Abstract
The bi-directional comorbidity between epilepsy and depression is associated with severe challenges for treatment efficacy and safety, often resulting in poor prognosis and outcome for the patient. We showed previously that rats selectively bred for depression-like behaviors (SwLo rats) also have increased limbic seizure susceptibility compared with their depression-resistant counterparts (SwHi rats). In this study, we examined the therapeutic efficacy of voluntary exercise in our animal model of epilepsy and depression comorbidity. We found that chronic wheel running significantly increased both struggling duration in the forced swim test and latency to pilocarpine-induced limbic motor seizure in SwLo rats, but not SwHi rats. The antidepressant and anticonvulsant effects of exercise were associated with an increase in galanin mRNA specifically in the locus coeruleus of SwLo rats. These results demonstrate the beneficial effects of exercise in a rodent model of epilepsy and depression comorbidity and suggest a potential role for galanin.
Keywords: epilepsy, depression, comorbidity, rat, animal model, exercise, antidepressant, anticonvulsant, galanin, locus coeruleus
Introduction
People with epilepsy are at increased risk for developing depression, and individuals with active or past depression, or a family history of depression, are also at elevated risk of developing epilepsy, particularly if there is also a history of suicide attempts [1]. The bi-directional comorbidity that exists between these diseases is associated with many challenges for treatment. Patients with this comorbidity are often refractory to many commonly used pharmacotherapies, require more frequent hospitalizations, and have worsened prognosis [2]. Particularly troubling is the association between many anticonvulsant medications and depressed mood, as well the relationship between antidepressants and increased risk of seizure, as has been reviewed extensively elsewhere [3, 4]. These factors make safe and effective treatment of comorbid epilepsy and depression very difficult, and clearly emphasize the need for novel therapeutic approaches. Given their relative lack of negative and potentially dangerous side effects as compared to many pharmacological approaches, non-pharmacological therapies, such as aerobic exercise, are gaining more widespread interest.
Several studies have demonstrated the ability of aerobic exercise to decrease seizure frequency and/or epileptiform discharges [5-8] and improve mood in patients with depression [9-12]. Furthermore, in many epilepsy cases, exercise improves mood even in the absence of changes in seizure frequency [13]. Many of these studies emphasize the low risk and relative safety of exercise in patients with epilepsy. As an added benefit, exercise is associated with other positive outcomes, particularly in cardiovascular and overall physical health [6, 14]. Combined, these studies indicate that exercise may be both safe and efficacious in treating simultaneous epilepsy and depression, although it has never been tested in a comorbidity model.
Interactions between the neuropeptide galanin and norepinephrine may underlie the antidepressant and anticonvulsant effects of exercise. Galanin mRNA is increased specifically in the noradrenergic locus coeruleus (LC) following chronic voluntary exercise [15-17]. In addition, mice overexpressing galanin in the LC are seizure-resistant [18], and the anticonvulsant effects of wheel running in a kainic acid seizure model are attenuated by the galanin antagonist, M-40 [17]. The antidepressant properties of exercise in animal models are well documented [19], and galanin may also have antidepressant-like properties, particularly via GalR2 receptor activation [20].
To test the antidepressant and anticonvulsant effects of exercise and the potential contribution of galanin in a comorbidity model, we compared FST activity, pilocarpine-induced seizure susceptibility, and galanin mRNA in SwLo and SwHi rats that were either sedentary or given ad libitum access to a running wheel for 3-4 weeks. SwLo rats were selectively bred for depression-like behavior in the forced swim test (FST) and later shown to be more sensitive to limbic seizures compared to their depression-resistant counterparts, the SwHi rats [21-24]. An advantage of testing the beneficial effects of exercise in this model is that the antidepressant and anticonvulsant properties of exercise have been primarily tested independently and in “normal” animals (i.e. those without inherent risk for developing depression-like behaviors or seizures), while SwLo rats have heritable susceptibility to both of these phenotypes.
Methods
Animals and housing
SwLo and SwHi rats were selectively bred based on forced swim test (FST) phenotype, as described previously [21]. Briefly, rats were fitted with “water wings” made from rectangular rubber and placed in a tank (65 cm high, 30 cm diameter) of 25°C water (14 cm from the top) for 10 min. Duration of struggling (active movement of all 4 paws, forepaws breaking surface of water) and floating (immobility) were measured by a trained researcher blinded to the line of the rat.
Twelve to fourteen male rats of each line were randomly assigned to the exercise condition, while 13-14 male rats of each line were randomly assigned to the sedentary condition. Rats were between 1.5-3.5 months of age at the beginning of the experiments, and were from generations 56-59 of the SwHi and SwLo rat lines. All rats used were experimentally naïve to the FST and pilocarpine, and were singly housed. Food and water were available ad libitum, with lights on from 0700 to 1900 hours. All experiments were conducted in accordance with Emory University IACUC approval.
Voluntary exercise
Rats in the exercise condition were given free 24-h access to a stainless steel rodent activity wheel (Mini Mitter, Bend, OR). Each wheel was connected to an electromagnetic counter that measured the number of wheel revolutions, which were recorded daily between approximately 0900 and 1000. The daily distance run was calculated for each rat by multiplying the number of wheel rotations by the wheel circumference (107.75 cm) and converting to km.
Forced swim test
Animals were exposed to a 15-min FST, as described above, following 3 weeks of exercise or sedentary conditions, and data for each 5-min time bin was analyzed for struggling and floating behavior. Following the test, rats were returned to their exercise or sedentary conditions for one additional week.
Pilocarpine-induced seizures
One week following the FST (during which the exercise group still had access to running wheels), rats were assessed for seizure susceptibility by measuring latency to first pilocarpine-induced behavioral seizure, limbic motor seizure, and status epilepticus, as described [23]. Briefly, rats were injected with atropine methyl bromide (2 mg/kg, s.c., Sigma-Aldrich, St Louis, MO) 30 min prior to pilocarpine hydrochloride administration (380 mg/kg, i.p., Sigma-Aldrich, St Louis, MO), and latencies were measured during continuous video monitoring for a maximum of two hours following pilocarpine administration, or until status epilepticus (SE) was achieved. First behavioral seizure was defined as the initial incidence of facial automatism and/or head shaking (i.e., stage 1 or 2 on a modified Racine scale) [25]. Limbic motor seizure was defined as rearing and falling with bilateral forelimb clonus (i.e., stage 3 or higher on a modified Racine scale) [25]. SE was defined as 30 min of continuous seizing. A booster dose of pilocarpine (190 mg/kg) was administered to any rat that did not demonstrate a limbic motor seizure within the first hour following pilocarpine administration. Significantly more SwLo rats in the exercise condition (10/14) required a pilocarpine booster compared with sedentary SwLo rats (2/14) (Fisher's exact test, p<0.01), indicative of an anticonvulsant effect of exercise in these rats. By contrast, there was not a significant difference between the number of exercising (5/12) and sedentary SwHi rats (10/13) that required a booster (Fisher's exact test, p=0.11). Animals were euthanized 2 h after pilocarpine administration or following 30 min of continuous seizing, whichever came first.
Galanin mRNA quantification
Galanin mRNA was measured in the LC and hippocampus by quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR). Following pilocarpine experiments, animals were anesthetized with isofluorane and decapitated. The LC and hippocampus were isolated on ice, flash-frozen, and stored at −80°C until further use. Tissue samples were homogenized using a motorized Kontes Pellet Pestle and passed through a QiaShredder (Qiagen), and RNA was isolated using the RNAqueous-Micro Kit (Invitrogen). RNA was eluted using RNAse-free water, and purity and concentration were verified using a Nanodrop spectrophotometer to measure A260/280. Values between 1.8 and 2.0 are considered acceptable, and all groups fell within this range (1.86 +/− 0.02 for LC, 1.94 +/− 0.01 for hippocampus). Furthermore, there were no significant differences between groups for LC (F(3,25)=1.203, p=0.33) or hippocampal samples (F (3,29)=0.11, p=0.33).
RNA samples were then used to synthesize cDNA for qRT-PCR using the SuperScript III First-Strand Synthesis System (Invitrogen). Primers for the reference gene β-actin and the target gene galanin were purchased from SABiosciences (Qiagen) and were tested on an agarose gel to verify single band products of expected size.
A Bio-Rad CFX96 was programmed to perform qRT-PCR amplification and melting curve analysis using SYBR® Green SuperMix for iQ™ (Quanta). 10-fold serial dilutions of cDNA from a non-selectively bred rat line were used to calculate the efficiency for both the β-actin and galanin primers. The mean efficiency value for each primer was then used for analysis with the Pfaffl method. All samples were run in triplicate, with galanin as the target gene and β-actin as the reference gene. A relative expression value was calculated for each sample as described by Pfaffl [26] using the Pfaffl spreadsheet found at http://pathmicro.med.sc.edu/pcr/realtime-home.htm, such that:
Statistical analysis
Wheel running data were analyzed by repeated measures two-way ANOVA. FST and pilocarpine data were analyzed using Student's t-tests. Galanin mRNA data were analyzed using a Student's t-test or Mann-Whitney, depending on variance. An F-test was used to compare variances between groups. If p<0.05, variances were considered unequal and a Mann-Whitney U test was used; otherwise, t-tests or ANOVAs were used. All data analysis was conducted using Prism GraphPad 6.0 and IBM SPSS 19.0.
Results
Distance run is comparable between SwLo and SwHi rats
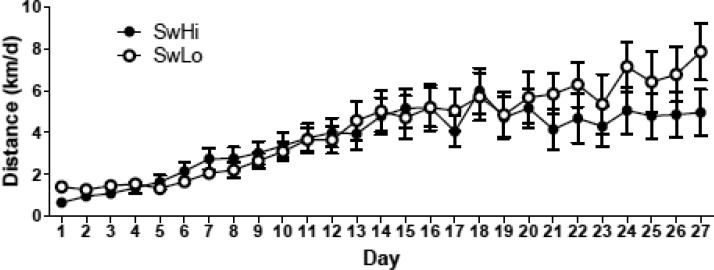
Average daily running distances increased over time and were similar between SwLo and SwHi rat lines (Fig. 1). There was a main effect of time on running distance, (F26, 624=19.82, p<0.0001, but no effect of rat line (F1, 624=0.26, p=0.61) or a rat line × time interaction (F26, 624=1.49, p=0.06).
Figure 1. Average daily voluntary exercise in SwLo and SwHi rats.
SwLo and SwHi rats were allowed 27 days of voluntary exercise on an activity wheel, and distance run (in km) was calculated daily. Shown is the mean ± SEM km per day. N=12-14 per group.
Exercise increases struggling in the forced swim test in SwLo rats
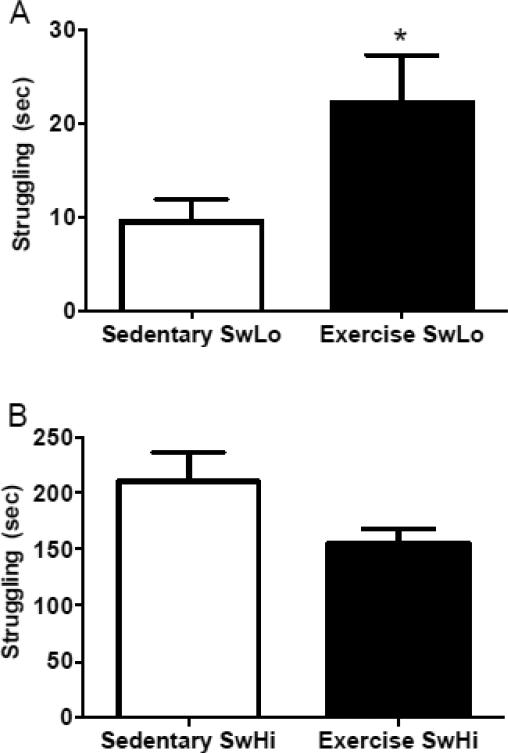
Following 3 weeks of running wheel exposure, struggling and floating duration were assessed in the FST. Exercise modestly, but significantly, increased struggling duration during the first 5 min of the FST in SwLo rats (t26=2.26, p=0.02) (Figure 2A). By contrast, exercise did not significantly affect struggling in SwHi rats (t23=1.848, p=0.08) (Figure 2B). No changes in struggling were observed at the 10 or 15 min time bins, and no changes were observed in floating behaviors in either rat line at any time point (data not shown). Additionally, there was no correlation between distance run and struggling behavior on the FST for either SwLo (data not shown; r=−0.08, p=0.78) or SwHi rats (data not shown; r=−0.16, p=0.62).
Figure 2. Effects of exercise on forced swim test perfomance in SwLo and SwHi rats.
Rats were given access to running wheels (Exercise) or a regular home cage (Sedentary) for 3 weeks, and behavior was assessed in a 15-min FST. Shown is the mean ± SEM struggling duration during the first 5 min for SwLo rats (A) and SwHi rats (B). *p<0.05 compared to the sedentary group. N=9-14 per group.
Exercise decreases seizure susceptibility in SwLo rats
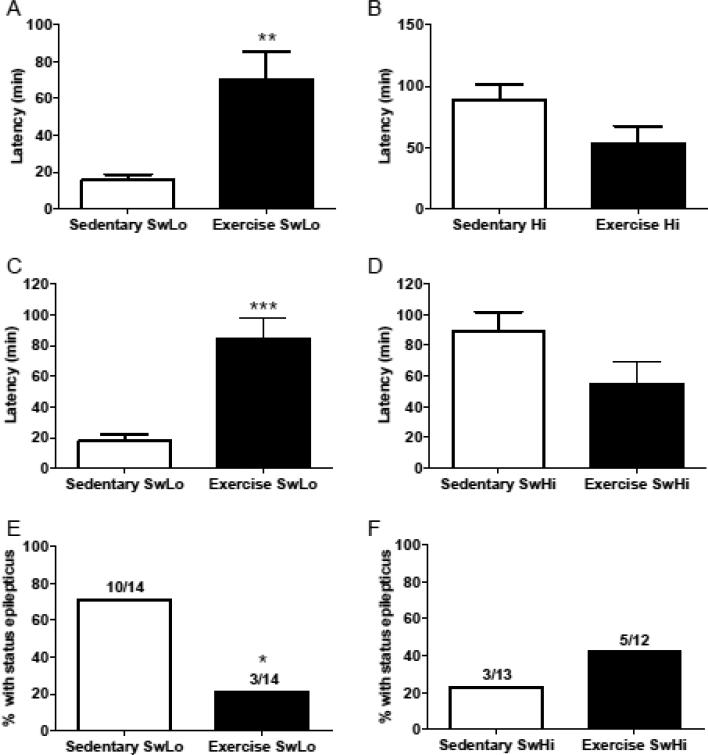
Following one additional week of running, pilocarpine (380 mg/kg, i.p.) was administered, and latency to first pilocarpine-induced behavioral seizure, limbic seizure, and status epilepticus (SE) were measured. Exercise significantly increased latency to first behavioral seizure in SwLo rats (t25=3.45, p=0.02) (Figure 3A), but did not significantly affect this measure in SwHi rats (t23=1.92, p=0.07) (Figure 3B). Exercise also significantly increased latency to limbic motor seizure in SwLo rats (t25=4.65, p<0.0001) (Figure 3C), but not SwHi rats (t23=1.84, p=0.08) (Figure 3D). There was no correlation between distance run and latency to pilocarpine-induced limbic motor seizure for SwLo (data not shown; r=0.15, p=0.61) or SwHi rats (data not shown; r=0.43, p=0.16). Exercise significantly decreased the fraction of SwLo rats that progressed to SE (10/14 sedentary vs. 3/14 exercise, Fisher's Exact Test, p=0.02) (Figure 3E) but had no effect on the fraction of SwHi rats that progressed to SE (sedentary 3/13, exercise 5/12, Fisher's Exact Test, p=0.41) (Figure 3F). Of the rats that achieved SE (sedentary SwLo, n=8; exercise SwLo, n=3; sedentary SwHi, n=3; exercise SwHi, n=5), there was no effect of exercise on latency to SE for SwLo (t9=0.57, p=0.59,) or SwHi rats (t6=1.39, p=0.21).
Figure 3. Effects of exercise on pilocarpine-induced seizure susceptibility in SwLo and SwHi rats.
After the forced swim test, rats were returned to their sedentary or exercise cages. One week later, seizure susceptibility was tested. Shown is the mean ± SEM latency to first behavioral seizure (Panels A & B), latency to limbic motor seizure (Panels C & D), and percent of rats progressing to status epilepticus (Panels E & F; raw number of rats progressing to status epilepticus over the total tested are shown above each bar) following administration of pilocarpine (380 mg/kg i.p.). *P<0.05, **p<0.01, ***p<0.001 compared to sedentary for that rat line. N=12-14 rats per group.
Exercise increases galanin mRNA in the locus coeruleus of SwLo rats
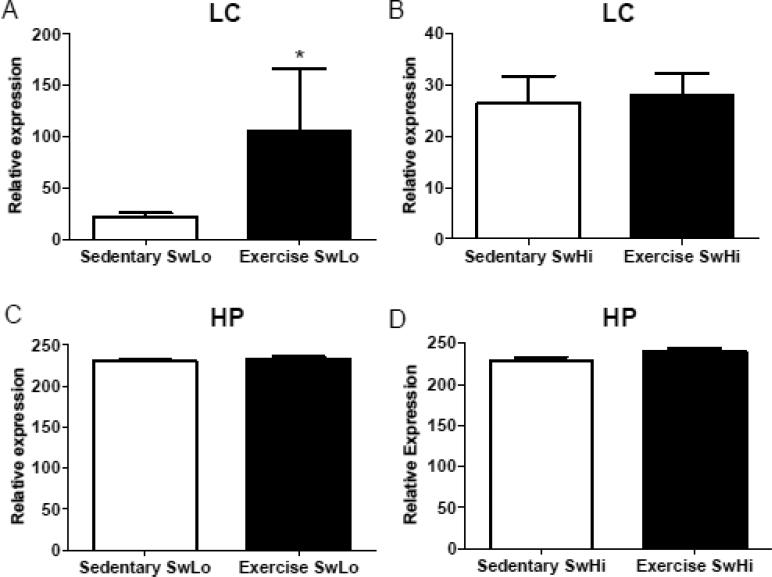
Galanin mRNA expression in both the LC and hippocampus of a subset of rats used for the behavioral experiments was quantified by qRT-PCR. Exercise significantly increased galanin mRNA in the LC (U=13, p<0.05) (Figure 4A), but not hippocampus (t15=0.66, p=0.52) (Figure 4B), of SwLo rats. Exercise did not significantly affect galanin mRNA in either the LC (t16=0.2389, p=0.81) (Figure 4A) or hippocampus (t16=1.70, p=0.11) (Figure 4B) of SwHi rats.
Figure 4. Effects of exercise on galanin mRNA abundance in SwLo and SwHi rats.
mRNA expression levels of galanin were assessed by qRT-PCR following exercise or sedentary conditions. Shown is the mean ± SEM relative expression of galanin (normalized to β-actin) in the (A) LC of SwLo rats, (B) LC of SwHi rats, (C) hippocampus (HP) of SwLo rats, and (D) hippocampus (HP) of SwHi rats. *p<0.05 compared to sedentary. N=8-10 rats per group.
Discussion
Antidepressant and anticonvulsant effects of exercise
Many antidepressants enhance the risk of seizure, particularly at high doses, and many anticonvulsant medications cause depressed mood [3, 4], severely limiting the pharmacotherapies available to patients with comorbid epilepsy and depression. Aerobic exercise has antidepressant and anticonvulsant effects in rodents and in humans. For example, exercise has been found clinically to reduce depressive symptoms in mild to moderately affected patients, and wheel running produces antidepressant-like behaviors in a variety of rodent models of stress and depression compared with sedentary controls [27]. Likewise, exercise has anticonvulsant effects in several rodent seizure models [17, 28, 29], and some clinical benefit has been seen in preliminary human studies [30-32]. Importantly for this proposal, epileptics who are physically active have significantly lower levels of depression than inactive subjects [33]. However, the benefits of exercise have never been tested in a model encompassing both disorders.
Antidepressant effects of exercise in SwLo rats
Originating from outbred Sprague-Dawley rats, the SwLo rat line has been selectively bred for nearly 60 generations for increased immobility and decreased struggling in the FST. Their counterparts, the SwHi rats, have been selectively bred for increased struggling and decreased floating in the FST and represent a model of depression resilience [21]. Although the SwLo rats were selectively bred using only FST criteria, they demonstrate other depression and anhedonic-like phenotypes, including decreased response to dopaminergic drugs and increased intracranial self-stimulation threshold [34, 35] (C. West, personal communication). We found that chronic wheel running modestly, but significantly, increased struggling in the FST only in SwLo rats. The small magnitude of the effect perhaps is not surprising, as the FST phenotype of the SwLo rats has been inbred over dozens of generation and is very robust. The long-term nature of the exercise was probably critical, as chronic, but not acute, antidepressant drug treatment is required for efficacy in these animals [36, 37]. The lack of effect of exercise on the performance of SwHi rats was also expected because conventional antidepressant drugs do not alter their FST behavior [36]. Interestingly, this does differ from other animal models in which even depression-resistant animals show response to antidepressants [38] However, while healthy control subjects in the human clinical population may experience the prosocial and anxiety-relieving effects of antidepressants, they usually do not respond to their mood-altering properties [39] This suggests that the SwHi and SwLo rat models more closely mimic the human population than other rodent models with regard to antidepressant efficacy. Further experiments are necessary to determine whether exercise can ameliorate other depression-like phenotypes in SwLo rats such as blunted responsiveness to dopaminergic drugs.
Anticonvulsant effects of exercise in SwLo rats
SwLo rats were never selected for seizure susceptibility phenotypes; they were bred based on low struggling activity and high immobility in the FST and only after several dozen generations were tested for, and found to have, increased sensitivity to seizure-inducing agents [24]. This suggests that the depression and seizure susceptibility observed in SwLo rats likely originates from a common genetic and neurobiological mechanism, although it is possible that these phenotypes are distinct have been inherited together via our selective breeding program because the genes for each are closely linked. Specifically, SwLo rats exhibited increased mortality following kainic acid-induced seizures [22] decreased latency to acute pilocarpine-induced limbic motor seizures, increased spontaneous seizures following pilocarpine-induced status epilepticus, and exacerbated seizure behaviors during hippocampal and amygdala electrical kindling as well as following electroshock [23]. We chose to measure latency to pilocarpine-induced limbic motor seizures in this study because (1) it is produces the most reliable and robust differences between SwLo and SwHi rats, (2) the clinical link between depression and limbic epilepsy is particularly strong [24], and (3) exercise can suppress limbic seizures [17, 40].
We found that wheel running potently suppressed pilocarpine-induced limbic motor seizures in SwLo rats, increasing their latency to that of sedentary SwHi rats that are relatively resistant to seizures at baseline. Interestingly, like the effects observed in the FST, exercise had no effect on seizure susceptibility in SwHi rats. It is unlikely this was due to a ceiling effect because rats that did not experience a limbic motor seizure in response to the initial dose of pilocarpine received a booster dose after 1 h, and 8 out of 12 exercising SwHi rats did have seizures within the 2 h test duration. Combined with the FST data, these results suggest that animals with a pre-existing susceptibility benefit the most from exercise and highlight the importance of testing therapeutics in models that encompass inherent disease risk. It will also be important to test the ability of exercise to suppress pilocarpine-induced epileptogenesis and spontaneous seizures in SwLo rats [33].
The role of galanin in the antidepressant and anticonvulsant effects of exercise in SwLo rats
While several non-pharmacological strategies, such as aerobic exercise, have demonstrated benefit in the clinical population with depression and/or epilepsy, much remains to be understood about their underlying mechanisms of action. Elucidation of these mechanisms would enhance our understanding of the diseases and suggest new targets for pharmacological therapies. This is important because not all patients are physically able to participate in an exercise program.
How can the antidepressant and anticonvulsant effects of exercise be explained on a neurological basis? One intriguing candidate is the neuropeptide galanin. Galanin is expressed in many different neuronal populations, including those implicated in mood and epilepsy. We found that exercise increased galanin mRNA, specifically in the LC of SwLo rats. We propose that LC-derived galanin mediates the beneficial effects of exercise in our model for four reasons. First, chronic exercise specifically increases galanin mRNA in the LC, but not tyrosine hydroxylase in the LC or galanin in other brain regions [15, 16, 41-43]. Second, galanin has anticonvulsant [44] and antidepressant [45] activity. Third, intracerebroventricular administration of a galanin receptor antagonist (M-40) attenuates the anticonvulsant effects of exercise in a kainic acid-induced seizure model [17]. Fourth, transgenic mice overexpressing galanin under the control of the dopamine β-hydroxylase (DBH) promoter, which is expressed predominantly in noradrenergic neurons, are resistant to epileptic seizures [46]. Future studies employing intracranial administration of galanin receptor antagonists into specific brain regions of exercising SwLo rats will be required to test this hypothesis.
Conclusion
This study demonstrates that aerobic exercise is antidepressant and anticonvulsant in a rodent model of depression and epilepsy comorbidity and suggests a potential role for galanin. However, many questions remain to be addressed. This study was limited in its assessment to 2 time points immediately following 3 or 4 weeks of exercise. Other exercise durations would also be informative because acute exercise may act as a stressor while longer exposure could result in habituation or sensitization to its antidepressant and anticonvulsant effects. It is also not clear how long the beneficial effects of exercise last following cessation of physical activity. It would also be informative to examine the contribution of neuromodulators and processes other than galanin that are implicated in mood and seizure susceptibility and are affected by exercise such as brain-derived neurotrophic factor and adult hippocampal neurogenesis [47, 48]. The SwLo rat will be a valuable tool for future research directed at understanding the neurobiological mechanisms underlying epilepsy and depression comorbidity and for screening novel therapeutics.
Highlights.
- Epilepsy and depression are comorbid disorders
- Wheel running has antidepressant and anticonvulsant effects in susceptible rats
- Wheel running increases galanin mRNA in the locus coeruleus of susceptible rats
- Aerobic exercise may be beneficial to patients suffering from epilepsy and depression
Acknowledgements
We thank Yvonne Ogbonmwan (Department of Human Genetics, Emory University) and the laboratory of Dr. Stephen Warren for technical assistance. These experiments were supported by the National Institute of Drug Abuse (DA027535 to DW) and the National Institute of Neurological Disease and Stroke (NS065663 to SAE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 2.Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Currents. 2006;6:141–6. doi: 10.1111/j.1535-7511.2006.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Kanner AM. The FDA alert on suicidality and antiepileptic drugs: Fire or false alarm? Epilepsia. 2009;50:978–86. doi: 10.1111/j.1528-1167.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 4.Judge BS, Rentmeester LL. Antidepressant overdose-induced seizures. Neurologic Clinics. 2011;29:565–80. doi: 10.1016/j.ncl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Nakken KO. Physical exercise in outpatients with epilepsy. Epilepsia. 1999;40:643–51. doi: 10.1111/j.1528-1157.1999.tb05568.x. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen HR, Ellertsen B, Gronningsaeter H, Nakken KO, Loyning Y, Ursin H. Physical exercise in women with intractable epilepsy. Epilepsia. 1994;35:1256–64. doi: 10.1111/j.1528-1157.1994.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 7.Vancini RL, de Lira CA, Scorza FA, de Albuquerque M, Sousa BS, de Lima C, Cavalheiro EA, da Silva AC, Arida RM. Cardiorespiratory and electroencephalographic responses to exhaustive acute physical exercise in people with temporal lobe epilepsy. Epilepsy & Behavior. 2010;19:504–8. doi: 10.1016/j.yebeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Denio LS, Drake ME, Jr., Pakalnis A. The effect of exercise on seizure frequency. Journal of Medicine. 1989;20:171–6. [PubMed] [Google Scholar]
- 9.Villaverde Gutierrez C, Torres Luque G, Abalos Medina GM, Argente del Castillo MJ, Guisado IM, Guisado Barrilao R, Ramirez Rodrigo J. Influence of exercise on mood in postmenopausal women. Journal of Clinical Nursing. 2012;21:923–8. doi: 10.1111/j.1365-2702.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- 10.Mata J, Thompson RJ, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. Walk on the bright side: physical activity and affect in major depressive disorder. Journal of Abnormal Psychology. 2012;121:297–308. doi: 10.1037/a0023533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62:633–8. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Arida RM, Cavalheiro EA, Scorza FA. From depressive symptoms to depression in people with epilepsy: contribution of physical exercise to improve this picture. Epilepsy Research. 2012;99:1–13. doi: 10.1016/j.eplepsyres.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 13.McAuley JW, Long L, Heise J, Kirby T, Buckworth J, Pitt C, Lehman KJ, Moore JL, Reeves AL. A Prospective Evaluation of the Effects of a 12-Week Outpatient Exercise Program on Clinical and Behavioral Outcomes in Patients with Epilepsy. Epilepsy & Behavior. 2001;2:592–600. doi: 10.1006/ebeh.2001.0271. [DOI] [PubMed] [Google Scholar]
- 14.Arida RM, Cavalheiro EA, da Silva AC, Scorza FA. Physical activity and epilepsy: proven and predicted benefits. Sports Medicine. 2008;38:607–15. doi: 10.2165/00007256-200838070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Sciolino NR, Dishman RK, Holmes PV. Voluntary exercise offers anxiolytic potential and amplifies galanin gene expression in the locus coeruleus of the rat. Behavioural Brain Research. 2012;233:191–200. doi: 10.1016/j.bbr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neuroscience Letters. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 17.Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Research. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Kokaia M, Holmberg K, Nanobashvili A, Xu ZQ, Kokaia Z, Lendahl U, Hilke S, Theodorsson E, Kahl U, Bartfai T, Lindvall O, Hokfelt T. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14006–11. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Ross B, Sanchez-Alavez M, Zorrilla EP, Bartfai T. Phenotypic analysis of GalR2 knockout mice in anxiety- and depression-related behavioral tests. Neuropeptides. 2008;42:387–97. doi: 10.1016/j.npep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss JM, Cierpial MA, West CH. Selective breeding of rats for high and low motor activity in a swim test: toward a new animal model of depression. Pharmacology, Biochemistry, and Behavior. 1998;61:49–66. doi: 10.1016/s0091-3057(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 22.Tabb K, Boss-Williams KA, Weiss JM, Weinshenker D. Rats bred for susceptibility to depression-like phenotypes have higher kainic acid-induced seizure mortality than their depression-resistant counterparts. Epilepsy Res. 2007;74:140–6. doi: 10.1016/j.eplepsyres.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epps SA, Tabb KD, Lin SJ, Kahn AB, Javors MA, Boss-Williams KA, Weiss JM, Weinshenker D. Seizure susceptibility and epileptogenesis in a rat model of epilepsy and depression co-morbidity. Neuropsychopharmacology. 2012 Aug 8; doi: 10.1038/npp.2012.141. 2012 doi: 10.1038/npp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epps SA, Weinshenker D. Rhythm and blues: animal models of epilepsy and depression comorbidity. Biochem Pharmacol. 2013;85:135–46. doi: 10.1016/j.bcp.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota KT, Duman RS. Environmental and pharmacological modulations of cellular plasticity: Role in the pathophysiology and treatment of depression. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arida RM, de Jesus Vieira A, Cavalheiro EA. Effect of physical exercise on kindling development. Epilepsy Res. 1998;30:127–32. doi: 10.1016/s0920-1211(97)00102-2. [DOI] [PubMed] [Google Scholar]
- 29.Arida RM, Scorza FA, Terra VC, Cysneiros RM, Cavalheiro EA. Physical exercise in rats with epilepsy is protective against seizures: evidence of animal studies. Arq Neuropsiquiatr. 2009;67:1013–6. doi: 10.1590/s0004-282x2009000600010. [DOI] [PubMed] [Google Scholar]
- 30.Arida RM, Scorza FA, Cavalheiro EA. Favorable effects of physical activity for recovery in temporal lobe epilepsy. Epilepsia. 2010;51(Suppl 3):76–9. doi: 10.1111/j.1528-1167.2010.02615.x. [DOI] [PubMed] [Google Scholar]
- 31.Vancini RL, de Lira CA, Scorza FA, de Albuquerque M, Sousa BS, de Lima C, Cavalheiro EA, da Silva AC, Arida RM. Cardiorespiratory and electroencephalographic responses to exhaustive acute physical exercise in people with temporal lobe epilepsy. Epilepsy Behav. 2010;19:504–8. doi: 10.1016/j.yebeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 32.de Lima C, Vancini RL, Arida RM, Guilhoto LM, de Mello MT, Barreto AT, Guaranha MS, Yacubian EM, Tufik S. Physiological and electroencephalographic responses to acute exhaustive physical exercise in people with juvenile myoclonic epilepsy. Epilepsy Behav. 2011;22:718–22. doi: 10.1016/j.yebeh.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Arida RM, de Almeida AC, Cavalheiro EA, Scorza FA. Experimental and clinical findings from physical exercise as complementary therapy for epilepsy. Epilepsy Behav. 2012 doi: 10.1016/j.yebeh.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 34.West CH, Bonsall RW, Emery MS, Weiss JM. Rats selectively bred for high and low swim-test activity show differential responses to dopaminergic drugs. Psychopharmacology (Berl) 1999;146:241–51. doi: 10.1007/s002130051113. [DOI] [PubMed] [Google Scholar]
- 35.Lin SJ, Epps SA, West CH, Boss-Williams KA, Weiss JM, Weinshenker D. Operant psychostimulant self-administration in a rat model of depression. Pharmacol Biochem Behav. 2012;103:380–5. doi: 10.1016/j.pbb.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West CH, Weiss JM. Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacol Biochem Behav. 1998;61:67–79. doi: 10.1016/s0091-3057(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 37.Weiss JM, Cierpial MA, West CH. Selective breeding of rats for high and low motor activity in a swim test: toward a new animal model of depression. Pharmacol Biochem Behav. 1998;61:49–66. doi: 10.1016/s0091-3057(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 38.Kaae SS, Chen F, Wegener G, Madsen TM, Nyengaard JR. Quantitative hippocampal structural changes following electroconvulsive seizure treatment in a rat model of depression. Synapse. 2012;66:667–76. doi: 10.1002/syn.21553. [DOI] [PubMed] [Google Scholar]
- 39.Serretti A, Calati R, Goracci A, Di Simplicio M, Castrogiovanni P, De Ronchi D. Antidepressants in healthy subjects: what are the psychotropic/psychological effects? Eur Neuropsychopharmacol. 2010;20:433–53. doi: 10.1016/j.euroneuro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Setkowicz Z, Mazur A. Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res. 2006;71:142–8. doi: 10.1016/j.eplepsyres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Murray PS, Groves JL, Pettett BJ, Britton SL, Koch LG, Dishman RK, Holmes PV. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides. 2010;31:2264–8. doi: 10.1016/j.peptides.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neal HA, Van Hoomissen JD, Holmes PV, Dishman RK. Prepro-galanin messenger RNA levels are increased in rat locus coeruleus after treadmill exercise training. Neurosci Lett. 2001;299:69–72. doi: 10.1016/s0304-3940(00)01780-8. [DOI] [PubMed] [Google Scholar]
- 43.Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118:1378–90. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- 44.Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. EXS. 2010;102:183–94. doi: 10.1007/978-3-0346-0228-0_13. [DOI] [PubMed] [Google Scholar]
- 45.Lu X, Sharkey L, Bartfai T. The brain galanin receptors: targets for novel antidepressant drugs. CNS Neurol Disord Drug Targets. 2007;6:183–92. doi: 10.2174/187152707780619335. [DOI] [PubMed] [Google Scholar]
- 46.Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–81. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49(Suppl 5):26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]