noribogaine as systemic administration of noribogaine does not induce tremor or ataxia (Glick et al. 1996b; Baumann et al. 2001), and does not alter locomotor activity in rats (Baumann et al. 2001). While intra-VTA infusion of noribogaine, and not systemic administration, was used in the present study, the present data suggest that noribogaine in the VTA reduces the motivation to self-administer ethanol.
Our results suggest that the ibogaine metabolite noribogaine exhibits similar activities as ibogaine in both increasing the level of GDNF and reducing ethanol operant self-administration after infusion of the drug into the VTA, the site of action of both ibogaine (He et al. 2005) and GDNF (Carnicella et al. 2008; Carnicella & Ron 2009). Interestingly, noribogaine is as potent as ibogaine in increasing GDNF expression in SH-SY5Y cells and in reducing operant ethanol self-administration (He et al. 2005). Also, like ibogaine, the decrease in ethanol self-administration induced by noribogaine is long-lasting, and although in this study we performed a 72-hour (four sessions) time course for noribogaine and previously we tested only a 48-hour (three sessions) time course for ibogaine (He et al. 2005), the results for both drugs are likely to be similar.
Ibogaine is metabolized to noribogaine (Obach et al. 1998) and the levels of noribogaine were reported to remain high for a prolonged period of time following oral administration of ibogaine in humans (Mash et al. 1995; Mash et al. 1998; Mash et al. 2000). In rats, however, in which metabolism of drugs in the liver and in the brain is generally much faster (Smith 1991; Woodland et al. 2008), only a small fraction of the noribogaine injected systemically is detected 24 hours after the administration (Baumann et al. 2001). Therefore, it seems unlikely that the decrease in operant ethanol self-administration observed 24 hours or more after the infusion results from a persistent level of noribogaine in the brain. However, this possibility cannot be excluded. We previously showed that ibogaine blocks or reverses biochemical and behavioral adaptations obtained in response to exposure of SH-SY5Y cells and rodents to ethanol via GDNF (He et al. 2005; He & Ron 2008). We also obtained evidence to suggest that the long-lasting increase in GDNF expression induced by ibogaine is due to the induction of a positive autoregulatory loop in which GDNF increases its own expression (He & Ron 2006). As GDNF in the VTA has been shown to be a potent inhibitor of ethanol-drinking and -seeking behaviors (Carnicella et al. 2008; Carnicella et al. 2009b), these data strongly suggest that the long-term action of noribogaine on operant ethanol self-administration, as with ibogaine, is mediated by GDNF via the activation of the same positive feedback loop.
In contrast, we did not observe a significant increase in GDNF expression upon treatment of cells with the synthetic derivative of ibogaine, 18-MC. In line with these results, infusion of 18-MC into the VTA did not lead to a reduction of operant self-administration of ethanol, even at high concentrations. Interestingly, systemic administration of 18-MC in selectively bred alcohol-preferring rats was found to dose-dependently decrease ethanol intake and preference in a two-bottle choice paradigm (Rezvani et al. 1997). These results thus suggest that the site of action of 18-MC is not the VTA, and that this synthetic derivative of ibogaine does not mediate its actions on ethanol consumption via the upregulation of the GDNF pathway. 18-MC has been reported to act as an antagonist of nicotinic α3β4 receptors (Glick et al. 2002b; Maisonneuve & Glick 2003). Indeed, low dose combinations of 18-MC with other pharmacological agents that antagonize the nicotinic α3β4 acetylcholine receptors (mecamylamine, dextromethorphan or burproprion), decrease morphine, methamphetamine and nicotine self-administration at doses that were ineffective when administered alone (Glick, Maisonneuve & Kitchen 2002a; Glick et al. 2002b). Moreover, 18-MC reduces morphine and methamphetamine self-administration after infusion into the medial habenula or interpeduncular nucleus (Glick et al. 2006; Glick et al. 2008), two diencephalon structures with a high level of nicotinic α3β4 receptors (Quick et al. 1999; Perry et al. 2002) that are known to influence the activity of the mesocorticolimbic system (e.g. Nishikawa, Fage & Scatton 1986; Jhou et al. 2009). In contrast, and in line with the present data, 18-MC is not effective in decreasing morphine and methamphetamine self-administration when infused into the VTA (Glick et al. 2006; Glick et al. 2008), in which the expression of the nicotinic α3β4 is low (Klink et al. 2001; Perry et al. 2002). Therefore, while the two ibogaine congeners noribogaine and 18-MC reduce drug self-administration when administered systemically, our data suggest that their site and mechanism of action are different. Like 18-MC, ibogaine is a potent antagonist of the nicotinic α3β4 receptors (Glick et al. 2002b). However, ibogaine also has a plethora of mechanisms of action in the brain, and it is proposed that this variety of effects accounts for its anti-addictive properties (Sweetnam et al. 1995; Glick & Maisonneuve 1998). It is, therefore, likely that both the effects of ibogaine on GDNF expression in the VTA, as well as its antagonist activity at the nicotinic α3β4 receptors, are likely to contribute significantly to its anti-addictive activities.
As described in the introduction, ibogaine’s side effects preclude its use as a medication. Noribogaine, as suggested by the present study and others (e.g. Glick et al. 1996b), shares the same desirable long-lasting actions and anti-addictive mechanisms as ibogaine. Importantly, however, noribogaine is safer than its parent compound and, for example, has no tremorigenic effects (Glick et al. 1996b; Baumann et al. 2001). As such, noribogaine may be considered to have a greater therapeutic profile than ibogaine. The present data also suggest that the effects of noribogaine on GDNF expression greatly contribute to its potential anti-addictive profile. We recently showed that another molecule, cabergoline, reduces ethanol consumption and relapse in rodent preclinical models by the upregulation of the GDNF pathway in the VTA (Carnicella et al. 2009a). Together, these results put forward the use of GDNF inducers as a valuable strategy to combat alcohol use and abuse disorders.
In summary, the present data strongly suggest that activation of the GDNF pathway in the VTA is a mechanism of action of noribogaine, but not 18-MC, to reduce ethanol taking. Taken with other studies, it emphasizes an important role of the GDNF pathway within the VTA to modulate ethanol-drinking behaviors. Understanding how ibogaine derivatives decrease drug intake and delineating the different signaling events mediating the anti-addictive properties of ibogaine derivatives may lead to the development of a new generation of drugs to treat addiction.
Acknowledgments
The authors thank the Addiction Research Institute for their generous gift of noribogaine. This work was supported by the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R.) and by the Department of the Army, Grant # W81XWH-07-1-0079 (D.R.) for which the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702-5014 is the awarding and administering acquisition office. The content of the information represented does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Footnotes
Authors’ Contribution
SC and DR designed research. SC, DYH and QVY performed research. SC, DYH and DR assisted with data analysis and interpretation of findings. SC and DR wrote the manuscript and SDG provided material consultation and discussion. All authors critically reviewed content and approved final version for publication.
References
- Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. Ibogaine in acute opioid withdrawal. An open label case series. Ann N Y Acad Sci. 2000;909:257–259. doi: 10.1111/j.1749-6632.2000.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Kaplan CD. The ibogaine medical subculture. J Ethnopharmacol. 2008;115:9–24. doi: 10.1016/j.jep.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB, Pablo JP, Mash DC. In vivo neurobiological effects of ibogaine and its O-desmethyl metabolite, 12-hydroxyibogamine (noribogaine), in rats. J Pharmacol Exp Ther. 2001;297:531–539. [PubMed] [Google Scholar]
- Cappendijk SL, Dzoljic MR. Inhibitory effects of ibogaine on cocaine self-administration in rats. Eur J Pharmacol. 1993;241:261–265. doi: 10.1016/0014-2999(93)90212-z. [DOI] [PubMed] [Google Scholar]
- Cappendijk SL, Fekkes D, Dzoljic MR. The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine. Behav Brain Res. 1994;65:117–119. doi: 10.1016/0166-4328(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009a;66:146–153. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009b;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF—a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–S23. quiz S24–25. [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996a;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW, Jr, Carlson JN. Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to trem-origenic effects and to effects on dopamine release in nucleus accumbens and striatum. Brain Res. 1994;657:14–22. doi: 10.1016/0006-8993(94)90948-2. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IS. Mechanisms of anti-addictive actions of ibogaine. Ann N Y Acad Sci. 1998;844:214–226. [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002a;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002b;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci. 2000;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Pearl SM, Cai J, Maisonneuve IM. Ibogaine-like effects of noribogaine in rats. Brain Res. 1996b;713:294–297. doi: 10.1016/0006-8993(95)01563-9. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur J Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Glick SD, Rossman K, Rao NC, Maisonneuve IM, Carlson JN. Effects of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor. Neuropharmacology. 1992;31:497–500. doi: 10.1016/0028-3908(92)90089-8. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, Janak PH, Ron D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20:2420–2422. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- He DY, Ron D. Glial cell line-derived neurotrophic factor reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of heat shock protein 90. J Biol Chem. 2008;283:12811–12818. doi: 10.1074/jbc.M706216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. 3. Upper, Saddla River, NJ: Prentice Hall; 1991. [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiliene A, Marksiene R, Kazlauskas S, Sadauskiene I, Razukas A, Ivanov L. Acute toxicity of ibogaine and noribogaine. Medicina (Kaunas) 2008;44:984–988. [PubMed] [Google Scholar]
- Maas U, Strubelt S. Fatalities after taking ibogaine in addiction treatment could be related to sudden cardiac death caused by autonomic dysfunction. Med Hypotheses. 2006;67:960–964. doi: 10.1016/j.mehy.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Maciulaitis R, Kontrimaviciute V, Bressolle FM, Briedis V. Ibogaine, an anti-addictive drug: pharmacology and time to go further in development. A narrative review. Hum Exp Toxicol. 2008;27:181–194. doi: 10.1177/0960327107087802. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn WL, Sanchez-Ramos J. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann N Y Acad Sci. 1998;844:274–292. [PubMed] [Google Scholar]
- Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, Singleton EG, Mayor M. Ibogaine: complex pharmaco-kinetics, concerns for safety, and preliminary efficacy measures. Ann N Y Acad Sci. 2000;914:394–401. doi: 10.1111/j.1749-6632.2000.tb05213.x. [DOI] [PubMed] [Google Scholar]
- Mash DC, Staley JK, Baumann MH, Rothman RB, Hearn WL. Identification of a primary metabolite of ibogaine that targets serotonin transporters and elevates serotonin. Life Sci. 1995;57:PL45–PL50. doi: 10.1016/0024-3205(95)00273-9. [DOI] [PubMed] [Google Scholar]
- Molinari HH, Maisonneuve IM, Glick SD. Ibogaine neurotoxicity: a re-evaluation. Brain Res. 1996;737:255–262. doi: 10.1016/0006-8993(96)00739-1. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Pablo J, Mash DC. Cytochrome P4502D6 catalyzes the O-demethylation of the psychoactive alkaloid ibogaine to 12-hydroxyibogamine. Drug Metab Dispos. 1998;26:764–768. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. San Diego: Academic Press; 2007. [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Popik P, Layer RT, Skolnick P. 100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug. Pharmacol Rev. 1995;47:235–253. [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Lee YW. Attenuation of alcohol intake by ibogaine in three strains of alcohol-preferring rats. Pharmacol Biochem Behav. 1995;52:615–620. doi: 10.1016/0091-3057(95)00152-m. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Ibogaine reduces preference for cocaine consumption in C57BL/6By mice. Pharmacol Biochem Behav. 1994;47:13–19. doi: 10.1016/0091-3057(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Sharpe LG, Jaffe JH. Ibogaine fails to reduce naloxone-precipitated withdrawal in the morphine-dependent rat. Neuroreport. 1990;1:17–19. doi: 10.1097/00001756-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Sheppard SG. A preliminary investigation of ibogaine: case reports and recommendations for further study. J Subst Abuse Treat. 1994;11:379–385. doi: 10.1016/0740-5472(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Smith DA. Species differences in metabolism and pharma-cokinetics: are we close to an understanding? Drug Metab Rev. 1991;23:355–373. doi: 10.3109/03602539109029764. [DOI] [PubMed] [Google Scholar]
- Sweetnam PM, Lancaster J, Snowman A, Collins JL, Perschke S, Bauer C, Ferkany J. Receptor binding profile suggests multiple mechanisms of action are responsible for ibogaine’s putative anti-addictive activity. Psychopharmacology (Berl) 1995;118:369–376. doi: 10.1007/BF02245936. [DOI] [PubMed] [Google Scholar]
- Vastag B. Addiction treatment strives for legitimacy. JAMA. 2002;288:3099–3101. doi: 10.1001/jama.288.24.3096. [DOI] [PubMed] [Google Scholar]
- Woodland C, Huang TT, Gryz E, Bendayan R, Fawcett JP. Expression, activity and regulation of CYP3A in human and rodent brain. Drug Metab Rev. 2008;40:149–168. doi: 10.1080/03602530701836712. [DOI] [PubMed] [Google Scholar]