Abstract
Ovarian cancer ranks as the second most common tumor of the female reproductive system, with a large burden on global public health. Therefore, the identification of novel molecular targets and diagnostics is an urgent need for many women affected by this disease. To this end, the human transcription factor SOX2 is involved in a wide range of pathophysiological roles, such as the maintenance of stem cell characteristics and carcinogenesis. To date, in most studies, SOX2 has been shown to promote the development of cancer, although its inhibitory roles in cancer have also been reported. However, to the best of our knowledge, the role of SOX2, specifically in ovarian cancer cells, has not been examined in detail. In this article, we report, for the first time, that SOX2 promotes migration, invasion, and clonal formation of ovarian cancer cells. We further observed that SOX2 targeted FN1, a key gene that regulates cell migration in ovarian cancer. Our findings collectively suggest that the SOX2-FN1 axis is a key pathway in mediating the migration and invasion of ovarian cancer cells. This pathway offers crucial molecular insights and promises to develop putative candidate therapeutic interventions in women with ovarian cancer.
Introduction
Ovarian cancer is the second most common tumor of the female reproductive system and has the highest overall mortality rate and 5-year survival rate, with a large burden on global public health (Siegel et al., 2012). Currently, the standard treatment of ovarian cancer consists of surgical resection in combination with postoperative chemotherapy with carboplatin and paclitaxel (Burges and Schmalfeldt, 2011). However, the vast majority of patients with advanced disease relapse within 5 years, often caused by the metastasis of ovarian cancer cells (Burges and Schmalfeldt, 2011). The identification of novel molecular targets and diagnostics is thus an urgent need for many women affected by this disease.
The human transcription factor SOX2 is involved in the maintenance of stem cell characteristics (Avilion et al., 2003). Its downregulation can lead to the loss of cell pluripotency and self-renewal characteristics (Xu et al., 2009). SOX2 is also involved in carcinogenesis. Most studies to date have found that SOX2 plays a role in promoting cancer. For example, the downregulation of SOX2 expression resulted in decreased tumor cell proliferation and colony formation in breast cancer (Stolzenburg et al., 2012), and the knockdown of SOX2 markedly suppressed invasion and metastasis of prostate cancer (Bae et al., 2011), colorectal cancer (Han et al., 2012), and gliomas (Alonso et al., 2011). However, the tumor suppressive role of SOX2 was reported in gastric cancer, in which the expression of SOX2 is frequently downregulated, and SOX2 inhibits cell growth through cell cycle arrest and apoptosis (Otsubo et al., 2011).
Ye et al. (2011) found that SOX2 is significantly overexpressed in ovarian cancer tissues compared with normal ovary tissues. Zhang et al. (2012) found that the SOX2 expression was associated with high-grade ovarian carcinoma and tumor recurrence. This association suggested that SOX2 might act as a tumor promoter in ovarian cancer. However, the mechanism of the action of SOX2 is not understood.
In this study, we investigated the role of SOX2 in the migration and invasion of ovarian cancer cells. We found that SOX2 promoted cell migration, invasion, and colony formation. We further demonstrated that one of the key genes in regulating SOX2-mediated invasion and migration is FN1, a gene that plays a role in tumor neovascularization and metastasis (Akiyama et al., 1995). Our study demonstrated that SOX2 targeted fibronectin 1 to promote cell migration and invasion in ovarian cancer, thus identifying the SOX2–FN1 axis as a key pathway in mediating the migration and invasion of ovarian cancer cells, and offering the potential of developing an effective therapeutic intervention based on this new finding.
Materials and Methods
Cell culture and transfection
Human ovarian cancer cell lines, including A2780, A2780-CP, CAOV3, IGROV1, IGROV1-CP and OVCAR3, were cultured in RPMI-1640 medium supplemented with 10% FBS. The other two human ovarian cancer cell lines, ES-2 and SKOV3, were cultured in McCoy's 5A medium containing 10% FBS. Transfection with Lipofectamine was performed according to the manufacturer's instructions (Invitrogen).
To generate GFP- and SOX2-EGFP-expressing cells, A2780 cells were transfected with the pEGFP-N1 and pEGFP-N1-SOX2 constructs, respectively. Twenty-four hours after transfection, the cells were selected with 500 μg/mL of G418 in the culture medium.
RNA isolation and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized by M-MLV Reverse Transcriptase (Takara) using 2 μg of RNA. Quantitative real-time PCR was performed using the SYBR Green real-time PCR Kit (Takara), and mRNA levels were normalized to GADPH or beta-actin levels.
Western blot analysis
Whole cell extracts were obtained according to the standard protocol using radio-immunoprecipitation assay buffer, followed by sonication for 5 min. Protein concentrations were measured using the Pierce BCA Protein Assay Kit. Proteins were resolved on 12% SDS-PAGE, transferred onto a PVDF membrane, and then blocked for 1 h in 5% nonfat powdered milk in TBST. Primary antibodies [anti-SOX2 (2683-1, Epitomics), 1:2000; anti-GAPDH (ab9483, Abcam), 1:5000; anti-alpha-tubulin (2871-1, Epitomics), 1:10000; and anti-FN1 (1573-1, Epitomics), 1:2000] were diluted to probe the target protein. Horseradish peroxidase-conjugated [anti-rabbit (1:5000); anti-mouse (1:5000) (Abcam)] or alkaline phosphatase-conjugated antibodies [anti-rabbit (1:5000); anti-mouse (1:5000) (Promega)] were used as secondary antibodies. Signals were detected using ECL reagents (Thermo Scientific) or BCIP/NBT alkaline phosphatase color development solution.
Immunofluorescence staining
Cells were cultured on cover slips, which were previously disinfected with potassium permanganate and then with 75% ethanol, and subjected to UV light for approximately 24 h. Cells were fixed with 4% paraformaldehyde for 15 min and then permeabilized with 0.1% Triton X-100. After blocking with 1% BSA for 30 min, the slides were incubated with the first antibody diluted in 1% BSA at 4°C overnight. Anti-SOX2 rabbit antibody (Epitomics) was used at 1:250 dilution. After washing with PBS, the slides were incubated with the Cy3-conjugated goat anti-rabbit IgG antibody (AP136C, Millipore) at 1:100 dilution for 1 h in the dark. The slides were then washed and mounted.
Cell proliferation assay and cell cycle analysis
Cells were seeded at 1000, 2000 or 3000 cells per well in 96-well plates. Ten repeat culture wells were set up for each treatment. MTT assay was performed as recommended by the manufacturer (PerkinElmer). Briefly, at 0, 1, 2, 3, and 4 days, 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) (5 mg/mL) dye solution was added to each well, and cells were incubated at 37°C for 4 h. After 4 h, the supernatant was removed, and 150 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the blue formazan crystals. Absorbance was then read at 490 nm using a 96-well plate reader.
For cell cycle analysis, cells were synchronized by serum deprivation for 24 h. Cells were harvested and washed with PBS, followed by fixation with 70% ethanol. After washing twice with PBS, the cells were resuspended in PBS containing 50 mg/mL propidium iodide and incubated for 30 min at room temperature in the dark. Samples were analyzed for DNA content by flow cytometry.
Wound-healing assay
Cells were seeded at 106 cells per well in 6-well plates and incubated at 37°C with 5% CO2 until confluent. The surface was scraped with a sterile pipette tip, followed by the addition of serum-free medium. The culture plates were incubated at 37°C with 5% CO2. Wound healing was observed and photographed at 0, 24, 48, and 72 h.
Transwell migration and invasion assay
For the migration assay, cells were serum-starved for 24 h and then harvested in serum-free medium. Thereafter, 104 cells suspended in 200 μL serum-free DMEM medium were seeded on the upper chamber of a Transwell device (24-well plate, Corning, NY), whereas the lower chamber was loaded with 500 μL of medium containing 20% fetal bovine serum. After 72 h of incubation at 37°C with 5% CO2, the cells were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. Photographs were taken using a Nikon Eclipse TS100 microscope. All experiments were performed in triplicate. To quantify the extent of cell migration in transwells, we individually counted the stained cells in 20 different areas. The cell numbers were then averaged.
The transwell cell invasion assay was performed using transwells that were pre-coated with a layer of matrigel (Sigma-Aldrich) on the upper chamber. The rest of the experimental procedure was same as the cell migration assay.
Gelatin zymography
Cells were seeded at 106 cells per well in 6-well plates. After 16 h, cells were washed with sterile PBS and then cultured in serum-free medium for 24 h. The medium was collected and centrifuged to remove cells and debris. Subsequently, 55 μL aliquots of conditioned medium were diluted in sample buffer and loaded for zymography on a 10% polyacrylamide gel containing 1 mg/mL gelatin. The gels were washed for at least 30 min in 2.5% Triton X-100 and incubated for 24 h at 37°C in 50 mM Tris, pH 7.5, 10 mM CaCl2, and 0.02% Na azide. The buffer was decanted, and the gels were stained. Areas where staining was absent indicate degradation of the gelatin by the proteolytic activities of MMPs.
Clonogenic assay
Cells were seeded in three gradients of 50, 100, and 200 cells in 10 cm dishes. After 15 days, the cells were stained with crystal violet to count the colonies formed.
Dual-Luciferase Reporter Assay System
The Dual-Luciferase Reporter Assay System provides an efficient means of performing dual-reporter assays. The activities of firefly (Photinus pyralis) and Renilla (Renilla reniformis, also known as sea pansy) luciferases are measured sequentially from a sample. The firefly luciferase reporter is measured first by adding Luciferase Assay Reagent II (LAR II) to generate a stabilized luminescence signal. After quantifying the firefly luminescence, this reaction is quenched, and the Renilla luciferase reaction is simultaneously initiated by adding Stop & Glo Reagent to the same tube.
We constructed the pGL3 (Promega) reporter vector containing the 4 kb promoter region of FN1. Cells were seeded in 12-well plates and then co-transfected with 300 ng FN1 reporter vector, 30 ng Renilla control vector (Promega), and 1500 ng SOX2- pEGFP-N1 construct or 1500 ng empty vector. After 48 h, the cultured cells were gently washed with PBS. Thereafter, 250 μL lysis reagent (Passive Lysis Buffer, PLB, Promega) was added to each well. The plates were rocked at room temperature for 15 min. In a 96-well luminometer plate, 100 μL of LAR II buffer (Luciferase Assay Reagent II, Promega) was pre-dispensed, and 20 μL of the lysed cells were then carefully transferred. Finally, 100 μL Stop & Glo reagent (Promega) was added to the wells, and the Renilla luciferase activities were measured by a luminometer (PerkinElmer).
siRNA-mediated knockdown of FN1 expression
We ordered two different siRNAs (Ribobio Inc.) to knock down the expression of FN1. The effectiveness of these siRNAs was confirmed by real-time PCR after 24 h of transfection.
Statistical analysis
The results are expressed as the mean±SD. Student's t-test was used to determine statistical significance.
Results
SOX2 promotes cell migration and invasion in ovarian cancer cells
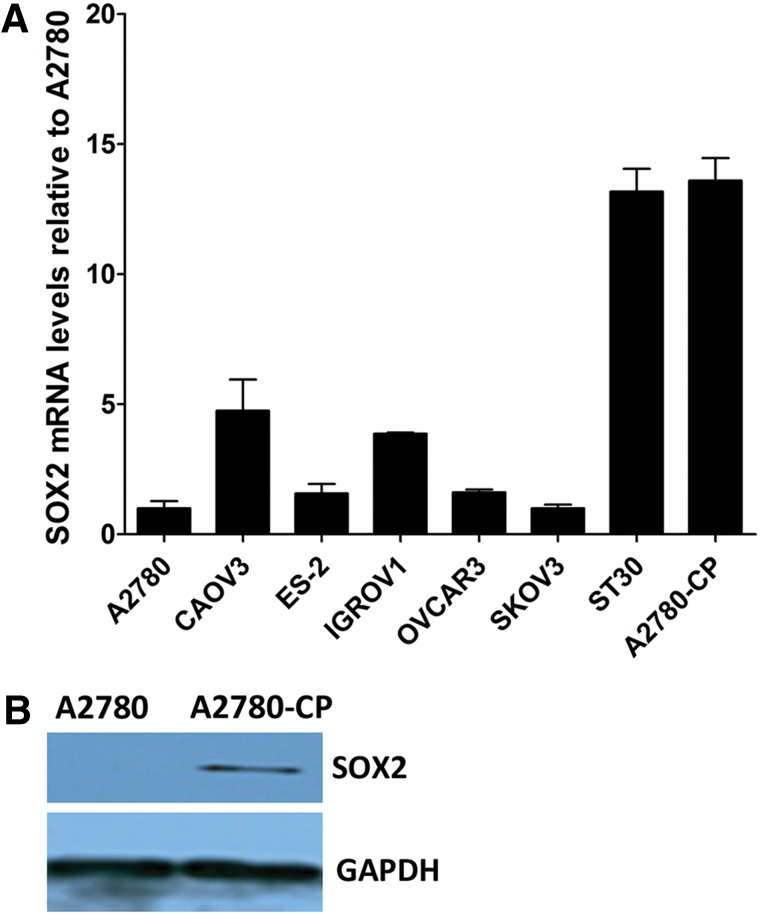
We found that the mRNA expression levels of SOX2 were greatly varied in different ovarian cancer cell lines (Fig. 1A) by RT-PCR. Interestingly, we noticed that the expression of SOX2 was significantly increased in the cisplatin-resistant cell line (A2780-CP) compared with its isogenic cisplatin-sensitive ovarian cancer cell line (A2780). By Western blot analysis, we confirmed that the SOX2 protein was overexpressed in cisplatin-resistant cells, whereas its expression was not detectable in cisplatin-sensitive cells (Fig. 1B). In addition, cell staining demonstrated that SOX2 was expressed in a much higher percentage of cells in the cisplatin-resistant cell line than that in the cisplatin-sensitive cell line (Fig. 2A).
FIG. 1.
SOX2 expression varies among different ovarian cancer cell lines. (A) Semi-quantitative RT-PCR analysis of SOX2 in eight ovarian cancer cell lines. Beta-actin was used for normalization. (B) Western blot analysis of the SOX2 protein expression in A2780 and A2780-CP cells. GAPDH was used as a control.
FIG. 2.
SOX2 correlates with cisplatin-resistant cells. (A) Immunofluorescence images of A2780 and A2780-CP cells stained with antibodies against SOX2. Secondary antibody was conjugated with Cy3. Cell nuclei were stained with Hoechst 33258. (B) Semi-quantitative RT-PCR analysis of SOX2 in A2780, A2780-GFP, and A2780-SOX2 cells. GAPDH was used for normalization. (C) Western blot analysis of the SOX2 protein expression in A2780-CP, A2780-GFP, and A2780-SOX2 cells. Alpha-Tubulin was used as a control.
To study the function of SOX2 in ovarian cancer cells, we first established stable clones that over express SOX2 in A2780 cells, in which the overexpression of SOX2 was confirmed by real-time PCR and Western blot analysis (Fig. 2B and 2C). The overexpression of SOX2 inhibited the growth of A2780 cells (Fig. 3A). Figure 3A shows the data with an initial seeding of 2000 cells per well with 10 replicates. In addition, seeding cells with initial densities of 1000 or 3000 cells showed the same trend (data not shown). The overexpression of SOX2 also reduced the percentage of cells in the S phase from 17.59% to 13.02% as demonstrated by cell cycle analysis (Fig. 3B).
FIG. 3.
SOX2 inhibits cell proliferation by reducing the percentage of cells in S phase in ovarian cancer cells. (A) Comparisons of the cell numbers of A2780-GFP and A2780-SOX2 at different time points. X-axis, time points. Y-axis, MTT absorbance at 490 nm. (B) The cell cycle analysis of A2780-GFP and A2780-SOX2 cells by flow cytometry.
We next assessed the role of SOX2 in cell migration using wound-healing and transwell assays. We found that the overexpression of SOX2 in A2780 cells enhanced cell migration in a wound-healing assay (Fig. 4A), which was further confirmed by a transwell migration assay (Fig. 4B and C). Figure 4C shows the average number of cells that migrated through the transwell membrane. The overexpression of SOX2 in A2780 cells also increased the number of colonies formed (Fig. 4D).
FIG. 4.
SOX2 promotes cell migration and invasion in ovarian cancer cells. (A) Wound-healing assay of A2780-GFP and A2780-SOX2 cells. Images at 0 hour and 72 hours after wound lines were introduced. (B) Cell migration assay of A2780-GFP and A2780-SOX2 cells by a transwell assay. (C) The numbers of stained A2780-GFP and A2780-SOX2 cells in 20 randomly chosen areas migrated through the transwells were quantified and averaged, p<0.05. (D) Clonogenic assay of A2780-GFP and A2780-SOX2 cells.
SOX2 is involved in the EMT process of ovarian cancer cells
The overexpression of SOX2 in A2780 cells (A2780-SOX2) changed the cell morphology from a round cell shape to an oval and a spindle cell shape. At the same time, SOX2 overexpressing cells (A2780-SOX2) did not attach to each other as tightly as those in the mock-transfected cells (A2780-GFP) (Fig. 5A). We previously showed that SOX2 regulated the epithelial to mesenchymal transition (EMT) in colorectal cancer cells (Han et al., 2012). Therefore, we assessed the expression of vimentin, which is a marker protein of mesenchymal cells, to examine if the morphological changes represented the EMT process. We found that vimentin was increased about 3-fold in A2780-SOX2 cells compared with A2780-GFP cells (Fig. 5B), suggesting that these morphology changes may involve the EMT process.
FIG. 5.
SOX2 is involved in the EMT process of ovarian cancer cells and induces MMP2 and MMP9 expression. (A) Cell morphology of A2780-GFP and A2780-SOX2 cells. (B) Semi-quantitative RT-PCR analysis of vimentin in A2780-GFP and 2780-SOX2 cells. GAPDH was used for normalization. (C) Transwell matrigel invasion assays of A2780-GFP and A2780-SOX2 cells. (D) Measurement of the MMP2 and MMP9 enzyme activities by gelatin zymography in A2780-GFP and A2780-SOX2 cells. (E) RNA expression of MMP2 and MMP9 in A2780-GFP and A2780-SOX2 cells measured by semi-quantitative RT-PCR. GAPDH was used for normalization.
SOX2 promotes invasion of ovarian cancer cells by increasing MMP2 and MMP9 production
A matrigel-coated transwell assay demonstrated that the overexpression of SOX2 promoted cell invasion by comparing the number of cells invaded through the matrigel in A2780-SOX2 cells (the lower panels) with those in A2780-GFP cells (the upper panels) in Figure 5C. To understand the mechanism underlying the increased cell invasion induced by SOX2 overexpression, we measured the proteolytic activity of MMP2 (matrix metalloproteinase 2) and MMP9 (matrix metalloproteinase 9) in the SOX2 overexpressing cells. Gelatinase, MMP2, and MMP9 have the highest enzymatic activities among all MMPs against type IV collagen, which is the main constituent of the extracellular matrix, and are associated with tumor invasion and metastasis (Toth and Fridman, 2001). By gelatin zymography, we showed that the overexpression of SOX2 increased the protein expression of both MMP2 and MMP9 (Fig. 5D). Real-time PCR analysis also showed an increase in their corresponding RNA expression levels (Fig. 5E).
SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer
In order to understand the mechanism of the SOX2-mediated increase in cell migration and invasion, we sought to find SOX2 targeted genes that are functionally important in cell migration and invasion. We picked 20 SOX2 bound genes (Table 1), including fibronectin 1 (FN1), from our previous ChIP-seq analysis of SOX2 in glioblastoma multiforme (GBM) (Fang et al., 2011) and colorectal cancer cells (Fang et al., 2010). We found that FN1 and S100P are the two most significantly induced genes by SOX2, when comparing the SOX2 overexpressing clones with the mock controls (Fig. 6A). As FN1 is important for cell migration (Akiyama et al., 1995), we further confirmed the changes in the expression of FN1 protein in A2780-GFP and A2780-SOX2 cells by Western blotting (Fig. 6B).
Table 1.
List of Genes Related to Cell Migration
| Gene | Descriptions |
|---|---|
| RAC1 | ras-related C3 botulinum toxin substrate 1 |
| VPS4B | vacuolar protein sorting 4 homolog B (S. cerevisiae) |
| PGK1 | phosphoglycerate kinase 1 |
| DDR2 | discoidin domain receptor tyrosine kinase 2 |
| PSME2 | proteasome (prosome, macropain) activator subunit 2 (PA28 beta) |
| MOCS1 | molybdenum cofactor synthesis 1 |
| EIF3S8 | eukaryotic translation initiation factor 3, subunit C |
| API5 | apoptosis inhibitor 5 |
| RPL36A | ribosomal protein L36a |
| TBC1D7 | TBC1 domain family, member 7 |
| DEGS1 | delta(4)-desaturase, sphingolipid 1 |
| RAFTLIN | raftlin, lipid raft linker 1 |
| HLC-8 | chromosome 17 open reading frame 80 |
| C14orf92 | TOX high mobility group box family member 4 |
| SH3KBP1 | SH3-domain kinase binding protein 1 |
| S100P | S100 calcium binding protein P |
| DHPS | deoxyhypusine synthase |
| TMSB10 | thymosin beta 10 |
| FN1 | fibronectin 1 |
| NBR1 | neighbor of BRCA1 gene 1 |
FIG. 6.
SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer. (A) Semi-quantitative RT-PCR analysis of SOX2 bound genes related to cell migration. GAPDH was used for normalization. (B) Western blot analysis of the expression of FN1 in A2780-GFP and A2780-SOX2 cells. GAPDH was used as a control. (C) Luciferase assay of the FN1 promoter activities in A2780 cells. (D) Semi-quantitative RT-PCR analysis of the FN1 knockdown efficiencies of two siRNAs in A2780-SOX2 cells. Beta-actin was used for normalization. A2780-SOX2-NC, the scramble control knockdown (NC) in A2780-SOX2 cells; A2780-SOX2-FN1-siRNA1, knockdown with siRNA oligo 1 of FN1 in A2780-SOX2 cells; A2780-SOX2-FN1-siRNA2, knockdown with siRNA oligo 2 of FN1 in A2780-SOX2 cells. (E) Western blot analysis of FN1 protein expression in siRNA-mediated FN1 knockdown (A2780-SOX2-FN1-siRNA2) and the scramble control knockdown (A2780-SOX2-NC) in A2780-SOX2 cells. GAPDH was used as a control. (F) Transwell assay of A2780-SOX2-NC (left panel) and A2780-SOX2-FN1-siRNA2 (right panel) cells. A2780-SOX2-NC, the scramble control knockdown (NC) in A2780-SOX2 cells; A2780-SOX2-FN1-siRNA2, knockdown with siRNA oligo 2 of FN1 in A2780-SOX2 cells. Images at 40X and 200X magnification are shown as the top and lower panels, respectively.
We then used the dual-luciferase reporter assay system to study the relationship between SOX2 and FN1. We amplified approximately 4 kb upstream promoter sequences from the transcription start site of FN1 and inserted it to the pGL3 reporter vector. Two ovarian cancer cell lines, A2780 and IGROV1, were co-transfected with either the SOX2 overexpression construct or the Renilla control vector and the FN1 reporter vector. We found that the FN1 promoter activity was activated in a dose-dependent manner in A2780 cells with different concentrations of the construct DNA (Fig. 6C). The activation was also confirmed in IGROV1 cells (data not shown). These data suggest that the FN1 promoter is responsive to SOX2 overexpression.
To determine if FN1 is necessary for SOX2-mediated cell migration, we evaluated two siRNA constructs, FN1-siRNA1 and FN1-siRNA2, to knockdown FN1 expression in A2780-SOX2 overexpressing cells. We found that only FN1-siRNA2 achieved a knockdown efficiency of approximately 50% by real-time PCR analysis (Fig. 6D). The knockdown effect of FN1-siRNA2 was also manifested at the protein level by Western blotting (Fig. 6E). Even with the overexpression of SOX2 in the A2780-SOX2-FN1-siRNA2 cells, the downregulation of FN1 resulted in the reversal of the role of SOX2 in promoting cell migration, as measured by a transwell migration assay (Fig. 6F).
Discussion
In this article, the first salient finding is that SOX2 is overexpressed in cisplatin-resistant cells (A2780-CP) compared with isogenic cisplatin-sensitive cells (A2780), most likely by increasing the percentage of cells expressing SOX2 in the cisplatin-resistant cells (Fig. 2A). As SOX2 is a stem cell marker, it is possible that the selection of cisplatin-resistant cells leads to the enrichment of the SOX2-expressing stem cell population. This hypothesis was supported by Ma et al.'s study (2010), which showed that a subpopulation of drug-resistant SKOV3 cells with high expression of stem cell markers, including SOX2, could be selected from ovarian cancer SKOV3 cells under drug selection (cisplatin and paclitaxel). Saigusa et al. (2011) also showed that after neoadjuvant chemo-radiotherapy (CRT) (30–40 Gy; 5-fluorouracil plus cisplatin) for esophageal squamous cell carcinoma (ESCC), high expression of SOX2 was correlated with lymphatic and vascular invasion, poorly differentiated tumors, and incomplete resection (P<0.05). Zhang et al. (2012) showed that SOX2 expression was associated with decreased disease-free survival durations (p=0.035; log-rank test), which we speculated might also be caused by a mechanism of increased chemotherapy resistance in the SOX2 overexpressing cells.
Although the phenotypical association of SOX2 with ovarian cancer as well as the association of SOX2 expression with the poor clinical outcome of ovarian cancer has been reported (Ye et al., 2011; Zhang et al., 2012), a mechanistic study was not previously conducted. For the first time, we demonstrated that SOX2 inhibited cell proliferation by reducing the percentage of cells in S phase (Fig. 3), but promoted the colony formation, migration, and invasion of ovarian cancer cells (Fig. 4). We further showed that the EMT process might be associated with SOX2-induced changes (Fig. 5A). Increased invasion might be related to the increased MMP2 and MMP9 proteolytic activities induced by SOX2 overexpression (Fig. 5D), a phenomenon we also observed in colorectal cancer cells (Han et al., 2012). Matrix metalloproteases (MMPs) are known to play essential roles in cell migration, differentiation, angiogenesis, apoptosis, and host defenses (Seiki, 2002). MMP2 and MMP9 are well known to be involved in regulating cell invasion and metastasis of human renal cell carcinoma (Zeliang et al., 2001) and non-small cell lung cancer (Dong et al., 2013).
We also demonstrated that fibronectin 1 is a mediator of the SOX2-induced invasion of ovarian cancer cells (Fig. 6). Fibronectin 1(FN1) is involved in cell migration processes, including embryogenesis, wound healing, blood coagulation, host defense, and metastasis (Akiyama et al., 1995). Using a dual-luciferase reporter assay system, we showed that SOX2 was able to induce FN1 promoter activities in IGROV1 and A2780 ovarian cancer cells (Fig. 6). Searching the promoter region of FN1 with SOX2 consensus binding motif, AACAA(A/T)G (Maruyama et al., 2005) or wwTGywTT (Fang et al., 2010), we identified two SOX2 binding sites that are located at −1950 and −3750 nucleotides upstream of the transcription start site of FN1 (data not shown). These data strongly confirmed that SOX2 directly regulates FN1 expression.
FN1 was previously shown to activate specific matrix metalloproteinases to promote breast cancer invasion and metastasis (Qian et al., 2011). In ovarian cancer, FN1 secreted from the human peritoneal tissue induces MMP9 expression and the invasion of ovarian cancer cell lines (Shibata et al., 1997). FN1 also induces MMP9 expression in human laryngeal carcinoma cells (Sen et al., 2010) and MMP2 expression in human prostate cancer cells (Moroz et al., 2012). We showed herewith that FN1 is a direct target of SOX2 (Fig. 6). We could not find any SOX2 binding sites in the promoter regions of MMP2 and MMP9, suggesting that SOX2 may induce the expression of both MMP2 and MMP9 in ovarian cancer cells through FN1.
Conclusions
Taken together, this study identified a SOX2-FN1-MMP pathway, which plays a role in promoting migration and invasion of ovarian cancer cells. This signaling pathway involves the following steps: (1) SOX2 targeting FN1 directly, and (2) FN1 inducing the expression of MMP2 and MMP9. These findings also suggest a role for SOX2 in mediating the EMT process and drug resistance in ovarian cancer cells.
Acknowledgments
The work was funded by Grant 81072060 from the National Natural Science Foundation of China, Grants 2008DFA11320 and 2012AA022705 from the Ministry of Science and Technology, China, Grant 20110101120153 by the Ministry of Education, China, and Grant 2012R10021 from the Zhejiang Provincial Government. The funders had no role in study design; the collection, analysis and interpretation of data; preparation of the manuscript; or decision to submit the manuscript for publication.
Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Akiyama SK. Olden K. Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- Alonso MM. Diez-Valle R. Manterola L, et al. Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PloS one. 2011;6:e26740. doi: 10.1371/journal.pone.0026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA. Nicolis SK. Pevny LH. Perez L. Vivian N. Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Devel. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae KM. Parker NN. Dai Y. Vieweg J. Siemann DW. E-cadherin plasticity in prostate cancer stem cell invasion. Am J Cancer Res. 2011;1:71–84. [PMC free article] [PubMed] [Google Scholar]
- Burges A. Schmalfeldt B. Ovarian cancer: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:635–641. doi: 10.3238/arztebl.2011.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong QZ. Wang Y. Tang ZP. Fu L. Li QC. Wang ED. Wang EH. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol. 2013;182:954–965. doi: 10.1016/j.ajpath.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Fang X. Yoon JG. Li L, et al. The SOX2 response program in glioblastoma multiforme: An integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genom. 2011;12:11. doi: 10.1186/1471-2164-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. Yu W. Li L, et al. ChIP-seq and functional analysis of the SOX2 gene in colorectal cancers. Omics. 2010;14:369–384. doi: 10.1089/omi.2010.0053. [DOI] [PubMed] [Google Scholar]
- Han X. Fang X. Lou X, et al. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS One. 2012;7:e41335. doi: 10.1371/journal.pone.0041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. Lai D. Liu T. Cheng W. Guo L. Cancer stem-like cells can be isolated with drug selection in human ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin (Shanghai) 2010;42:593–602. doi: 10.1093/abbs/gmq067. [DOI] [PubMed] [Google Scholar]
- Maruyama M. Ichisaka T. Nakagawa M. Yamanaka S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J Biol Chem. 2005;280:24371–24379. doi: 10.1074/jbc.M501423200. [DOI] [PubMed] [Google Scholar]
- Moroz A. Delella FK. Lacorte LM. Deffune E. Felisbino SL. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem Biophys Res Commun. 2012;430:1319–1321. doi: 10.1016/j.bbrc.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Otsubo T. Akiyama Y. Hashimoto Y. Shimada S. Goto K. Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PloS One. 2011;6:e16617. doi: 10.1371/journal.pone.0016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P. Zuo Z. Wu Z, et al. Pivotal role of reduced let-7g expression in breast cancer invasion and metastasis. Cancer Res. 2011;71:6463–6474. doi: 10.1158/0008-5472.CAN-11-1322. [DOI] [PubMed] [Google Scholar]
- Saigusa S. Mohri Y. Ohi M, et al. Podoplanin and SOX2 expression in esophageal squamous cell carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep. 2011;26:1069–1074. doi: 10.3892/or.2011.1408. [DOI] [PubMed] [Google Scholar]
- Seiki M. The cell surface: The stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- Sen T. Dutta A. Maity G. Chatterjee A. Fibronectin induces matrix metalloproteinase-9 (MMP-9) in human laryngeal carcinoma cells by involving multiple signaling pathways. Biochimie. 2010;92:1422–1434. doi: 10.1016/j.biochi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Shibata K. Kikkawa F. Nawa A. Suganuma N. Hamaguchi M. Fibronectin secretion from human peritoneal tissue induces Mr 92,000 type IV collagenase expression and invasion in ovarian cancer cell lines. Cancer Res. 1997;57:5416–5420. [PubMed] [Google Scholar]
- Siegel R. Naishadham D. Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Stolzenburg S. Rots MG. Beltran AS, et al. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012;40:6725–6740. doi: 10.1093/nar/gks360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Med. 2001;57:163–174. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N. Papagiannakopoulos T. Pan G. Thomson JA. Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Ye F. Li Y. Hu Y. Zhou C. Chen H. Expression of Sox2 in human ovarian epithelial carcinoma. J Cancer Res Clin Oncol. 2011;137:131–137. doi: 10.1007/s00432-010-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeliang L. Tongcai L. Zhixi S. Ping W. Kong Chuize. Qiang W. Expressions of matrix metalloproteinases 2 and 9 in human renal cell carcinoma. J Chin Med Univ. 2001;30:299–300. 306. [Google Scholar]
- Zhang J. Chang DY. Mercado-Uribe I. Liu J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Human Pathol. 2012;43:1405–1412. doi: 10.1016/j.humpath.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]