Abstract
Sponsored by the New York Academy of Sciences, the Warren Alpert Medical School of Brown University and the University of Massachusetts Boston, “Behavioral Epigenetics” was held on October 29–30, 2010 at the University of Massachusetts Boston Campus Center, Boston, Massachusetts. This meeting featured speakers and panel discussions exploring the emerging field of behavioral epigenetics, from basic biochemical and cellular mechanisms to the epigenetic modulation of normative development, developmental disorders, and psychopathology. This report provides an overview of the research presented by leading scientists and lively discussion about the future of investigation at the behavioral epigenetic level.
Keywords: behavior, epigenetics, chromosome, gene regulation, transcription, methylation
Background and perspectives
What is behavioral epigenetics?
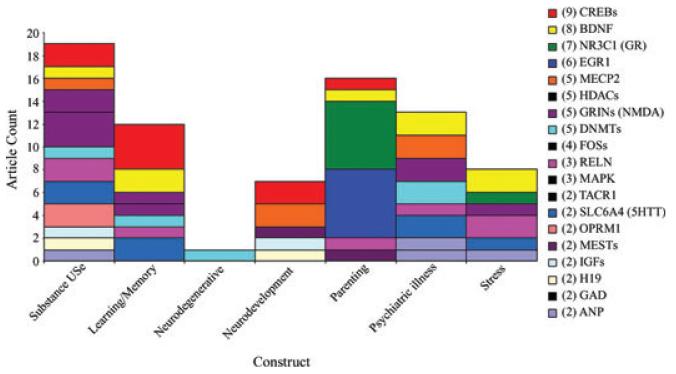
Barry M. Lester (Alpert Medical School of Brown University) introduced the topic of the conference, behavioral epigenetics, by describing research on the developmental origins of adult diseases, suggesting that the fetus is actually making adaptations through programming to “prepare” for the postnatal environment in response to environmental signals. These effects are due, in part, to epigenetic mechanisms, raising the fascinating question of whether these mechanisms can also explain behavioral outcomes, thus providing an example of the kind of research that could lead to a new field—behavioral epigenetics. Behavioral epigenetics was described as the application of the principles of epigenetics to the study of physiological, genetic, environmental, and developmental mechanisms of behavior in human and nonhuman animals. Investigations typically focus at the level of chemical changes, gene expression, and biological processes that underlie normal and abnormal behavior. This includes how behavior affects and is affected by epigenetic processes. Interdisciplinary in its approach, it draws on sciences, such as neuroscience, psychology and psychiatry, genetics, biochemistry, and psychophar-macology. Whereas there are thousands of studies of epigenetics that have been conducted over the last 40 years, the application of epigenetics to the study of behavior is just beginning. A literature search of citations found only 96 articles to date on behavioral epigenetics (see Appendix). These articles were analyzed according to the behavioral construct that was studied (e.g., substance use, psychiatric illness, learning/memory, neurodevelopment, parenting, stress, and neurodegenerative disorders), the species studied (e.g., human, mouse, rat), the tissue that was analyzed (e.g., brain, blood), the epigenetic mechanisms that were studied (e.g., methylation, histone modifications), and the particular genes investigated (Fig. 1). For example, in relation to parenting, the most commonly studied genes were the glucocorticoid receptor and FOS genes. The presentation concluded with cautionary notes about the unique issues involved in the study of behavioral epigenetics in humans.
Figure 1.
The figure shows the 96 articles on behavioral epigenetics grouped by the behavioral construct studied and the genes that were studied in each of the behavioral construct categories.
Epigenetics: basic processes and mechanisms
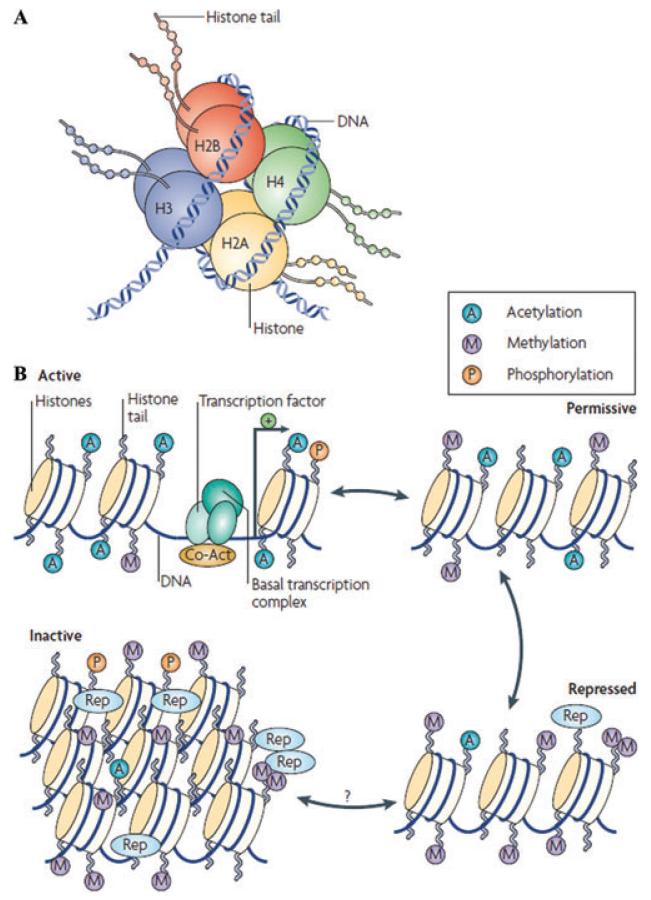
Eric Nestler (Mount Sinai School of Medicine) presented an overview of basic epigenetic processes and mechanisms.1,2 A broad perspective of epigenetics includes any structural adaptation in chromosomal regions that mediate altered rates of gene transcription. Epigenetic regulation, also known as chromatin remodeling, in neurons, describes a process where the activity of a particular gene is controlled by the structure of chromatin in that gene’s proximity (Fig. 2). Chromatin remodeling is complex, involving multiple covalent modifications of histones (e.g., acetylation, phosphorylation, methylation), ATPase-containing protein complexes that move histone oligomers along a strand of DNA, methylation of DNA, and the binding of numerous transcription factors and transcriptional coactivators and corepressors, all of which act in a concerted fashion to determine the activity of a given gene. Epigenetic regulation is crucial for nervous system development. Specifically, it can help elucidate how genes are affected by environmental stimuli, including several common mental retardation syndromes and related neurodevelopmental disorders that are caused by abnormalities in chromatin-remodeling mechanisms.
Figure 2.
General scheme of chromatin remodeling. (A) DNA double helix wrapped around an octomer of histone proteins forming the unit of chromatin, the nucleosome. (B) Chromatin can be conceptualized as existing in two primary structural states: as active, or open, euchromatin in which histone acetylation opens up the nucleosome to allow binding of the basal transcriptional complex and other activators of transcription; or as inactive, or condensed, heterochromatin, where all gene activity is permanently silenced. In reality, chromatin exists in a continuum of several functional states (active, permissive, repressed, and inactivated). Enrichment of histone modifications, such as acetylation (A) and methylation (M) at histone N-terminal tails and related binding of coactivators (Co-Act) or repressors (Rep), to chromatin modulates the transcriptional state of the nucleosome.
Epigenetic regulation also occurs in the mature, fully differentiated brain and provides unique mechanisms that may underlie the stable changes in gene expression under both normal conditions (e.g., learning and memory) and in several pathological states (e.g., depression, drug addiction, schizophrenia, and Huntington’s disease, among others). In some rare cases (e.g., gene imprinting), epigenetic modifications can be transmitted to offspring, which raises the possibility that behavioral experience in adult life might influence gene expression in subsequent generations. However, there has not yet been definitive evidence for epigenetic transmission of behavioral experience. While work on epigenetic mechanisms in the brain is still in early stages, it promises to improve our understanding of brain plasticity, the pathophysiology of neuropsychiatric disorders, and may lead to the development of fundamentally new treatments for these conditions.
Epigenetics, intergenerational inertia, and human adaptation
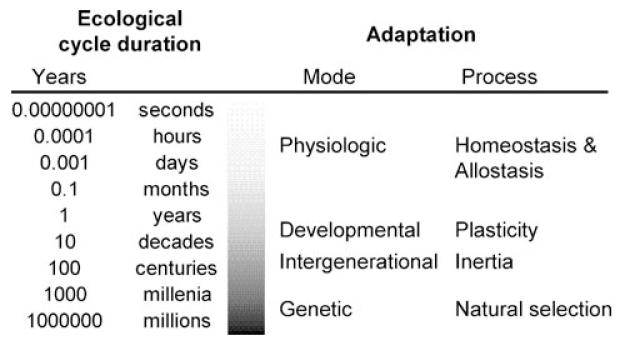
Christopher W. Kuzawa (Northwestern University) explored the importance of the dynamic nature of epigenetic change as a means by which organisms adapt to environmental change.3,4 He emphasized that organisms must cope with everything from very rapid and acute fluctuations (e.g., overnight fast followed by breakfast) to chronic conditions that change only gradually (e.g., ice ages or migrating to a new environment). A range of adaptive mechanisms allows human populations to adjust to these various timescales of change (Fig. 3). Natural selection sifts through the gene pool to select gene variants well suited to the most stable features of local ecologies. Rapid and reversible homeostatic processes lie at the other extreme, maintaining internal constancy against a backdrop of dynamic environmental conditions, such as food intake or psychosocial stress. He noted the adaptive importance of developmental plasticity or the capacity grounded in epigenetic and other changes that allows a single genome to create a range of possible traits in interaction with the environment (e.g., growing larger lungs when raised at high altitude). Because organisms only develop once, changing development in response to environmental conditions is generally an irreversible process; thus, developmental plasticity is a suitable mode of adaptation to environmental features that are too chronic to be buffered by homeostasis, but that are also too transient for genetic adaptations to consolidate around.
Figure 3.
The timescales of human adaptability. Light gray, more rapidly responsive/less durable; black, slowest to respond/most durable. Epigenetic changes contribute to multiple modes of adaptation, including developmental and intergenerational processes that allow adjustment to gradual environmental change occurring on a decadal or multigenerational timescale. Modified after Kuzawa’s work.4
Kuzawa pointed out that many documented examples of epigenetic sensitivity involve the adoption of stable changes in gene regulation in response to experiences during limited, early stages of development (sensitive periods). Might it make adaptive sense for a long-lived species like humans to commit to a strategy for life so early in the life cycle? Limiting epigenetic sensitivity to early developmental windows may, in fact, create opportunities to adjust biology to more reliable environmental cues in the form of the mother’s own phenotype. Examples that suggest an ability of the current generation to entrain development to maternal characteristics that reflect her cumulative experiences include the setting of infant growth rate to breast milk leptin as a cue of maternal energetic history, and work in the Philippines suggesting that fetal growth may be calibrated to a woman’s cumulative nutritional experiences across her lifetime. In both examples, offspring developmental biology is not sensitive to potentially transient (and thus unreliable) conditions during the brief period of pregnancy or lactation. Rather, the maternal resources and signals that are transferred to offspring may be more integrative and cumulative in nature and, thus, potentially provide a more reliable basis for adaptive adjustment. Kuzawa hypothesized that the timing of early sensitive periods, during which many epigenetic settings are established, may be more than accidental, but reflect the evolution of a conduit of sorts, allowing nongenomic information to be transferred between generations.
Learning and memory
The second session, moderated by J. David Sweatt (University of Alabama at Birmingham), served as an overview of the roles for epigenetic mechanisms in long-term learning and memory processes and highlights one of the most exciting contemporary areas in the behavioral epigenetics field. The session was comprehensive in scope, covering cognition and behavior, synaptic function and cellular plasticity, biochemical signaling mechanisms, and molecular epigenetic mechanisms. The work described largely emphasized the specific epigenetic mechanisms of histone posttranslational modification and DNA methylation.
Epigenetic mechanisms in memory formation
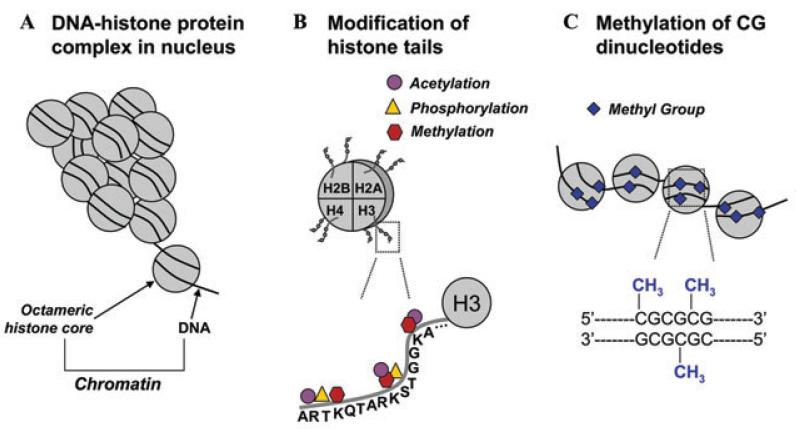
Sweatt addressed the idea that conservation of epigenetic mechanisms for information storage represents a unifying model in biology, with epigenetic mechanisms being used for cellular memory at levels from behavioral memory to development to cellular differentiation.5,6 As background, Sweatt discussed how DNA methylation and histone modifications are the two most extensively investigated epigenetic mechanisms. As Sweatt described, until recently it was thought that once laid down, these epigenetic marks would remain unchanged for the lifetime of the organism; recent studies, however, including those from the Sweatt laboratory, have challenged this view. Nevertheless, it is clear that DNA methylation and attendant changes in chromatin structure are capable of self-regeneration and self-perpetuation, necessary characteristics for a stable molecular mark. Thus, Sweatt discussed the broad hypothesis that DNA methylation marks can be modified in response to an organism’s experience and that these marks play a role in dynamically regulating the gene transcription supporting synaptic plasticity and long-term memory formation and maintenance (Fig. 4).
Figure 4.
Schematic representation of epigenetic modifications. (A) In the nucleus, DNA coils and condenses around histones. Each octameric histone core contains two copies each of histones H2A, H2B, H3, and H4. The DNA-protein complex is referred to as chromatin. (B) The DNA-histone interaction occurs at the N-terminal tail of a histone, where, for example, on the H3 N-terminal tail, there are several sites for epigenetic marking via acetylation, methylation, and phosphorylation. (C) In and around gene promoters that are rich in cytosine-guanine nucleotides (CpG islands), methyl groups are transferred to CpG sites. This process, called DNA methylation, is catalyzed by a class of enzymes known as DNA methyltransferases.
Sweatt’s presentation also described several pieces of evidence supporting the idea that DNA methylation plays a role in memory function in the adult central nervous system (CNS).6 Thus, he described how general inhibitors of DNA methyltransferase (DNMT) activity alter DNA methylation in the adult brain and alter the DNA methylation status of the plasticity-promoting genes reelin and bdnf. Additional studies demonstrated that de novo DNMT expression is upregulated in the adult rat hippocampus after contextual fear conditioning and that blocking DNMT activity blocks contextual fear conditioning. In addition, results were presented demonstrating that fear conditioning is associated with rapid methylation and transcriptional silencing of the memory suppressor gene protein phosphatase 1 (PP1) and demethylation and transcriptional activation of the plasticity gene reelin. These findings have the surprising implication that both active DNA methylation and active demethylation might be involved in long-term memory consolidation in the adult CNS.
Finally, a recent series of studies were described5 that found that the bdnf gene locus is also subject to memory-associated changes in DNA methylation, and, moreover, that this effect is regulated by the NMDA receptor. Data were also presented indicating that neuronal DNMT-deficient animals have deficits in contextual fear conditioning, the Morris maze learning task, and hippocampal longterm potentiation (LTP). Overall, Sweatt concluded that DNA methylation is dynamically regulated in the adult CNS in response to experience and that this cellular mechanism is a crucial step in memory formation.
Chromatin-modifying enzymes in long-term memory
In the second presentation of the session, Marcelo A. Wood (University of California, Irvine) discussed the role of chromatin-modifying enzymes in regulating gene expression required for long-term memory formation. Why are chromatin-modifying enzymes needed to regulate gene expression? A simplistic answer comes from the level that compaction genomic DNA undergoes when being compressed to fit into a nucleus. Genomic DNA is two meters in length, yet has to fit into a six-micron nucleus, and, thus, must undergo an approximately 10,000-fold compaction. This generates an access and indexing problem, which is solved in part by chromatin-modifying enzymes. The best-studied chromatin-modifying enzymes in the field of learning and memory are histone-modifying enzymes, especially histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Fig. 5).
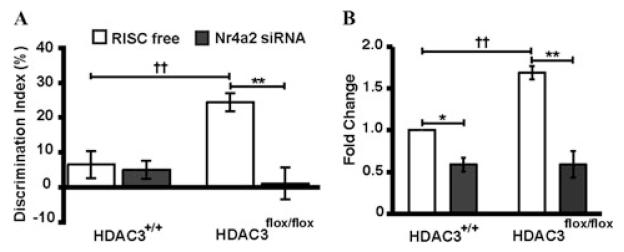
Figure 5.
HDAC3 modulates memory formation in a Nr4a2-dependent manner. (A) HDAC3-FLOX mice with dorsal hippocampal deletion of Hdac3 exhibit significantly enhanced long-term memory as compared to wild-type littermates (both groups received RISC free control). In contrast, siRNA-Nr4a2–treated HDAC3-FLOX mice exhibit no enhanced memory. (B) qRT-PCR shows that siRNA against Nr4a2 significantly reduces Nr4a2 expression in both HDAC3-FLOX and wild-type littermates.
In the first part of his talk, Wood presented his lab’s research in examining the role of the CREB-binding protein (CBP), a potent HAT and transcriptional coactivator, in long-term memory. One limitation in studying the role of CBP in learning and memory has been the lack of genetically modified mice with sufficient spatial and temporal regulation. The Wood lab used genetically modified CBP-FLOX mice, in combination with adeno-associated virus (AAV)–expressing Cre recombinase, to generate homozygous focal Cbp deletions in only area CA1 of the dorsal hippocampus. This novel approach resulted in the necessary spatial restriction to study the role of CBP in one brain region and its effect on long-term memory; additionally, it provided the temporal restriction to study a homozygous deletion of Cbp in adult mice, which avoids confounds from developmental or performance issues. The Wood lab found that homozygous deletions of Cbp resulted in hippocampus-dependent long-term memory impairments associated with decreased levels of specific histone modifications and decreased gene expression.7
In the second part of his talk, Wood presented research examining the role of a specific HDAC in long-term memory formation. To date, the function of HDAC3, one of the most highly expressed class I HDACs in the brain, has never been examined. Again, using AAV-expressing Cre recombinase and HDAC3-FLOX genetically modified mice, the Wood lab demonstrated that HDAC3 is a key negative regulator of long-term memory formation in the hippocampus. Homozygous deletion of Hdac3 in area CA1 of the hippocampus led to enhanced long-term memory associated with increased levels of specific histone modifications and increased gene expression. Similar results were observed when an HDAC3 selective inhibitor, called RGFP136, was site specifically delivered to the dorsal hippocampus. Together, the genetic and pharmacologic data demonstrated that HDAC3 is a negative regulator of long-term memory formation.8
In summary, Wood posited that HDACs represent a type of molecular brake pad that is normally engaged but transiently removed by sufficient activity-dependent signaling to regulate the transcription required for long-term memory formation.9 Wood concluded by suggesting that this process may represent a molecular mechanism to explicate why we do not encode everything we experience into long-term memory. Moreover, impaired function of these molecular brake pads may be associated with disorders including drug addiction and posttraumatic stress disorder.
Signaling and epigenetic mechanisms in stress-related memory formation
In the final presentation, Johannes (Hans) Reul (University of Bristol, UK) presented a novel mechanism that may explain why we make such strong memories of psychologically stressful and emotional events in our lives. The mechanism he proposed involves crosstalk between different signaling pathways influencing epigenetic processes in neurons of the hippocampus, a limbic brain region involved in learning and memory. Stressful events, for example, a domestic dispute or a job interview, or in animals, an attack by a predator, evoke the secretion of glucocorticoid hormones from the adrenal gland. Classically, these hormones regulate metabolic and other physiological processes that enable an individual to cope with the challenge in the best possible way.
Reul reported, however, that research spanning the last 25 years has provided evidence that glucocorticoids secreted during a psychologically stressful challenge enhance the consolidation of memories related to the event—a long-standing observation that has remained unexplained until now. A finding made in the 1980s by de Kloet’s group10 pointed to the dentate gyrus, the gateway of the hippocampus, as a site of action for glucocorticoids in stress-related memory formation.
In his presentation, Reul showed that the hormone’s action required distinct epigenetic modifications of the chromatin: the phosphorylation of serine10 (S10), in combination with the acetylation of lysine14 (K14) of histone H3 (H3S10p-K14ac), leading to the induction of the immediate-early genes c-Fos and Egr-1 in dentate gyrus granule neurons in rats and mice in vivo.11,12 As the gluco-corticoid receptor (GR) cannot bring about these histone modifications directly, Reul suggested that the GR acts indirectly via interaction with other signaling molecules. More specifically, he postulated that GR interacts with the NMDA receptor-activated ERK (extracellular signal-regulated kinase) MAPK (mitogen-activated protein kinase) signal pathway, which has a marked role in learning and memory processes (Fig. 6; Refs. 12 and 13). Supporting this notion, Reul presented unpublished in vivo data clearly showing that in activated dentate granule neurons, that is, those exhibiting phosphorylated ERK1/2 (pERK1/2), GRs are required to activate the downstream histone-modifying enzymes MSK1 (mitogen and stress-activated kinase 1), and Elk-1 (Ets-like protein-1) (Fig. 6; Refs. 12-14).
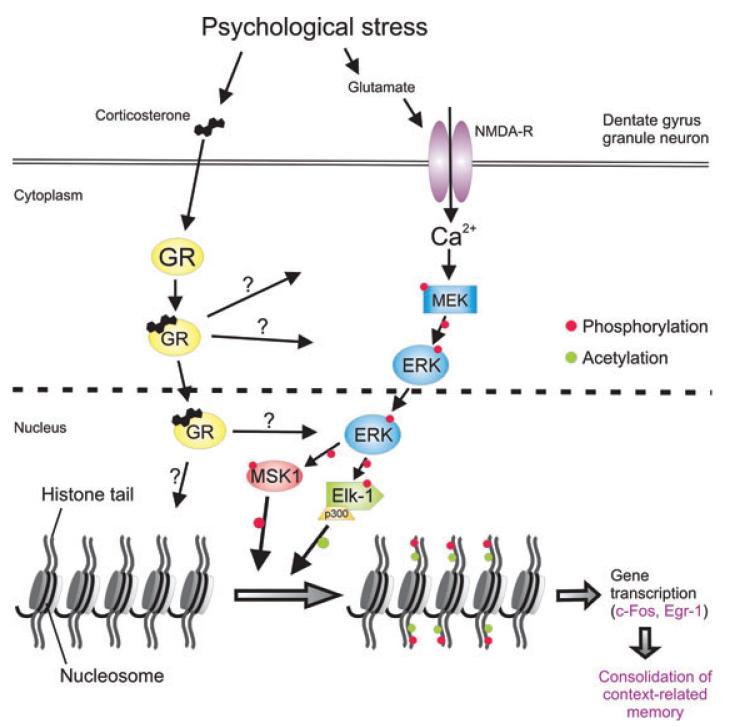
Figure 6.
Glucocorticoid hormones, secreted as a result of a stressful event, enhance the consolidation of behavioral responses, including memories related to the event. Until recently, the underlying mechanisms of these effects were unknown. Recent work of the Reul group at the University of Bristol shows that glucocorticoids act by binding to glucocorticoid receptors (GRs) that interact with the NMDA receptor-activated ERK1/2/MSK1-Elk-1 signaling pathway enhancing the formation of epigenetic modifications (i.e., the serine10 phosphorylation and lysine14 acetylation in histone H3 (H3S10p-K14ac)) and the induction of the neuroplasticity-associated immediate-early genes c-Fos and Egr-1 in sparsely distributed mature dentate gyrus neurons. Evidence has been accumulating that these signaling, epigenetic, and genomic phenomena are of critical importance for the consolidation of memories related to the endured stressful event.
Reul went on to describe pMSK1, a kinase that can phosphorylate histone H3 at serine10, whereas pElk-1 binds the HAT p300, which can acetylate histone tails. Further, showing a series of immunofluorescence images, he demonstrated that, during the consolidation phase of memory formation, all participating signaling molecules (pERK1/2, pMSK1, pElk-1), modified histone molecules (H3S10p-K14ac), and induced intermediate-early gene products (c-Fos, Egr-1) can be found in the same dentate gyrus granule neurons.12,13 Furthermore, he showed that blocking GRs led to a substantially decreased formation of pMSK1 and pElk-1, but not pERK1/2, in dentate granule neurons after forced swim stress.12 He concluded that stressful events are strongly encoded into memory because of the marked activating role of GRs on ERK MAPK, signaling to the chromatin in dentate gyrus neurons (Fig. 6; Ref. 12). These findings may be of significance for stress-related psychiatric disorders, such as major depression and anxiety, including PTSD.
The formal presentations were followed by a wide-ranging and lively discussion of the roles and regulation of epigenetic mechanisms in longterm synaptic plasticity and behavioral memory in vivo.
Development
This section, chaired by Edward Tronick (University of Massachusetts Boston and Children’s Hospital Boston), focused on the emerging environmental epigenetics hypothesis, which suggests that environmental signals operate during early development to alter epigenetic marks across the genome, thus influencing neural development and function.
Alterations of DNA methylation, growth restriction, and infant neurobehavior
The first talk was by Carmen J. Marsit (Brown University) and focused on altered epigenetic marks within the placenta. Epidemiological studies identify variations in birth weight as a predictor of health over the lifespan, including the risk for neuropsychiatric disorders.15 Marsit discussed a novel way in which to consider the effects of the intrauterine environment on infant neurodevelopment in human populations, focusing on how differences in DNA methylation at specific genomic regions in the human placenta are associated with infant neurobehavior. The placenta acts as the master regulator of the intrauterine environment, not only through nutrients, gas, water, and waste exchange, but also through the production of pregnancy-related hormones, proteins, and growth factors, including neuropeptide hormones analogous to those produced by the hypothalamus and pituitary. Finally, the placenta also acts as a barrier commonly metabolizing maternal hormones to inactive forms and, thus, stabilizing the intrauterine endocrine environment. Such considerations have led to the concept that the placenta acts as the “third brain” by linking the developed maternal physiological state with the developing fetus.
Importantly, placental gene expression is subject to environmental regulation. Marsit’s group considered how changes to the patterns of DNA methylation in the placenta may alter the function of the placenta in these critical roles and, in turn, how these alterations manifest in neurobehavioral phenotypes in infants, characterized using the well-established Neonatal Intensive Care Unit Network Neurobehavioral Scales (NNNS).
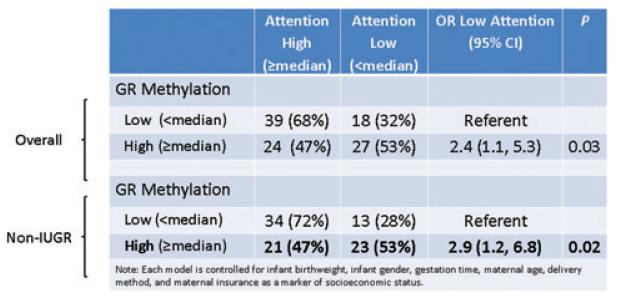
Marsit highlighted work linking patterns of DNA methylation in the placenta to the intrauterine environment represented by infant growth, showing strong and significant associations between profiles of DNA methylation, identified using genome-wide array-based approaches, and infant birth weight.16 He went on to demonstrate that increasing methylation of the human GR 1F was strongly and significantly associated with decreased measures of infant attention on the NNNS (Fig. 7). Importantly, the methylation of an analogous receptor (rat exon 17) in rat pup hippocampus has linked to maternal behaviors (see Meaney presentation below).
Figure 7.
Association between greater than median glucocorticoid receptor exon 1F methylation and infant attention score is specific to nongrowth restricted infants.
Marsit also showed that these effects were most pronounced among infants of normal weight for gestational age, suggesting that there may be normal variability in placental methylation that accounts for variation in infant neurobehavior. As Marsit expands his studies of the role of the intrauterine environment captured in the placenta epigenome, links between the methylation of key genes involved in HPA axis control and infant neurobehavior, and associations between genome-wide profiles of DNA methylation and infant neurodevelopment are being pursued. These studies are of particular importance as multiple environmental exposures, such as nutrient deprivation, are known to affect infant growth and are associated with an increased risk for neurocognitive conditions, including attention deficit hyperactivity disorder (ADHD).
Epigenetic alterations and exposure to cocaine in utero
Barry Kosofsky (Weill Cornell Medical College) discussed how developmental brain disorders and the consequences of prenatal exposure to drugs of abuse (cocaine, in particular) are associated with sustained changes in CNS gene expression and have lasting consequences for brain structure and function (Fig. 8).17 Prenatal exposure to toxins, including substances of abuse, is associated with developmental effects in children. Kosofsky suggested that such aberrant effects might be considered “molecular malformations,” leading to conditions where neural signaling pathways are rendered dysfunctional. Such molecular changes may “feed forward” to produce alterations in the behavioral repertoire of affected infants, children, and young adults—changes that are sculpted by environmental interactions. Kosofsky’s research explores the hypothesis that the resulting molecular maladaptations are, in part, mediated by epigenetic mechanisms.
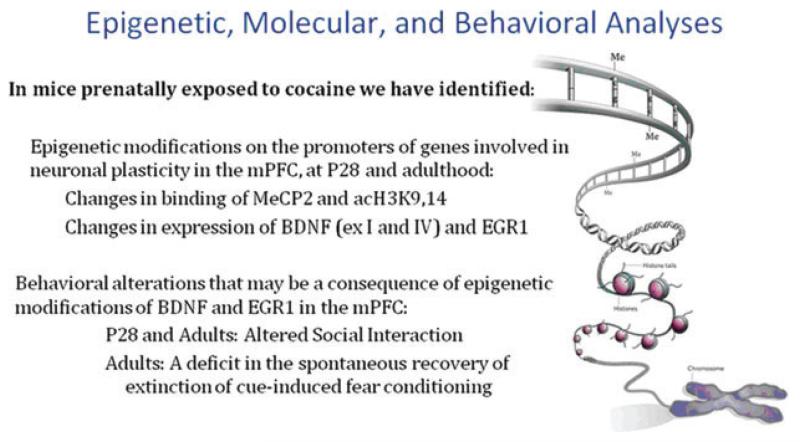
Figure 8.
Epigenetic mechanisms underlying persistent alterations on promoters of genes involved in neuronal plasticity.
Kosofsky presented data from a mouse model of transplacental cocaine exposure. These findings suggest that changes are expressed in a gene-specific, region-specific, and time-specific fashion; when apparent in the medial prefrontal cortex (mPFC), these changes appear to result in altered structural and functional maturation of that brain region. When compared with control animals (i.e., mice with no prenatal drug exposure), the cocaine-exposed mice showed a differential pattern of performance on a social interaction (SI) task: increased SI relative to controls at P28 (juvenile) and decreased SI relative to controls as adults. A parallel pattern of expression of the mRNA for the transcription factor EGR1 (also known as NGF-1a and zif 268) was observed in mPFC corresponding with these ages. In adult animals, changes in EGR1 expression correlated with decreased binding of MeCP2 to the EGR1 promoter; the same pattern was not observed at P28. Variations in MeCP2 occupancy suggest that an epigenetic mechanism may underlie changes in gene expression and behavior.
Kosofsky presented additional behavioral studies using an “extinction of conditioned fear” model, demonstrating that mice exposed to cocaine prenatally demonstrated impaired spontaneous recovery of extinction, a form of learning that relies on the mPFC. The prenatally cocaine-exposed animals showed a decrease in the binding of MeCP2 to the promoter of exons I and IV of the bdnf gene, which was associated with decreased mRNA expression of those transcripts in mPFC, likewise suggesting an epigenetic mechanism underlying the behavioral alterations. These findings are consistent with the presentation of David Sweatt in an earlier session on epigenetic mechanisms for learning and memory that highlighted the importance of epigenetic regulation of the bdnf gene for fear conditioning. The implication from these findings is that perinatal environmental conditions might determine the capacity for neural plasticity in later life through epigenetic regulation of genes critical for synaptic remodeling. Kosofsky’s group is currently pursuing “rescue experiments” to further explore the link between the proposed molecular mechanisms in the animals prenatally treated with cocaine. The findings may provide an opportunity for translational benefit regarding the diagnosis and treatment for the offspring of woman who abuse drugs during pregnancy.
Epigenetic programming by maternal care
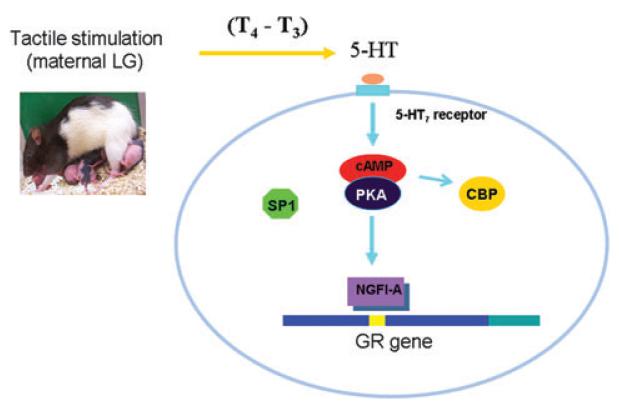
Michael J. Meaney (McGill University) summarized previous studies showing that variations in maternal care in the rat, specifically in the frequency of pup licking/grooming (LG), is associated with increased methylation of the exon 17 GR promoter in the hippocampus, decreased hippocampal FR expression, and increased hypothalamic-pituitary-adrenal (HPA) responses to stress.18,19 Previous work suggests that reversing the effects of differential DNA methylation of the exon 17 promoter, in turn, can reverse the effects of maternal care on hippocampal FR expression and HPA responses to stress. Meaney also presented findings from studies in the postmortem human hippocampus showing that a developmental history of child abuse was associated with an increase in the methylation of the exon 1F GR promoter (also see Marsit summary) and decreased GR expression. The focus of the talk concerned the mechanisms by which the environmental signal, pup LG, might generate the difference in DNA methylation, and gene expression. Meaney summarized in vitro and in vivo evidence for the importance of serotonin (5-HT)- and 5-HT–induced increases in hippocampal NGFI-A expression for the alterations in the methylation state of the exon 17 promoter. A shRNA targeting NGFI-A blocks both the effects of 5-HT on the methylation state of the exon 17 promoter and effects on GR expression. Pup LG provides tactile stimulation of the pup, resulting in an increase in circulating levels of triiodoithyronine (T3), the most biologically active thyroid hormone. T3 increases central 5-HT activity in the rat pup, and its administration is sufficient to increase the association of NGFI-A with the exon 17 promoter. Pup LG from the mother directly increases NGFI-A association with the exon 17 promoter, and artificial tactile stimulation mimics this effect. These findings suggest that the tactile stimulation derived from pup LG increases 5-HT activity at the level of the hippocampus, thus increasing NGFI-A expression and its association with the exon 17 promoter, which then initiates an alteration of the methylation state of the exon 17 GR promoter (Fig. 9). The results are consistent with previous studies in vitro showing that overexpression of NGFI-A alters the methylation of the exon 17 promoter. Interestingly, NGFI-A also regulates the expression of the GAD1 gene that encodes glutamic acid decaroxylase 1, and maternal care regulates the methylation of the GAD1 and GAD1 expression in a manner comparable to that of the GR. These studies are consistent with earlier reports of alterations in DNA methylation associated with increased transcription factor binding, and suggest that environmental conditions can directly alter epigenetic states through the activation of intracellular signaling pathways. Meaney also noted important caveats, most notably, the importance of identifying the enzyme directly responsible for the alteration in the methylation state.
Figure 9.
Tactile stimulation derived from pup LG increases 5-HT activity at the level of the hippocampus, thus increasing NGFI-A expression and its association with the exon 17 promoter, which then initiates an alteration of the methylation state of the exon 17 glucocorticoid receptor promoter.
Summary of symposium on development
Each of these presentations focused on well-established environmental influences, including pre- and postnatal maternal effects and drugs of exposure. This research reflects an emerging body of science examining epigenetic states as candidate mechanisms that link environmental conditions in early development with sustained changes in gene expression and neural development. Predictably, the discussion focused on the enthusiasm for the potential benefits of interventions targeting epigenetic mechanisms. The speakers noted that the current period marks a very early stage for research linking environmental conditions to alterations in gene expression and brain function. Nevertheless, the translational science presented within this symposium suggests that epigenetics represents a fruitful area of research bridging epidemiological findings with studies of biological mechanism.
Neuropsychiatry
Histone methylation in cocaine-induced behavioral and structural plasticity
Ian Maze presented research from Eric Nestler’s laboratory (Mount Sinai School of Medicine) that directly implicates a role for repressive histone methylation, specifically dimethylation of Lys9 on histone H3 (H3K9me2), in cocaine addiction (Fig. 10).
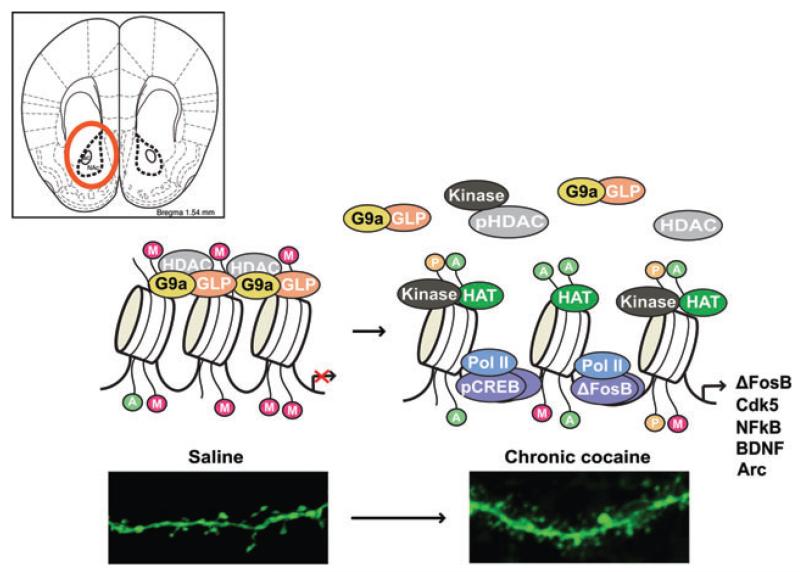
Figure 10.
Mechanism of increased cocaine sensitivity.
Maze et al. have shown that chronic cocaine administration to mice reduces global levels of H3K9me2 in nucleus accumbens (NAc), a key brain region involved in processing reward and implicated in addiction.20 In NAc, the reduction in H3K9me2 is mediated through decreased expression of G9a, a histone methyltransferase that specifically catalyzes H3K9me2. The repression of G9a, in turn, is mediated by the cocaine-induced transcription factor, ΔFosB. Using conditional mutagenesis and viralmediated gene transfer, the group found that G9a downregulation increases dendritic spine plasticity of NAc neurons and enhances rewarding responses to cocaine by decreasing repressive H3K9me2 at specific target genes and, therefore, increasing those genes’ expression. Cocaine-induced downregulation of G9a and H3K9me2 also promotes an individual’s vulnerability to stressful experiences and the development of depression-like behavioral abnormalities. These findings are consistent with clinical data that drug addiction and depression often occur together. This work has defined new mechanisms by which drugs of abuse and stress produce long-lasting alterations in gene expression and behavior.20 Moreover, identifying common regulatory mechanisms in cocaine and stress models may aid in the development of therapeutics aimed at alleviating addiction and depression syndromes.
Epigenetic targets in neurodegenerative and psychiatric disorders
Ted Abel (University of Pennsylvania) focused on another type of crucial histone modification, namely, histone acetylation, which is generally associated with transcriptional activation. Histone acetylation is catalyzed by HATs and reversed by HDACs. One of the major HATs present in the brain is the transcriptional coactivator, termed CBP. Abel et al. have shown that CBP is involved in synaptic plasticity in the hippocampus and in specific forms of long-term memory mediated via hippocampal circuits. Thus, mutant mice, in which CBP activity in neurons is reduced, exhibit deficits in spatial and contextual memory and in long-lasting forms of hippocampal synaptic plasticity. A complementary method to study the role of histone acetylation in synaptic plasticity and memory is to examine the effects of HDAC inhibitors, which increase histone acetylation and transcriptional activation. The Abel laboratory and other groups have found that administration of an HDAC inhibitor, such as trichostatin A, enhances long-term contextual memory and facilitates synaptic plasticity via the transcription factor CREB. Among important target genes induced by HDAC inhibition and CREB in the hippocampus are certain nuclear receptor transcription factors that are critical for the enhanced cognitive ability observed. Histone acetylation may, therefore, provide an epigenetic mechanism for establishing gene-specific modifications that result in the coordinate expression of genes required for long-term memory storage. As well, HDAC inhibitors may provide a novel therapeutic approach to treat the cognitive deficits that accompany many neuropsychiatric disorders.
Epigenetic mechanisms regulating synapse function and behavior
Lisa M. Monteggia (The University of Texas Southwestern Medical Center at Dallas) discussed her laboratory’s studies of loss-of-function mutations in the gene methyl-CpG-binding protein-2 (MeCP2) that cause Rett syndrome, a neurodevelopmental disorder characterized by reduced cognitive function and autism spectrum-like behavioral abnormalities, among other deficits.
Monteggia et al., along with other groups, have demonstrated that mice lacking MeCP2 exhibit abnormal cognitive and social behavior. The group has also demonstrated that loss of MeCP2 decreases excitatory glutamatergic transmission in the hippocampus, primarily by reducing glutamate release. By contrast, no deficit in inhibitory GABAergic function is seen. The results suggest that some Rett abnormalities are caused by an imbalance between excitatory and inhibitory neurotransmission in particular brain circuits, a possibility supported by work from the Monteggia laboratory. MeCP2, encoded by the X chromosome, functions predominantly by binding to methylated CpG islands in the promoter region of certain genes and thereby silencing those genes’ expression. This occurs, in part, by forming a protein complex with HDACs, which also repress gene activation, as noted above. The Monteggia laboratory has, therefore, started to investigate the coordinated role of histone deacetylation and DNA methylation in the regulation of synaptic function. The regulation of these two key epigenetic mechanisms by synaptic activity, and how such alterations affect neurotransmission, will be critical to elucidate the mechanisms underlying Rett syndrome as well as the roles these factors have in basic cellular processes. This work is also essential in understanding abnormalities in neurotransmission that underlie Rett syndrome and other neuropsychiatric disorders.
Epigenetic risk factors in social-communication disorders
David H. Skuse (University College London in the UK) discussed genomic imprinting, which involves epigenetic modifications that result in differential gene expression from certain genes (or even entire chromosomes) that are of paternal versus maternal origin.
Importantly, Skuse et al. have proposed that imprinting of the X-chromosome could result in sexually dimorphic characteristics. This hypothesis predicts that sexually dimorphic (male) vulnerability to some neurodevelopmental disorders, such as autism, could occur on the basis of whether the silencing of alleles is confined to chromosomes that were either paternally or maternally derived.21
The X-chromosome is enriched for genes that are involved in brain function. X-monosomy in humans results in Turner syndrome, which provides a model by which these putative mechanisms can be studied. In general, studies have shown that females with a single X-chromosome of paternal origin have better social communication skills, and are more empathic, than those whose single X-chromosome is maternal in origin.22 Autosomal gene expression may be regulated by X-linked genes.23
Skuse’s laboratory has shown in longitudinal studies that these differences persist from childhood into adulthood. These human observations have more recently been followed-up by studies of X-monosomic mice. Replicated findings include preferential expression of alleles from the maternally derived X-chromosome. One particular allele, invariably expressed in males, is associated with perseverative behavior.24 No evidence for preferential expression of the paternally derived X-chromosome has yet been observed, although recent work from Catherine Dulac’s laboratory at Harvard has provided support for the role of X-linked imprinting in brain development.25
Better understanding the mechanisms of genomic imprinting has the potential of providing new insight into the molecular basis of individual variability in personality traits as well as features of neuropsychiatric disorders.
Concluding Remarks
This conference brought together cutting-edge animal and human research in the emerging field of behavioral epigenetics. As exciting as these findings are, there are a number of challenges that need to be addressed as the field moves forward. Ed Tronick (University of Massachusetts and Harvard Medical School) pointed out that given what is now known about environmental effects that there needs to be as much effort put into characterizing the details of the experience of the animal or human and its environment as has gone into characterizing molecular mechanisms. At the moment experiential and environmental “phenotyping” is crude. Even in the best animal experimental studies, factors in addition to the study proper, such as housing conditions, events during animal housing, handling regimes, light cycles, social contacts, to name a few are not well specified. Yet any or all of these environmental factors, and the animals’ engagement with them, may lead to epigenetic effects. Indeed the presumption of epigenetics is that such factors do and will have effects. For example, the stability of epigenetic changes may be the result of changes in environmental factors that function to stabilize, destabilize, or even reiteratively induce epigenetic changes rather than the stability being “inherent” to the epigenetic change in and of itself. Moreover, the dynamics of change need to be better understood; that is, epigenetic changes related specifically to external environmental events in turn can become causal elements that go on to amplify, stabilize, or inhibit other epigenetic changes. These dynamics have come to be appreciated in studies of physiologic systems such as the HPA axis, where physiologic and behavioral feedback and feed-forward loops operating over time are critical to understanding how the organism functions. Similar dynamic thinking needs to be introduced into our models of epigenetic changes.
Tronick noted that most of the models we have for epigenetic mechanisms related to behavior are models of abnormal processes, such as toxic exposures or deprivation. We know less about epigenetic processes that affect normal behavior as was shown in work of Marsit. Thus much of what we know may be related to aberrant processes that fall outside the range of normal epigenetic processes. For example, the finding of high levels of methylation early in development could suggest that the timing of the effects of experience may be critical to understanding epigenetic effects on behavior. Moreover, the idea of developmental change as being, in part, a process related to a release from methylation would have profound effects for our understanding of development, such as identifying the events and their timing that trigger the release from methylation, as well as for therapeutic interventions.
Tronick recognized that at the present time our ability to specify the chain of causality of epigenetic changes in human behavior is limited because of our inability to access brain tissue. One can only hope that new techniques will be developedthat overcome this limitation and that with a better understanding of tissue may lead to the finding of “substitutes tissue” and correlated changes in other physiological systems. For example, epigenetic changes in the glucocorticoid receptor detected in plasma or buccal cells accompanied by parallel changes in ACTH, CRF or cortisol would strengthen the role of the HPA axis. But while we wait for these new innovations there needs to be an appreciation of the limitations of human epigenetic work compared to the research done on animals. The state of the art of the two areas is not the same, and applying state-of-the-art animal standards to human work will only limit progress. Tracing human behavior to epigenetic changes will be difficult, but we can make every effort to have as much detailed characterization of the epigenetic, physiologic, behavioral, and environmental links as possible and then to fill in the hidden links with work using animal models. That said, whatever the organism, we need to appreciate the full complexity of epigenetic changes.
Certainly, part of the excitement of this conference was the coming together of scientists from different disciplines, such as molecular biologists and behavioral scientists, who are capable of developing models that will elucidate both the hidden links and the complexity to further advance this relatively new field of behavioral epigenetics.
Acknowledgments
The Behavioral Epigenetics conference was presented by the New York Academy of Sciences, the Warren Alpert Medical School of Brown University, and the University of Massachusetts Boston, and supported in part by the University of Massachusetts Boston and the Life Technologies™ Foundation (Silver), the Massachusetts Life Sciences Center (Bronze), and Bristol-Myers Squibb R&D and Genomatix Software, Inc (Academy Friends). Funding for this conference was also made possible by (i) Grant 1 R13 DA029985-01 from the National Institute on Drug Abuse, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Mental Health, and the National Institutes of Health, Office of the Director (Barry M. Lester, Principal Investigator); (ii) an Independent Medical Education Grant from AstraZeneca; (iii) March of Dimes Foundation Grant No. 4-FY10-458; and (iv) an educational grant from Janssen, a division of Ortho-McNeilJanssen Pharmaceuticals, Inc., administered by Ortho-McNeil-Janssen Scientific Affairs, LLC.
Appendix: ninety-six articles published to date on behavioral epigenetics
- 1.Fischer A, Sananbenesi F, Wang X, et al. Recovery of learning and memory is associated with chromatin remodeling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 2.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 4.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. J. Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakobsson J, et al. KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron. 2008;60:818–831. doi: 10.1016/j.neuron.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 6.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberlander TF. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 8.Vecsey CG. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J. Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey SC, Ugale R, Zhang H, et al. Brain chromatin remodeling: a novel mechanism of alcoholism. J. Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in Cbp/ mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 13.Weaver MJ, Meaney M, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl. Acad. Sci. USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver ICG, et al. Reversal of maternal programming of stress responses in adult off-spring through methyl supplementation: altering epigenetic marking later in life. J. Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayson DR, et al. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan PO, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS ONE. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 20.Weaver ICG, et al. The transcription factor nerve growth factor-inducible protein A mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J. Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur. J. Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- 22.Collins A, et al. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS ONE. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth TL, et al. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatr. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst C, et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch. Gen. Psychiatr. 2009;66:22–32. doi: 10.1001/archpsyc.66.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Alter MD, et al. Variation in the largescale organization of gene expression levels in the hippocampus relates to stable epigenetic variability in behavior. PLoS ONE. 2008;3:e3344. doi: 10.1371/journal.pone.0003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romieu P. Histone deacetylase inhibitors decrease cocaine but not sucrose selfadministration in rats. J. Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassel S, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol. Pharmacol. 2006;70:487–492. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- 28.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Poulter MO, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol. Psychiatr. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Bönsch D, et al. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J. Neural Trans. 2006;113:1299–1304. doi: 10.1007/s00702-005-0413-2. [DOI] [PubMed] [Google Scholar]
- 31.Onishchenko N, Karpova N, Sabri F, et al. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- 32.Frieling H, et al. Epigenetic downregulation of atrial natriuretic peptide but not vasopressin mRNA expression in females with eating disorders is related to impulsivity. Neuropsychopharmacology. 2008;33:2605–2609. doi: 10.1038/sj.npp.1301662. [DOI] [PubMed] [Google Scholar]
- 33.Philibert RA, et al. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagarajan RP, et al. MECP2 Promoter methylation and X chromosome inactivation in autism. Autism Res. 2008;1:169–178. doi: 10.1002/aur.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine AA, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. PNAS. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breton CV, et al. Prenatal tobacco smoke exposure affects global and genespecific DNA methylation. Am. J. Respir. Crit. Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mill J, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am. J. Hum. Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philibert RA, Gunter TD, Beach SRH, et al. MAOA methylation is associated with nicotine and alcohol dependence in women. Am. J. Med. Genet. Pt. B. Neuropsychiatr. Genet. Vol. 147B. 2008;5:565–570. doi: 10.1002/ajmg.b.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in lateonset alzheimer’s disease. PLoS One. 2008;16:e2698. doi: 10.1371/journal.pone.0002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwamoto K, et al. DNA methylation status of SOX10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J. Neurosci. 2005;1:5376–5381. doi: 10.1523/JNEUROSCI.0766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champagne FA, et al. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen DA, et al. Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology. 2009;34:867–873. doi: 10.1038/npp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bönsch D, Lenz B, Kornhuber J, Bleich S. DNA hypermethylation of the alpha synuclein promoter in patients with alcoholism. Neuroreport. 2005;8:167–170. doi: 10.1097/00001756-200502080-00020. [DOI] [PubMed] [Google Scholar]
- 45.Bleich S, et al. Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin. Exp. Res. 2006;30:587–591. doi: 10.1111/j.1530-0277.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 46.Bönsch D, Lenz B, Reulbach U, et al. Homocysteine associated genomic DNA hypermethylation in patients with chronic alcoholism. J. Neura.l Transm. 2004;111:1611–1616. doi: 10.1007/s00702-004-0232-x. [DOI] [PubMed] [Google Scholar]
- 47.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J. Neurosci. 2007;8:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels WM, et al. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metab. Brain Dis. 2009;24:615–627. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuchikamia M, Morinobua S, Kurataa1 A, Yamamotoa S, Yamawakia S. Single immobilization stress differentially alters the expression profile of transcripts of the brainderived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int. J. Neuropsychopharmacol. 2009;12:73–82. doi: 10.1017/S1461145708008997. [DOI] [PubMed] [Google Scholar]
- 50.Klempan TA, Ernst C, Deleva, et al. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol. Psychiatry. 2009;66:824–831. doi: 10.1016/j.biopsych.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Huang HS, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J. Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deutsch SI, et al. Sodium butyrate, an epigenetic interventional strategy, attenuates a stress-induced alteration of MK-801’s pharmacologic action. Eur. Neuropsychopharmacol. 2008;8:565–568. doi: 10.1016/j.euroneuro.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Ferrante RJ, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guipponi M, et al. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am. J. Med. Genet. Pt B: Neuropsychiatr. Genet. Vol. 150 B. 2009;6:799–807. doi: 10.1002/ajmg.b.30901. [DOI] [PubMed] [Google Scholar]
- 57.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 58.Renthal W, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J. Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillemacher T, et al. Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. J. Psychiatr. Res. 2009;43:388–392. doi: 10.1016/j.jpsychires.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Burmistrova OA, et al. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc) 2007;72:578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
- 61.Azami S, et al. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea. J. Neurosci. Res. 2006;15:1610–1620. doi: 10.1002/jnr.21045. [DOI] [PubMed] [Google Scholar]
- 62.Marutha Ravindran CR, Ticku MK. Effect of 5-azacytidine on the methylation aspects of NMDA receptor NR2B gene in the cultured cortical neurons of mice. Neurochem. Res. 2009;34:342–350. doi: 10.1007/s11064-008-9783-9. [DOI] [PubMed] [Google Scholar]
- 63.Baek MN, et al. Artificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in mice. Neurosci. Lett. 2010;475:124–128. doi: 10.1016/j.neulet.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, et al. CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Developmental Cell. 2010;18:114–125. doi: 10.1016/j.devcel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 65.Kaminen-Ahola N, et al. Maternal ethanol consumption alters the epigenotype and the phenotype offspring in a mouse model. PLoS Genet. 2010;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dash PK, Orsi SA, Moore AN. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163:1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hager R, Cheverud JM, Wolf JB. Change in maternal environment induced by cross-fostering alters genetic and epigenetic effects on complex traits in mice. Proc. Biol. Sci. 2009;276:2949–2954. doi: 10.1098/rspb.2009.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doe CM, et al. Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c preRNA editing and altered 5HT2CR-mediated behaviour. Hum. Mol. Genet. 2009;18:2140–2148. doi: 10.1093/hmg/ddp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esler M, et al. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann. N.Y. Acad. Sci. 2008;1148:338–348. doi: 10.1196/annals.1410.064. [DOI] [PubMed] [Google Scholar]
- 70.Lasek AW, Janak PH, He L. Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 2007;6:728–735. doi: 10.1111/j.1601-183X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 71.Liljelund P, Handforth A, Homanics GE, Olsen RW. GABAA receptor beta3 subunit gene-deficient heterozygous mice show parent-of-origin and gender-related differences in beta3 subunit levels, EEG, and behavior. Brain Res. Dev. Brain Res. 2005;157:150–161. doi: 10.1016/j.devbrainres.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Plagge A, et al. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat. Genet. 2004;36:818–826. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- 73.Fernández-Gonzalez R, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc. Natl. Acad. Sci. USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miura K, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3amaternal-deficientmice. Neurobiol.Dis. 2002;9:149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- 75.Lefebvre L, et al. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene. Mest. Nat. Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 76.Peleg S, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 77.Tochigi M, et al. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol. Psychiatr. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Bromberg A, Bersudsky Y, Levine J, Agam G. Global leukocyte DNA methylation is not altered in euthymic bipolar patients. J. Affect. Disord. 2009;118:234–239. doi: 10.1016/j.jad.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 79.Davies W, et al. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat. Genet. 2005;37:625–639. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- 80.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. USA. 2010;23:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee CT, Ma YL, Lee EH. Serum- and glucocorticoid-inducible kinase1 enhances contextual fear memory formation through down-regulation of the expression of Hes5. J. Neurochem. 2007;100:1531–1542. doi: 10.1111/j.1471-4159.2006.04284.x. [DOI] [PubMed] [Google Scholar]
- 82.Olsson CA, et al. Prospects for epigenetic research within cohort studies of psychological disorder: a pilot investigation of a peripheral cell marker of epigenetic risk for depression. Biol. Psychol. 2010;83:159–165. doi: 10.1016/j.biopsycho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Hillemacher T, et al. Epigenetic regulation and gene expression of vasopressin and atrial natriuretic peptide in alcohol withdrawal. Psychoneuroendocrinology. 2009;34:555–560. doi: 10.1016/j.psyneuen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 84.de Mooij-van Malsen JG, van Lith HA, Oppelaar H, et al. Evidence for epigenetic interactions for loci on mouse chromosome 1 regulating open field activity. Behav. Genet. 2009;39:176–182. doi: 10.1007/s10519-008-9243-y. [DOI] [PubMed] [Google Scholar]
- 85.Ouko LA, et al. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 2009;33:1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 86.Phiel CJ, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;28:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 87.Skinner MK, Anway MD, Savenkova MI, et al. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chertkow-Deutsher Y, Cohen H, Klein E, Ben-Shachar D. DNA methylation in vulnerability to post-traumatic stress in rats: evidence for the role of the postsynaptic density protein Dlgap2. Int. J. Neuropsychopharmacol. 2010;3:347–359. doi: 10.1017/S146114570999071X. [DOI] [PubMed] [Google Scholar]
- 89.Dzitoyeva S, Dimitrijevic N, Manev H. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc. Natl. Acad. Sci. USA. 2003;29:5485–5490. doi: 10.1073/pnas.0830111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senechal Y, et al. Increased exploratory activity of APP23 mice in a novel environment is reversed by siRNA. Brain Res. 2008;1243:124–133. doi: 10.1016/j.brainres.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 91.Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr. Res. 2008;98:111–117. doi: 10.1016/j.schres.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DiFiglia M, et al. Therapeutic silencing of mutant huntington with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. USA. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Itaba-Matsumoto N, et al. Imprinting status of paternally imprinted DLX5 gene in Japanese patients with Rett syndrome. Brain Develop. 2007;29:491–495. doi: 10.1016/j.braindev.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Uddin M, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA. 2010;18:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marutha Ravindran CR, Ticku MK. Role of CpG islands in the up-regulation of NMDA receptor NR2B gene expression following chronic ethanol treatment of cultured cortical neurons of mice. Neurochem. Int. 2005;46:313–327. doi: 10.1016/j.neuint.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Hunter RG, McCarthy KJ, Milne TA, et al. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. USA. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 2.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 3.Kuzawa CW, Quinn EA. Developmental origins of adult function and health: evolutionary hypotheses. Annu. Rev. Anthropol. 2009;38:131–147. [Google Scholar]
- 4.Kuzawa CW. The fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am. J. Hum. Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 5.Miller CA, et al. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Barrett RM, Malvaez M, Kramar E, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharm. 2011 Apr 20; doi: 10.1038/npp.2011.61. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuown SC, Barrett RM, Matheos DP, et al. HDAC3 is a critical negative regulator of long-term memory formation. J. Neuorsci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McQuown SC, Wood MA. HDAC3 and the molecular brake pad hypothesis. Learn. Mem. 2011 Apr 16; doi: 10.1016/j.nlm.2011.04.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinol. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- 11.Bilang-Bleuel A, et al. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptordependent behavioural response. Eur. J. Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Mecinas M, Collins A, Qian X, et al. Forced swimming-evoked histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons involves ERK1/2-mediated MSK1 and Elk-1 phosphorylation. Soc. Neurosci. Abst. 2009;777:17. [Google Scholar]
- 13.Reul JMHM, Hesketh SA, Collins A, Gutierrez-Mecinas M. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory function. Epigenetics. 2009;4:434–439. doi: 10.4161/epi.4.7.9806. [DOI] [PubMed] [Google Scholar]
- 14.Chandramohan Y, Droste SK, Arthur JS, Reul JMHM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signalregulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur. J. Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav. Immun. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Filiberto AC, et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011 May 1;6(5) doi: 10.4161/epi.6.5.15236. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crandall JE, Hackett HE, Tobet SA, et al. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence of the cerebral cortex in mice. Cereb. Cort. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang TY, Hellstrom IC, Bagot RC, et al. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J. Neurosci. 2010;39:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat. Neurosci. 2010;11:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- 20.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skuse D. Genetic influences on the neural basis of social cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:2129–2141. doi: 10.1098/rstb.2006.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skuse DH, et al. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- 23.Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;4:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Davies W. Genomic imprinting on the X chromosome: implications for brain and behavioral phenotypes. Ann. N.Y. Acad. Sci. 2010;1204(Suppl.):E14–E19. doi: 10.1111/j.1749-6632.2010.05567.x. [DOI] [PubMed] [Google Scholar]
- 25.Gregg C, et al. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]