Abstract
Pediatric autoimmune neuropsychiatric disorders associated with streptococcus infections (PANDAS) originated from the observational work of Swedo and collaborators, who formalized their definition in 1998 in a set of operational criteria. The application of these criteria, which focuses on tics and obsessive-compulsive symptoms as core symptoms, has encountered difficulties, eventually leading to a high rate of misdiagnosis. In particular, the core feature represented by the association between newly diagnosed infections and neuropsychiatric symptom relapses in youths with this diagnosis could not be demonstrated by longitudinal studies. Exploratory studies aiming to identify clinical or cognitive features that could discriminate PANDAS from other pediatric obsessive-compulsive and tic disorders present methodological limitations, and therefore are not conclusive. Other behavioral features, in addition to obsessive-compulsive symptoms and tics, have been included in pediatric acute-onset neuropsychiatric syndromes (PANS) and childhood acute neuropsychiatric syndromes (CANS), two new concepts recently proposed in order to define a much broader clinical spectrum encompassing etiologically diverse entities. Given the uncertainties on the clinical definition of PANDAS, it is not surprising that evidence in support of a post-infectious, immune-mediated pathophysiology is also insufficient. Anti-dopamine receptor antibodies might be relevant to both Sydenham’s chorea (SC)—the prototypical post-streptococcal neuropsychiatric disorder—and some rare forms of encephalitis targeting the basal ganglia specifically, but studies exploring their association with children fulfilling Swedo’s criteria for PANDAS have been inconclusive. Moreover, we lack evidence in favor of the efficacy of antibiotic prophylaxis or tonsillectomy in patients fulfilling Swedo’s criteria for PANDAS, whereas a response to immune-mediated treatments like intravenous immunoglobulins has been documented by one study, but needs replication in larger trials. Overall, the available evidence does not convincingly support the concept that PANDAS are a well-defined, isolated clinical entity subdued by definite pathophysiological mechanisms; larger, prospective studies are necessary to reshape the nosography and disease mechanisms of post-streptococcal acute neuropsychiatric disorders other than SC. Research is also under way to shed further light on a possible relationship between streptococcal infections, other biological and psychosocial stressors, and the complex pathobiology of chronic tic disorders.
Keywords: Group-A beta-hemolytic streptococcal infection, autoimmunity, PANDAS, PANS, CANS, Tourette syndrome, obsessive-compulsive symptoms
Introduction
The term pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) refers to children with abrupt onset of tics and/or obsessive-compulsive symptoms (OCS) associated with a recent group-A beta-hemolytic streptococcal (GABHS) infection.1 Sydenham’s chorea (SC), a well-characterized manifestation of rheumatic fever (RF), is considered the prototype of neurologic disorders caused by aberrant immune responses to GABHS. SC patients often exhibit OCS together with other behavioral abnormalities. This contributed to the hypothesis that SC and PANDAS might be two distinct presentations of cortico-subcortical network dysfunction triggered by GABHS. Molecular mimicry, in which antibodies targeting bacterial antigens cross-react with brain (basal ganglia) antigens, was proposed as the pathogenic basis of post-streptococcal neuropsychiatric disorders.2,3
PANDAS have become a popular concept among patients, clinicians, and researchers. However, their clinical definition and prevalence are still debated. During the last 15 years, several limitations of the working diagnostic criteria for PANDAS have been highlighted. Moreover, different attempts to ascertain their frequency within the general population of youths with tics and OCS were not successful. Reliable diagnostic biomarkers are still not available and their pathogenesis remains undefined. This led to a recent nosographic reappraisal of PANDAS, implying that further work is needed to define the clinical boundaries of post-streptococcal disorders within the rubric of acute pediatric neuropsychiatric symptoms. In this review, we summarize the main points of debate around the clinical and pathophysiological features of PANDAS, with additional considerations on the possible role of infections and immunity in the natural history of tic disorders.
Brief historical preface
In his monograph “On Chorea and Choreiform Affectations” (1894), Osler first described obsessive-compulsive behavior in SC. Half a century later, this observation was confirmed in larger case series.4,5 In 1965, Langlois and Force reported in a 6-year-old the coexistence of tics and SC precipitated by infections, subsequently treated successfully with antibiotics and neuroleptics. In 1978, Kondo and Kabasawa reported in an 11-year-old boy a tic disorder started abruptly about 10 days after a febrile illness associated with elevated antistreptolysin O (ASO) antibody titers and good response to corticosteroids;6 this case prompted the discussion on the role of biological stressors in tic disorders, representing relevant background information for the first description of PANDAS. Kiessling7 reported an association of tics during pediatric GABHS outbreaks. During the same period, clinical researchers at the National Institutes of Mental Health (NIMH) reported SC cases that often exhibited OCS with a fluctuating clinical course.8–10 Allen et al11 identified a subgroup of children who presented obsessive-compulsive disorder (OCD) and/or tic disorders following an infectious illness without fulfilling the criteria for SC; they summarized the essential features of their cases in the acronym PITANDs (pediatric, infection-triggered, autoimmune neuropsychiatric disorders). The PITANDs subgroup was soon renamed “PANDAS” by Swedo and colleagues12 in 1998 in their seminal article where they proposed their set of working diagnostic criteria.
Clinical phenomenology
The original series of 50 PANDAS patients presented with episodic OCD and/or tic disorders with abrupt onset, following GABHS pharyngeal infections and exhibiting a remitting–relapsing course in association with infections (not necessarily streptococcal).12 Their natural history was similar to that of SC, which may also relapse, often triggered by infectious or hormonal factors. Swedo et al12 suggested five operational criteria to define PANDAS, which have been extensively discussed after their first publication (see Table 1 caption for further details). In this report it is underscored that, given the potentially long duration of antistreptococcal antibody titer elevation, longitudinal screening of patients is required to confirm that clinical exacerbations occur in association with rising titers and clinical remissions in association with falling titers. Beside the core features, these children also manifested emotional lability (66%), deteriorated school performance (60%), personality change (54%), separation anxiety (46%), nightmares (18%), bedtime rituals (50%), deterioration in handwriting (36%), oppositional behaviors (32%), and motoric hyperactivity (50%).12 Interestingly, the majority of these features are also detectable in acute SC.
TABLE 1. Operational Criteria for PANDAS, PANS, and CANS12,25,26.
| PANDAS | PANS | Idiopathic CANS |
|---|---|---|
| 1. Presence of OCD and/or a tic disorder | 1. Abrupt, dramatic onset of obsessive-compulsive disorder or severely restricted food intake | Acute onset before age 18 of behavioral and motor signs encompassing |
| The patient must meet lifetime diagnostic criteria (DSM-III-R or DSM-IV) for OCD or a tic disorder. | 1) Primary criterion | |
| Obsessive-compulsive disorder | ||
| 2. Pediatric onset | 2. Concurrent presence of additional neuropsychiatric symptoms, with similarly severe and acute onset, from at least two of the following seven categories | 2) Secondary criteria |
| 1) Anxiety | 1) Anxiety | |
| 2) Emotional lability and/or depression | 2) Psychosis | |
| 3) Irritability, aggression and/or severely oppositional behaviors | 3) Developmental regression | |
| 4) Behavioral (developmental) regression | 4) Sensitivity to sensory stimuli | |
| Symptoms of the disorder first become evident between 3 years of age and the beginning of puberty. | 5) Deterioration in school performance | 5) Emotional lability |
| 6) Sensory or motor abnormalities | 6) Tics | |
| 7) Somatic signs and symptoms, including sleep disturbances, enuresis or urinary frequency. | 7) Dysgraphia | |
| 8) Clumsiness | ||
| 9) Hyperactivity | ||
| 3. Episodic course of symptom severity | 3. Symptoms are not better explained by a known neurologic or medical disorder | 3. Mono- or polyphasic course |
| Clinical course is characterized by the abrupt onset of symptoms or by dramatic symptom exacerbations. Often, the onset of a specific symptom exacerbation can be assigned to a particular day or week, at which time the symptoms seemed to “explode” in severity. Symptoms usually decrease significantly between episodes and occasionally resolve completely between exacerbations.1 | Such as Sydenham’s chorea, systemic lupus erythematosus, Tourette disorder, or others. | |
| 4. Association with Streptococcal infection | ||
| Symptom exacerbations must be temporally related to Streptococcal infection, i.e., associated with positive throat culture and/or rising anti-streptococcal antibody titers.2 | ||
| 5. Association with neurological abnormalities | ||
| During symptom exacerbations, patients will have abnormal results on neurological examination. Motoric hyperactivity and adventitious movements (including choreiform movements) are particularly common.3 |
Abbreviations: OCD, Obsessive-Compulsive Disorder; PANDAS, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections; PANS, Pediatric Acute-Onset Neuropsychiatric Syndrome; CANS, Childhood Acute Neuropsychiatric Syndromes.
Parents are often able to indicate precisely the time of symptom onset or exacerbations.
According to Swedo et al12 the association between group-A beta-hemolytic streptococcal infection and neuropsychiatric symptoms should be preferably observed on at least two occasions (i.e., two exacerbations). The time lag between infection and exacerbations may vary within and across individuals, often between several days and a few weeks.
The presence of frank chorea, however, suggests a diagnosis of Sydenham’s chorea rather than PANDAS.
Course of illness and diagnosis
Probably the main weakness of the proposed criteria for PANDAS—and the principal source of debate around their definition—is the difficulty in demonstrating an association between new GABHS infections and exacerbations of tics/OCS throughout the course of the illness. Recent work has underscored that unequivocal bona fide new GABHS infections with expected immune response require a precisely documented acquisition of GABHS followed by significant increase in both ASO and anti-deoxyribonuclease B (anti-DNAseB) antibody titers.13 The detection of only one antibody rise may be neither sensitive nor specific enough for accurately diagnosing a new GABHS infection; moreover, it is crucial to demonstrate the rise in antibody titer, which requires serial monitoring, since antibody titers may remain elevated for more than 1 year after the inciting infection.13 As a result of the difficulty in performing appropriate prospective analyses of clinical symptoms and infectious markers, the diagnosis of PANDAS has often been erroneously formulated in the community setting. In many instances, this overdiagnosis originated from inaccurate diagnosis of a new GABHS infection, often based on single-point-in-time detection of antibody titers at the upper limit of normal range.14
Besides this frequent, inappropriate attribution of the PANDAS diagnostic label, multicenter studies that applied rigorous prospective evaluations failed to confirm a clearly higher rate of GABHS-related exacerbations in PANDAS than in non-PANDAS tic disorder and/or OCD. Kurlan et al15 followed up 40 matched PANDAS/non-PANDAS case–control pairs for 24 months. PANDAS patients met all the five diagnostic criteria; in particular, an antecedent bona fide GABHS infection had to be present at onset plus one symptom exacerbation or for two exacerbations; exacerbations were declared according to group consensus criteria (i.e., when the site investigator identified a clinically relevant worsening of OCS or tics lasting for >5 days, unrelated to medication changes). In this study, PANDAS cases presented with a slightly higher rate of GABHS-related exacerbations, but the majority of exacerbations in both groups were not associated with this pathogen. Leckman et al16 compared in a 25-month longitudinal study 31 subjects with PANDAS (diagnosed as in Kurlan et al15) to 53 non-PANDAS, assessing them for clinical symptoms, throat cultures, and antistreptococcal antibody titers. The authors did not find any group difference in the number of clinical exacerbations (confirmed through a rigorous two-stage algorithm based on rating scale score changes) or in the number of diagnosed GABHS infections; moreover, in patients with PANDAS, only 12% of newly diagnosed GABHS infections were associated with exacerbations of tics or OCS within 2 months. Although the selection of PANDAS cases may vary across specialty clinics within a multicenter study17 and the inter-rater reliability of data collection was not clearly reported, these longitudinal studies used published operational criteria and a rigorous definition of exacerbations. These results do not support the existence of a chronic tic/OCS disorder with a relapsing–remitting course associated with GABHS infections, suggesting that this core diagnostic criterion of PANDAS should be reconsidered.
Is there a specific clinical phenotype that distinguishes PANDAS?
Subsequent studies have attempted to characterize in more detail the phenomenology of PANDAS, in order to better discriminate PANDAS from non-PANDAS acute OCS/tics already at first presentation, and reduce the need for costly and potentially unrewarding follow-up. Bernstein and colleagues18 compared 21 children from their center who fulfilled Swedo et al’s PANDAS criteria to 19 children with non-PANDAS OCD. The fulfillment of the PANDAS diagnostic criteria, however, was mostly based only on retrospective review of clinical records. A correction for multiple comparisons was applied to only 19 of 51 yes/no items of a “PANDAS questionnaire”, specifically developed for this study and not properly validated, and comprising a mix of clinical features either reported in the literature as associated with PANDAS or observed in PANDAS patients from their center. This part of the analysis showed that separation anxiety, urinary urgency, oppositional defiant behavior, mood swings, inattention, hyperactivity, impulsivity, deterioration in handwriting, and decline in school performance were more likely to occur in PANDAS. However, the fact that these symptoms were preselected based also on their common occurrence in children with PANDAS from the same center suggests circular reasoning. Murphy et al19 conducted an exploratory analysis on 109 children with tics and/or OCD, aged 4–17 years, with personal and family history, diagnostic interview, physical examination, medical record review, and measurement of baseline levels of streptococcal antibodies; 41 fulfilled Swedo’s PANDAS criteria and 68 did not. As in Bernstein et al’s study, this study also retrospectively reviewed clinical records to assign the diagnosis of PANDAS based on the five criteria. An independent clinician reviewed a subsample of clinical records, yielding high inter-rater reliability (intraclass correlation coefficient = 0.86); to our knowledge, this is the only study to date reporting a reliability measure for the application of the five operational criteria for PANDAS, but the statistical power of this reliability analysis was not clear. Murphy et al’s19 results can only be interpreted as preliminary, given the high number of comparisons performed and the lack of correction for multiple testing. A history of tonsillectomies/adenoidectomies and remission of symptoms during antibiotic therapy were more frequent in PANDAS (p<0.01). Among motor/behavioral features, clumsiness was slightly more common among PANDAS (p<0.05). Some of the features highlighted in Bernstein et al’s study18 were also analyzed by Murphy et al,19 who failed to demonstrate significant difference according to PANDAS caseness. Overall, neither of these exploratory studies allows definitive conclusions on the existence of “PANDAS-specific” phenomenological features, and larger, more adequately powered studies are warranted. Fine motor skills underlying handwriting, manual dexterity, and related school performance deserve special attention.
Is there a neurocognitive endophenotype of PANDAS?
Neurocognitive functioning in PANDAS has been investigated by two independent studies. Hirschtritt et al20 administered a battery of tests measuring response execution, response selection, stimulus selection, and executive functioning (Wisconsin Card Sorting Test and Tower of Hanoi) to PANDAS patients (aged 5–16 years, and diagnosed according to the five criteria, assessed in extensive clinical interviews) and healthy volunteers accurately matched by age, sex, and IQ. Owing to limitations of the study design, sample sizes differed across cognitive tests, ranging between 22 and 45 case–control pairs. PANDAS subjects differed from controls only on the response accuracy in a test of attention and suppression, whereas attention deficit hyperactivity disorder (ADHD) comorbidity and age predicted reaction times within the response execution task. A subsequent study from Lewin and colleagues21 provided a more comprehensive exploratory analysis of visuospatial and executive functioning in 26 well-characterized young patients with PANDAS with OCD, whose performance was compared with normative data obtained from test manuals or a compendium for pediatric neuropsychological testing. The diagnosis of PANDAS was based on the fulfillment of the five criteria, assessed through the review of clinical records by a child–adolescent psychiatrist expert in psychoneuroimmunology. The only difference between PANDAS patients and the normative population was an impairment of visual–constructive and visual–spatial recall memory (Rey–Osterrieth Complex Figure test [ROCF]: copy, immediate recall, and delayed recall tasks). Moreover, compared to those with normal titers, patients with current ASO titer elevations had elevated OCD severity, lower speeded dexterity, and scored worse on the immediate and delayed recall tasks of the ROCF test, as well as on the Stroop Color–Word Interference test, that measures inhibitory control. This study was limited by the use of normative control data rather than ad hoc selection of a control group, small sample sizes, lack of correction for multiple comparisons and correlational analyses using single-point-in-time ASO titers, lack of inclusion of patients with PANDAS without OCD (i.e., tic disorder only), and lack of evaluation of the influence of comorbidities such as ADHD or of IQ measures on cognitive performance. Despite both studies identifying defective cognitive performances in PANDAS, the lack of a control group of patients with non-PANDAS tic disorder and/or OCD does not allow conclusions either in favor or against a specific cognitive endophenotypic trait of PANDAS.
PANDAS and rheumatic fever
Another aspect that raised concerns on the definition of PANDAS is its counterintuitive lack of association with other clinical features of RF, which assimilates PANDAS to another isolated post-streptococcal illness like post-streptococcal reactive arthritis.22 Unlike SC patients, for whom the co-occurrence of rheumatic manifestations is important to confirm the diagnosis, children with PANDAS do not exhibit echocardiographic abnormalities typical of rheumatic carditis, suggesting that PANDAS are unlikely to be part of the RF spectrum. Snider et al conducted a study on 60 children with PANDAS, recruited at the NIMH according to the five criteria. These authors failed to find an increased risk of rheumatic carditis in these patients.23 This was confirmed by Segarra and Murphy,24 who did observe any valvular insufficiency in these children, leading to the conclusion that their cardiac risk is similar to that of the general population.
Childhood acute neuropsychiatric syndromes and pediatric acute-onset neuropsychiatric syndrome
Over the years, it has become evident that, given the difficulties in consistently and reliably applying PANDAS diagnostic criteria in routine clinical practice, a reappraisal of their definition was necessary. More recently, two groups independently emphasized the need to move beyond PANDAS, postulating a broader definition of the whole spectrum of acute neuropsychiatric syndromes with young onset. Singer et al25 developed childhood acute neuropsychiatric syndromes (CANS), including in this concept all neuropsychiatric symptoms with acute, fulminant onset in childhood, and highlighting that a variety of conditions (infectious, post-infectious, drug-induced, toxic, traumatic, vascular, autoimmune, hypoxic, psychogenic) could present with acute OCS and other neuropsychiatric symptoms.25 Therefore, the etiologic evaluation of CANS would require a comprehensive diagnostic battery that, according to the characteristics of each individual case, may include routine blood tests and autoimmune screening, urinalysis, toxicological screening, cerebrospinal fluid analyses, brain imaging, electroencephalogram, and review of videotaped symptoms if movement disorders are prominent. Given the breadth of the spectrum, future work should operationalize the diagnostic work-up through algorithmic flow-charts; this would also be useful to evaluate whether a ‘post-streptococcal’ variant of CANS, defined by precise, new diagnostic criteria, can be identified. In this article, “idiopathic CANS” consists of all patients for whom a precise etiology could not be identified after a comprehensive examination, and their definition is summarized in Table 1. Notably, they de-emphasized the importance of tics, which became a secondary criterion (Table 1). In their conclusions, Singer et al25 strongly recommend “that a national centralized registry be established for the collection of standardized and longitudinal information on this cohort” of idiopathic CANS, since this is the only way to better define diagnostic criteria, identify possible subtypes, and delineate trials of rational therapy.
The second group of authors discussed the assessment of more than 400 youths diagnosed with PANDAS by six expert clinicians.26 These clinicians were asked to identify the symptoms which best characterized the collective group of patients, and the approved key clinical feature was “acute and dramatic symptom onset”, and in some particular cases “severe enough that parents took the child to the emergency room”. On this consensual basis, they proposed criteria for pediatric acute-onset neuropsychiatric syndrome (PANS), summarized in Table 1. A new interesting conclusion of this article is the relevance given to anorexia, which constitutes, together with OCD, a primary criterion. As in CANS, tics lost prominence in the diagnostic definition.26
Overall, CANS and PANS represent very similar concepts, and it seems unlikely they will eventually co-exist in routine clinical practice. Both might be useful conceptualizations of the wide spectrum of acute neuropsychiatric syndromes in childhood associated with basal ganglia dysfunction, helping clinical researchers to improve the nosology of this group of disorders. Greater efforts should, however, be made to integrate these two concepts.
“Post-streptococcal” neurologic and psychiatric disorders beyond SC and PANDAS: Where is the evidence?
The characterization of PANDAS incited a number of anecdotal case reports and small case series of motor and behavioral disorders with abrupt onset in association with a recent GABHS infection. Many of these cases merely demonstrated an intriguing co-occurrence between the neuropsychiatric disorder and the infection, generally without providing robust evidence in favor of an etiologic link. These cases included patients presenting with acute myoclonus,27 generalized dystonia associated with infantile bilateral striatal necrosis,28 paroxysmal dystonic choreoathetosis,29 restless legs syndrome,30 and even a combination of “functional” and organic movement disorders.31 A larger case series of different hyperkinetic movement disorders rapidly following GABHS pharyngeal infection was reported by Dale and colleagues,32 comprising 40 children manifesting chorea (n = 20), motor tics (n = 16), dystonia (n = 5), tremor (n = 3), stereotypies (n = 2), opsoclonus (n = 2), and myoclonus (n = 1); of note, emotional disorders occurred in 47.5%, including OCD (27.5%), generalized anxiety (25%), and depressive episodes (17.5%). In a relevant proportion of these cases, the temporal association with GABHS infections appeared to be strengthened by the detection of serum anti-striatum autoantibodies; however, the clinical and pathophysiological significance of these antibodies has been, since then, widely reconsidered and remains unclear. In line with the PANS concept, four cases of anorexia nervosa linked by an antecedent streptococcal infection were described by Sokol, who postulated the existence of a “PANDAS-anorexia nervosa” presentation, identified by specific descriptive criteria that require validation.33
Over the last decade, the work done by Dale and collaborators has led to the description of a form of acute disseminated encephalomyelitis (ADEM) possibly triggered by GABHS infections. The phenotype of this putatively “post-streptococcal” ADEM showed very minimal spinal involvement, and was mainly characterized by movement disorders (50%, mainly dystonia) and behavioral changes (70%) including emotional lability, inappropriate laughter, separation anxiety, confusion, and hypersomnolence.34 Again, in addition to raised ASO titers, most patients showed antibodies to human striatal proteins. A proportion of these patients responded to immunotherapy. This phenotype seems very similar to the recently defined spectrum of “basal ganglia encephalitis”, in which motor, psychiatric, sleep, and autonomic disturbances coexist, often in association with antibodies to human dopamine receptors. The etiology of “basal ganglia encephalitis” is, nevertheless, likely heterogeneous, and GABHS infections could only be one amongst a wide spectrum of immunogenic triggers linked to its onset.35
Finally, an autoimmune sleep disorder like narcolepsy/cataplexy has intriguingly been put in relationship with GABHS infections. A retrospective case–control study compared 200 patients with a recent onset of narcolepsy/hypocretin deficiency and 200 age-matched healthy controls for ASO and anti-DNAseB titers. The highest anti-streptococcal antibody titers were observed close to disease onset, whereas these tended to be lower as the disease progressed.36 Kleine–Levin syndrome, another rarer sleep disorder characterized by recurrent episodes of hypersomnia and behavioral disturbances, has been diagnosed in co-occurrence with PANDAS in an 11-year-old girl. Her sleep disorder responded to penicillin prophylaxis.37
Overall, the association of this miscellaneous group of motor and behavioral disorders with GABHS infections remains unproven, as it is based exclusively on cross-sectional or retrospective data, often derived from relatively small and uncontrolled observational case series. Whether other pathogens might also show an association with some of these disorders or immune regulatory mechanisms might predispose to abnormal immune responses to, or reinfection by, GABHS remain unanswered questions.
Considerations on pathophysiology
The pathophysiology of PANDAS has been put in relationship to disease mechanisms in SC. The latter condition is putatively associated with autoimmune mechanisms triggered by GABHS pharyngitis. This is primarily based on the very strong clinical association between the motor and behavioral disorder typical of SC, the previous inciting GABHS infection, and the frequent coexistence of other manifestations of RF; moreover, several studies suggest SC responds to immune-modifying treatments.38–40 Nevertheless, the exact mechanisms underlying SC are still incompletely defined, although, based on available evidence, an autoantibody-mediated process is favored as the most likely. An early model suggested that neuropsychiatric disturbances occurring suddenly after GABHS infections result from basal ganglia dysfunction secondary to autoimmune processes consisting of antibodies or immune cells cross-reacting between GABHS and cerebral cells, a hypothesis classically known as “molecular mimicry”.41 Pathological data from PANDAS patients are lacking, and imaging studies on these subjects are also very limited. Magnetic resonance imaging on the original cohort from Swedo et al14 revealed enlargement of caudate, putamen, and globus pallidus, most pronounced early in the course of illness.42 This was interpreted as a consequence of regional inflammatory change,42 but it has never been properly replicated. Only very few of these studies assessed circulating anti-striatum autoantibodies in patients fulfilling PANDAS criteria, and a reliable immunological marker for this subgroup of patients has not been as yet identified. When direct comparisons were performed between PANDAS and TS patients, it appeared that circulating autoantibodies did not differentiate between these two groups.43 Moreover, serial measurement of cytokine levels in children with a rigorously applied diagnosis of PANDAS did not yield significant differences between time at exacerbations associated with GABHS infections and time at exacerbations not associated with GABHS infections.44 Finally, other potential immunological markers, e.g., the B-cell marker D8/17 (a monoclonal antibody directed against a non-HLA-B-cell marker), have been explored over the years with disappointing results.45
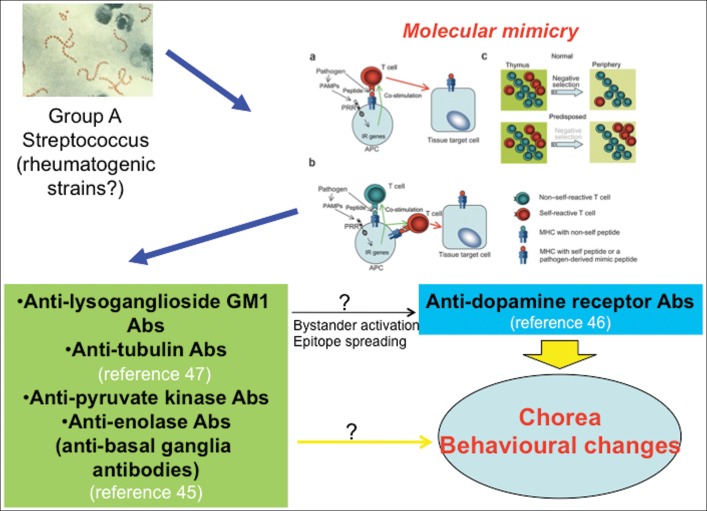
More recently, a new pathogenic model of SC has been proposed, in which antibodies to dopamine D1 and D2 receptors bind to striatal neurons, leading to alterations in dopaminergic neurotransmission and post-synaptic intracellular signaling.46 This model does not support the hypothesis of molecular mimicry, at least until a streptococcal “dopamine receptor-mimicking” antigen is clearly identified. The longer latency between the inciting infection and SC onset, compared with other features of RF, might be consistent with other immune-mediated phenomena, e.g., epitope spreading, eventually leading to the production of pathogenic anti-D1 and anti-D2 antibodies (Figure 1). PANDAS differ, however, from SC in that the latency between infection and neuropsychiatric onset seems shorter. Hence, it might be expected that, if an immune-mediated mechanism is relevant in PANDAS, this might partially differ from the pivotal mechanism of SC. Not surprisingly, there is controversy also in respect to anti-dopamine receptor antibodies expression in PANDAS. Antibodies binding to the neuronal surface were originally found in the sera of patients with SC and PANDAS, the latter diagnosed according to the five criteria; these antibodies caused elevated calcium/calmodulin-dependent protein kinase II signaling in neuronal cell lines, and were proposed to target dopamine D1 and D2 receptors.47 However, when a highly specific quantitative methodology was implemented to identify autoantibodies against neuronal surface antigenic targets (i.e., a flow cytometry-based approach on human embryonic kidney cells transfected with dopamine receptors), Dale et al35 detected anti-D2 receptor antibodies in 12 of 17 patients with “basal ganglia encephalitis” and in 10 of 30 SC patients, but in none of the 22 patients labeled as PANDAS. In this study, serum samples were taken from the latter group of patients during acute exacerbations related to a GABHS infection; however, although all patients had one or more exacerbations associated with GABHS, not all patients had a GABHS-related relapsing–remitting course, and the five criteria were not rigorously applied. Similar findings were obtained by the same group using the same methodology on a neuroblastoma cell line (SH-SY5Y).48 Assay methodology, patient selection, and limitations in the clinical definition of PANDAS might have accounted for this discrepancy.
FIGURE 1. Post-streptococcal Neuropsychiatric Disorders (including PANDAS) Might Be Associated with Antineuronal Antibodies.
The molecular mimicry hypothesis is one of the mechanisms through which autoantibodies targeting brain structures might be abnormally produced in these conditions. Not all the autoantigens targeted by these antibodies in Sydenham’s chorea or PANDAS seem, however, to be involved in antigenic mimicry between group A streptococcus and brain cells. Other mechanisms, such as bystander activation or epitope spreading, may also be relevant to the synthesis of pathogenic autoantibodies. Part of the figure adapted from Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol 2010;11:28–35.
The attempts to replicate the behavioral disorder of PANDAS in rodents using passive transfer based on stereotactic striatal micro-infusion of patients’ immunoglobulins has also led to inconsistent results.49,50 A more convincing approach is the active immunization of mice with GABHS protein homogenate, which generated repetitive behavior similar to human stereotypies that correlated with peripheral anti-neural antibodies and immune deposits in the brain;51 moreover, mice transfused with IgG1 from previously immunized mice developed a similar phenotype, suggesting that immune activation against this pathogen might lead to antibody-mediated neuronal dysfunction.52 More work is needed to understand the mechanisms linking antibody deposition to neuronal dysfunction, and to clarify whether dopamine receptors are indeed a relevant antigenic target. Also, more evidence is needed from clinical studies on the relative contribution of antibody-mediated and cytokine-mediated mechanisms. Finally, hematopoietic-derived microglia were shown to play a role in the pathogenesis of repetitive behaviors in rodents, e.g., trichotillomania, but its involvement in tic disorders and OCS in humans needs to be explored further.53
Considerations on treatment
Given the existing uncertainties around the clinical definition and pathophysiology of PANDAS, it is not surprising that aspects related to the treatment of these patients are still undefined. Penicillin prophylaxis is known to be effective in reducing the frequency of RF recurrences, including SC. Because of the hypothesized pathophysiologic similarities between SC and PANDAS, it was hypothesized that penicillin prophylaxis would reduce neuropsychiatric exacerbations in children with PANDAS. Indeed, several studies revealed mixed results. Early uncontrolled observational case series suggested significant and rapid improvements of OCD, anxiety, and tics in PANDAS patients following antibiotic treatment, which in some cases was associated with good long-term prognosis.54 The first randomized controlled trial conducted by Garvey et al55 failed to provide support for the use of this treatment in patients specifically diagnosed with PANDAS. In this early trial, however, active treatment did not meet its primary endpoint of reducing the frequency of GABHS infections, and therefore it cannot be considered really informative. In a subsequent clinical trial, Snider et al56 showed that antibiotic prophylaxis, using either penicillin or azithromycin, reduced neuropsychiatric symptom exacerbations in children with PANDAS. However, this was an active comparator study that lacked a placebo arm, and clinical data at baseline were collected on a retrospective basis. Therefore, randomized placebo-controlled trials testing safety and efficacy of antibiotic prophylaxis in PANDAS are currently still not available. New studies are necessary on this topic and currently under way (ClinicalTrials.gov Identifier: NCT01860300). Besides the bactericidal effect, it has been intriguingly suggested by Murphy17 that penicillins could exert a protective effect in these patients through less explored immunomodulatory, or even neuroprotective, mechanisms.
Earlier anecdotal reports presented cases of children who fulfilled PANDAS criteria and were treated with tonsillectomy. Orvidas and Slattery57 originally reported on two siblings with a history of more than three episodes of streptococcal pharyngitis in 1 year despite adequate antibiotic treatment. These patients manifested behavioral changes (mainly tics and OCS), concomitant with recurrent infectious episodes. After tonsillectomy, both were free from GABHS infection recurrences and OCS/tic exacerbation for a follow-up of 11 months. Similarly, Heubi and Shott58 described clinical improvement after tonsillectomy of a fluctuating tic disorder and OCD in two patients with recurrent tonsillitis. In the first of these cases, the strength of the antistreptococcal immune response is unclear, given that antistreptococcal antibody titers were only minimally raised and the duration of follow-up was only 2 months. The second case did not present with relevant behavioral oscillations temporally linked to recurrent infections. Hence, it is doubtful whether the diagnosis of PANDAS had been appropriately assigned to these patients. Batuecas Calerio et al59 described a PANDAS patient with symptoms of obstructive sleep apnea syndrome and recurrent bacterial tonsillitis. Following tonsillectomy, his obstructive sleep apneas and ocular-facial tics underwent complete remission. Overall, these and other anecdotal cases reported in the literature57–60 provide a tenuous link between this treatment and symptom remission, since possible confounders such as pharmacological treatment (antibiotics, anti-tic medications), stress, or fatigue were not adequately taken into account. An important contribution was recently provided by Murphy et al,61 who enrolled 112 patients in a prospective study (the majority within a 7–12 years age range) with DSM-IV-R criteria for OCD and/or tic disorder, 43 of whom classified as PANDAS (based on infection-related behavioral abnormalities, regression, deterioration in school performance, emotional lability, and urinary symptoms) and 69 classified as non-PANDAS. Within this cohort, clinical and laboratory measures were compared between the 36 patients who received tonsillectomy and/or adenoidectomy and the 76 who did not. The group of surgical patients did not differ from the non-surgical group on antistreptococcal antibody titers (ASO, anti-DNAse B, and anti-A carbohydrate), as well as on OCS and tic severity scores. Neuropsychiatric symptom onset occurred after a mean time period of 2.4–2.9 years after surgery in more than 50% of patients in the surgical group; however, PANDAS cases were two times more likely to have undergone surgery than non-PANDAS, possibly as a consequence of a higher rate of GABHS infections in the former group. The main limitation of this study is that patients whose OCD/tic disorder remitted between surgery and the start of the study could not be enrolled due to design characteristics, thus possibly under-representing a clinical subgroup highly relevant to the study aims. Nevertheless, the results of this cohort study, the largest to date on this topic, do not support a positive impact of tonsillectomy/adenoidectomy on the course of children with OCD/tics, regardless of whether they had been diagnosed with PANDAS or not.
Over the last 25 years, first-line immune-modulating treatments (steroids, plasma exchange [PE], intravenous immunoglobulins [IVIg]) have all demonstrated efficacy in the treatment of acute SC. Perlmutter et al62 conducted a randomized sham-controlled trial of PE and IVIg in 30 children fulfilling the five criteria; sham control was implemented only for the IVIg active arm for ethical reasons. At 1-month follow-up, IVIg led to a 45% decrease in OCS severity (superior to placebo), whereas PE led to a 58% decrease; for tics, PE decreased severity by 42%. Interestingly, this improvement was maintained in 82% of cases after an average 12-month follow-up period. This study had a relatively small sample size and has not been replicated since; a new trial is, however, currently under way (ClinicalTrials.gov Identifier: NCT01281969).
Until the clinical definition of PANDAS is definitely settled and more robust trials evaluating potential etiologic treatments become feasible, children fulfilling Swedo et al’s criteria for PANDAS should receive the mainstay of treatment for OCS, tics, and associated behavioral symptoms. Anecdotal evidence confirms that serotonin reuptake inhibitors (SSRIs), drugs of choice for the treatment of OCD, may be effective also in patients fulfilling these criteria. Interestingly, SSRIs may also exhibit anti-inflammatory effects, suppressing the synthesis of interferon-gamma.17,63 Likewise, cognitive-behavioral therapy has also been explored in PANDAS-associated OCD. Storch et al64 reported on a small series of seven children with PANDAS (range 9–13 years) treated for 3 weeks with intensive cognitive-behavioral therapy. Six of seven showed improvement on the Children’s Yale-Brown Obsessive-Compulsive Scale and Anxiety Disorder Interview Schedule for DSM-IV Child Interview Schedule-Parent version; at long-term follow-up, three of these six patients continued to show clinical improvement. Albeit limited, this anecdotal evidence suggests that patients fulfilling PANDAS criteria are not refractory to routine treatments for OCS and tics.
What is the role of infections and immunity in chronic tic disorders?
Research focused on the clinical and pathophysiological characterization of PANDAS has led to increased interest in the involvement of GABHS and other pathogens (e.g., common cold viruses,65 Mycoplasma pneumoniae,66 Borrelia burgdorferi,67 herpesviruses68) as biological stressors modulating the clinical course of tic disorders and OCD, regardless of whether they fulfill or not the proposed diagnostic criteria for PANDAS. Cross-sectional studies showed that TS patients across different ages exhibit higher circulating levels of antistreptococcal antibodies and higher GABHS carriage rate than healthy peers.69 One sophisticated array-profiling study addressing antistreptococcal antibody reactivity in children with tic disorders found that these patients express higher rates of antibodies directed against a significantly broader number of antigens than healthy children or children with acute pharyngitis, indicating abnormal reactivity to the pathogen.70 Large, community-based retrospective studies in the United States have shown that children with OCD or tic disorders were more likely to have contracted a GABHS throat infection in the 3 months prior to onset,71,72 although this could not be replicated by a similar UK-based study from Schrag and coworkers.73 Finally, community- and service-based prospective studies looking at this association have been largely inconsistent so far. In a longitudinal observation of 693 elementary school-age children (ages 3–12 years), Murphy and colleagues74 failed to report an association between tics and GABHS infections during the observation period, probably due in part to the intrinsic fluctuations of tic severity over time. Interestingly, they found that, over 8 months, repeated GABHS infections represent a risk factor on the development of behavioral symptoms consistent with attention deficit hyperactivity disorder (relative risk 1.71; p<0.0001), suggesting that GABHS infections could modulate the clinical course of a broader spectrum of disorders related to fronto-striatal networks. In our Italian survey on 168 TS children and 177 controls, we detected a higher GABHS carriage rate, and antistreptococcal and antineural antibodies in TS patients, but prospective follow-up failed to show an effect of new infections on rigorously defined clinical exacerbations.69 Interestingly, ASO titers were persistently elevated in almost 60% of TS patients in this study, confirming enhanced antistreptococcal immune response.69 Finally, the prospective study from Lin et al75 in Yale on 45 patients and 41 controls followed up for 2 years showed that GABHS infections could triple the effect of psychosocial stress in predicting short-term future tic severity, suggesting interaction between streptococcal infections and stress.
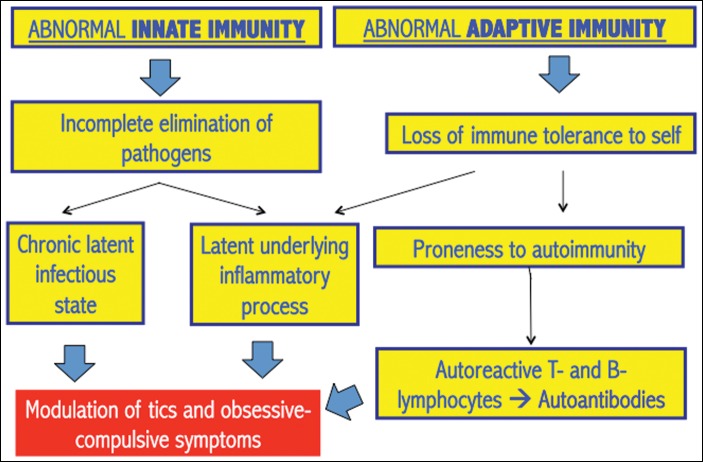
Overall, the relationship between TS and GABHS may be rather complex. On one hand, these patients show a very strong antibody response to this pathogen, even stronger than those in patients with acute pharyngitis;70 on the other hand, there is preliminary evidence that the regulation of immune responses may be altered in these patients and this might make them more prone to develop both infections (including streptococcal infections) and autoimmune processes, thus giving rise to a complex vicious cycle. A summary of these abnormalities, involving both innate and adaptive immune responses, is provided in Table 2 and Figure 2. Dysfunctional innate responses are supported by gene expression profiling of peripheral lymphocytes,76,77 and by measurements of monocyte-derived cytokines.78 Innate immunity dysfunction might cause incomplete elimination of pathogens with subsequent chronic immune-inflammatory activity: this is supported by the markedly raised antibody response to GABHS,69,70 Chlamydia trachomatis, and Toxoplasma gondii.79 Acute inflammatory markers like neopterin were raised in independent cohorts of TS patients.78,80,81 Cytokine levels may also covary with symptom severity in “garden-variety” youth with tics/OCS, but not in PANDAS.44,82 Abnormal regulation of adaptive responses are suggested by increased numbers of B and T lymphocytes with activated phenotype.85–85 Some findings also suggest predisposition to autoimmunity, e.g., decreased numbers of T regulatory lymphocytes, important regulators of immune tolerance,88 reduced IgG3 and IgA levels,86,87 and higher degree of maternal family history of autoimmune diseases.88 Finally, TS patients could be prone to common allergic illnesses, including asthma, atopic dermatitis, conjunctivitis, and rhinitis.89
TABLE 2. Summary of Evidence of Immunological Changes in Tourette Syndrome from Case–Control Cross-sectional or Case-only Prospective Studies with ≥10 Subjects per Group.
| References | |
|---|---|
| Gene expression profiling of peripheral blood mononuclear cells | |
| Age-related overexpression of genes related to natural killer cell pathways and regulation of anti-viral responses | 76, 77 |
| Cytokine expression | |
| Increased concentration of interleukin-12 and tumor necrosis factor- α in serum during symptom exacerbations | 82 |
| Increased concentration of interleukins 4, 5, 6, and 10 in serum during symptom exacerbations (only statistical trend) | 82 |
| Decreased concentration of monocyte-derived cytokines (interleukin 2 receptor antagonist, soluble CD14) | 78 |
| Immune cell subpopulations in peripheral blood | |
| Increased number of CD4+CD95+ and CD8+CD95+ T-cells | 84 |
| Increased number of CD69+ B-cells | 84 |
| Decreased number of T regulatory cells | 85 |
| Immunoglobulin synthesis | |
| Decreased concentration of serum IgG3 and IgA (the latter in patients fulfilling criteria for PANDAS) | 86, 87 |
| Oligoclonal bands of intrathecal synthesis in the cerebrospinal fluid of 40% of patients with Tourette syndrome | 90 |
| Past medical and family history | |
| Higher rate of maternal history of autoimmune diseases | 88 |
| Higher rate of past history of common allergic illnesses | 89 |
Abbreviations: PANDAS, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections.
Adapted from Martino D. Immunity and stress response in Tourette syndrome. In: Martino D, Leckman JF (editors). Tourette syndrome New York: Oxford University Press; 2013. p 301–328.
FIGURE 2. Hypothesized Immune Regulatory Abnormalities.
Summary diagram of the hypothesized immune regulatory abnormalities in patients with Tourette syndrome (see text for details).
The complex relationship between biological (including GABHS) and psychosocial stressors and the natural course of chronic tic disorders and related obsessive-compulsive symptoms is currently being explored by a pan-European study, which is following up hundreds of patients and unaffected relatives (www.emtics.eu). We also look forward to the results of ongoing novel transcriptomic analyses on post-mortem specimens of TS patients, which might provide a deeper insight into the role of immune competent cells in the central nervous system (CNS) in this condition.
Conclusions
Almost two decades after their first characterization, PANDAS (and, more generally, the whole group of “post-streptococcal neuropsychiatric disorders”—with the exception of SC) still need diagnostic criteria that are of relatively simple applicability and at the same time with a proven high reliability across raters. Advances in knowledge on this putative entity could have been hindered by the uncontrolled application of this diagnostic label to a “garden-variety” of patients with tics and OCS. The development of concepts such as PANS or CANS, if adequately integrated, may help in defining the boundaries of their “post-streptococcal” subgroup, but other approaches, such as focusing on a direct revision of the Swedo operational criteria for PANDAS, could also be rewarding. We believe this nosographical problem is a priority issue, and its solution may be crucial to improve our understanding of the prevalence and pathomechanisms of post-streptococcal acute neuropsychiatric disorders other than SC.
Footnotes
Funding: None.
Conflict of Interests: The authors report no conflict of interest.
Financial disclosures: D. Martino received honoraria for speaking engagements from Chiesi Farmaceutici, UCB pharma and the Movement Disorders Society, and served on the editorial advisory board of Frontiers in Movement Disorders.
References
- 1.Giovannoni G. PANDAS: overview of the hypothesis. Adv Neurol. 2006;99:159–165. [PubMed] [Google Scholar]
- 2.Swedo SE. Sydenham’s chorea (SC): a model for childhood autoimmune neuropsychiatric disorders. J Am Med Assoc. 1994;272:1788–1791. doi: 10.1001/jama.1994.03520220082035. [DOI] [PubMed] [Google Scholar]
- 3.Garvey MA, Giedd JN, Swedo SE. PANDAS: the search for environmental triggers of pediatric neuropsychiatric disorders. Lessons from rheumatic fever. J Child Neurol. 1998;13:413–423. doi: 10.1177/088307389801300901. [DOI] [PubMed] [Google Scholar]
- 4.Chapman AH, Pilkey L, Gibbons MJ. A psychosomatic study of eight children with Sydenham’s chorea. Pediatrics. 1958;21:582–595. [PubMed] [Google Scholar]
- 5.Freeman JM, Aron AM, Collard JE, Mackay MC. The emotional correlates of Sydenham’s chorea. Pediatrics. 1965;35:42–49. [PubMed] [Google Scholar]
- 6.Kondo K, Kabasawa T. Improvement in Gilles de la Tourette syndrome after corticosteroid therapy. Ann Neurol. 1978;4:387. doi: 10.1002/ana.410040423. [DOI] [PubMed] [Google Scholar]
- 7.Kiessling LS. Tic disorders associated with evidence of invasive group A beta-hemolytic streptococcal disease. Dev Med Child Neurol Suppl. 1989;59:48. [Google Scholar]
- 8.Swedo SE, Rapoport JL, Cheslow DL, et al. High prevalence of obsessive-compulsive symptoms in patients with Sydenham’s chorea. Am J Psychiatry. 1989a;146:246–249. doi: 10.1176/ajp.146.2.246. [DOI] [PubMed] [Google Scholar]
- 9.Swedo SE, Rapoport JL, Leonard H, Lenane M, Cheslow D. Obsessive-compulsive disorder in children and adolescents. Arch Gen Psychiatry. 1989b;46:335–341. doi: 10.1001/archpsyc.1989.01810040041007. [DOI] [PubMed] [Google Scholar]
- 10.Swedo SE, Leonard HL, Schapiro MB, et al. Sydenham’s chorea: physical and psychological symptoms of St Vitus dance. Pediatrics. 1993;91:706–713. [PubMed] [Google Scholar]
- 11.Allen AJ, Leonard HL, Swedo SE. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1995;34:307–311. doi: 10.1097/00004583-199503000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DR, Kurlan R, Leckman J, Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis. 2010;50:481–490. doi: 10.1086/650167. [DOI] [PubMed] [Google Scholar]
- 14.Gabbay V, Coffey BJ, Babb JS, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcus: comparison of diagnosis and treatment in the community and at a specialty clinic. Pediatrics. 2008;122:273–278. doi: 10.1542/peds.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurlan R, Johnson D, Kaplan EL; Tourette Syndrome Study Group Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. 2008;121:1188–1197. doi: 10.1542/peds.2007-2657. [DOI] [PubMed] [Google Scholar]
- 16.Leckman JF, King RA, Gilbert DL, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2011;50:108–118. doi: 10.1016/j.jaac.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy TK. Infections and tic disorders. In: Martino D, Leckman JF, . In. New York: Oxford University Press; 2013. pp. p. 168–201. editors). Tourette syndrome. [Google Scholar]
- 18.Bernstein GA, Victor AM, Pipal AJ, Williams KA. Comparison of clinical characteristics of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2010;20:333–340. doi: 10.1089/cap.2010.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK. Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr. 2012;160:314–319. doi: 10.1016/j.jpeds.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschtritt ME, Hammond CJ, Luckenbaugh D, et al. Executive and attention functioning among children in the PANDAS subgroup. Child Neuropsychol. 2009;15:179–194. doi: 10.1080/09297040802186899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin AB, Storch EA, Mutch PJ, Murphy TK. Neurocognitive functioning in youth with pediatric autoimmune neuropsychiatric disorders associated with streptococcus. J Neuropsychiatry Clin Neurosci. 2011;23:391–398. doi: 10.1176/appi.neuropsych.23.4.391. [DOI] [PubMed] [Google Scholar]
- 22.Arnold MH, Tyndall A. Poststreptococcal reactive arthritis. Ann Rheum Dis. 1989;48:686–688. doi: 10.1136/ard.48.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snider LA, Sachdev V, MaCkaronis JE, St Peter M, Swedo SE. Echocardiographic findings in the PANDAS subgroup. Pediatrics. 2004;114:e748–e751. doi: 10.1542/peds.2004-0308. [DOI] [PubMed] [Google Scholar]
- 24.Segarra AR, Murphy TK. Cardiac involvement in children with PANDAS. J Am Acad Child Adolesc Psychiatry. 2008;47:603–604. doi: 10.1097/CHI.0b013e3181676b82. [DOI] [PubMed] [Google Scholar]
- 25.Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R. Moving from PANDAS to CANS. J Pediatr. 2012;160:725–731. doi: 10.1016/j.jpeds.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome) Pediatr Therapeut. 2012;2:2. [Google Scholar]
- 27.Di Fazio MP, Morales J, Davis R. Acute myoclonus secondary to group A beta-hemolytic streptococcus infection: a PANDAS variant. J Child Neurol. 1998;13:516–518. doi: 10.1177/088307389801301010. [DOI] [PubMed] [Google Scholar]
- 28.Dale RC, Church AJ, Benton S, et al. Post-streptococcal autoimmune dystonia with isolated bilateral striatal necrosis. Dev Med Child Neurol. 2002;44:485–489. doi: 10.1111/j.1469-8749.2002.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 29.Dale RC, Church AJ, Surtees RA, Thompson EJ, Giovannoni G, Neville BG. Post-streptococcal autoimmune neuropsychiatric disease presenting as paroxysmal dystonic choreoathetosis. Mov Disord. 2002;17:817–820. doi: 10.1002/mds.10169. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo M, Tsuchiya K, Hamasaki Y, Singer HS. Restless legs syndrome: association with streptococcal or mycoplasms infection. Pediatr Neurol. 2004;31:119–121. doi: 10.1016/j.pediatrneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Squintani G, Tinazzi M, Gambarin M, et al. Post-streptococcal ‘complex’ movement disorders: unusual concurrence of psychogenic and organic symptoms. J Neurol Sci. 2010;288:68–71. doi: 10.1016/j.jns.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Dale RC, Heyman I, Surtees RAH, et al. Dyskinesias and associated psychiatric disorders following streptococcal infections. Arch Dis Child. 2004;89:604–610. doi: 10.1136/adc.2003.031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokol MS. Infection-triggered anorexia nervosa in children: clinical description of four cases. J Child Adolesc Psychopharmacol. 2000;10:133–145. doi: 10.1089/cap.2000.10.133. [DOI] [PubMed] [Google Scholar]
- 34.Dale RC, Church AJ, Cardoso F, et al. Poststreptococcal acute disseminated encephalomyelitis with basal ganglia involvement and auto-reactive antibasal ganglia antibodies. Ann Neurol. 2001;50:588–595. doi: 10.1002/ana.1250. [DOI] [PubMed] [Google Scholar]
- 35.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 36.Aran A, Lin L, Nevsimalova S, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–983. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das A, Radhakrishnan A. A case of PANDAS with Kleine-Levin type periodic hypersomnia. Sleep Med. 2012;13:319–320. doi: 10.1016/j.sleep.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Oosterveer DM, Overweg-Plandsoen WC, Roos RA. Sydenham’s chorea: a practical overview of the current literature. Pediatr Neurol. 2010;43:1–6. doi: 10.1016/j.pediatrneurol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Cardoso F. Sydenham’s chorea. Handb Clin Neurol. 2011;100:221–229. doi: 10.1016/B978-0-444-52014-2.00014-8. [DOI] [PubMed] [Google Scholar]
- 40.Baizabal-Carvallo JF, Jankovic J. Movement disorders in autoimmune diseases. Mov Disord. 2012;27:935–946. doi: 10.1002/mds.25011. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol. 2008;609:29–42. doi: 10.1007/978-0-387-73960-1_3. [DOI] [PubMed] [Google Scholar]
- 42.Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- 43.Singer HS, Hong JJ, Yoon DY, Williams PN. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology. 2005;65:1701–1707. doi: 10.1212/01.wnl.0000183223.69946.f1. [DOI] [PubMed] [Google Scholar]
- 44.Singer HS, Gause C, Morris C, Lopez P; Tourette Syndrome Study Group Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics. 2008;121:1198–1205. doi: 10.1542/peds.2007-2658. [DOI] [PubMed] [Google Scholar]
- 45.Martino D, Dale RC, Gilbert DL, Giovannoni G, Leckman JF. Immunopathogenic mechanisms in Tourette syndrome: a critical review. Mov Disord. 2009;24:1267–1279. doi: 10.1002/mds.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brimberg L, Benhar I, Mascaro-Blanco A, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37:2076–2087. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signalling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Brilot F, Merheb V, Ding A, Murphy T, Dale RC. Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology. 2011;76:1508–1513. doi: 10.1212/WNL.0b013e3182181090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor JR, Morshed SA, Parveen S, et al. An animal model of Tourette’s syndrome. Am J Psychiatry. 2002;159:657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- 50.Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioral changes. Mov Disord. 2004;19:390–396. doi: 10.1002/mds.10522. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004;24:1780–1791. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaddanapudi K, Hornig M, Serge R, et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. 2010;15:712–726. doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]
- 53.Chen SK, Tvrdik P, Peden E, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS) Arch Pediatr Adolesc Med. 2002;156:356–361. doi: 10.1001/archpedi.156.4.356. [DOI] [PubMed] [Google Scholar]
- 55.Garvey MA, Perlmutter SJ, Allen AJ, et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry. 1999;45:1564–1571. doi: 10.1016/S0006-3223(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 56.Snider LA, Lougee L, Slattery M, Grant P, Swedo SE. Antibiotic prophylaxis with azithromycin or penicillin for childhood onset neuropsychiatric disorders. Biol Psychiatry. 2005;57:788–792. doi: 10.1016/j.biopsych.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Orvidas LJ, Slattery MJ. Pediatric autoimmune neuropsychiatric disorders and streptococcal infections: role of otolaryngologist. Laryngoscope. 2001;111:1515–1519. doi: 10.1097/00005537-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Heubi C, Shott SR. PANDAS: pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections—an uncommon, but important indication for tonsillectomy. Int J Pediatr Otorhinolaryngol. 2003;67:837–840. doi: 10.1016/S0165-5876(03)00158-7. [DOI] [PubMed] [Google Scholar]
- 59.Batuecas Calerio A, Sanchez Gonzalez F, Santa Cruz Ruiz S, Santos Gorjon P, Blanco Perez P. PANDAS Syndrome: a new tonsillectomy indication? Acta Otorrinolaringol Esp. 2008;59:362–363. doi: 10.1016/S0001-6519(08)75557-2.. in Spanish. [DOI] [PubMed] [Google Scholar]
- 60.Arostegui S, Aguero JA, Escar C. PANDAS following amygdalectomy. An Sist Sanit Navar. 2003;26:287–290. doi: 10.23938/ASSN.0456. [DOI] [PubMed] [Google Scholar]
- 61.Murphy TK, Lewin AB, Parker-Athill EC, Storch EA, Mutch PJ. Tonsillectomies and adenoidectomies do not prevent the onset of pediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Pediatr Infect Dis J. 2013;32:834–838. doi: 10.1097/INF.0b013e31829062e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- 63.Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette’s disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. J Child Adolesc Psychopharmacol. 2010;20:317–331. doi: 10.1089/cap.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storch EA, Murphy TK, Geffken GR, et al. Cognitive-behavioral therapy for PANDAS-related obsessive-compulsive disorder: findings from a preliminary waitlist controlled open trial. J Am Acad Child Adolesc Psychiatry. 2006;45:1171–1178. doi: 10.1097/01.chi.0000231973.43966.a0. [DOI] [PubMed] [Google Scholar]
- 65.Hoekstra PJ, Manson WL, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of common cold with exacerbations in pediatric but not adult patients with tic disorder: a prospective longitudinal study. J Child Adolesc Psychopharmacol. 2005;15:285–292. doi: 10.1089/cap.2005.15.285. [DOI] [PubMed] [Google Scholar]
- 66.Muller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele-Horn M. Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 2004;129:119–125. doi: 10.1016/j.psychres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Riedel M, Straube A, Schwarz MJ, Wilske B, Muller N. Lyme disease presenting as Tourette’s syndrome. Lancet. 1998;351:418–419. doi: 10.1016/S0140-6736(05)78357-4. [DOI] [PubMed] [Google Scholar]
- 68.Turley JM. Tourette-like disorder after herpes encephalitis. Am J Psychiatry. 1988;145:1604–1605. doi: 10.1176/ajp.145.12.1604a. [DOI] [PubMed] [Google Scholar]
- 69.Martino D, Chiarotti F, Buttiglione M, et al. The relationship between group A streptococcal infections and Tourette syndrome: a study on a large service-based cohort. Dev Med Child Neurol. 2011;53:951–957. doi: 10.1111/j.1469-8749.2011.04018.x. [DOI] [PubMed] [Google Scholar]
- 70.Bombaci M, Grifantini R, Mora M, et al. Protein array profiling of tic patient sera reveals a broad range and enhanced immune response against Group A Streptococcus antigens. PLoS One. 2009;4:e6332. doi: 10.1371/journal.pone.0006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics. 2005;116:56–60. doi: 10.1542/peds.2004-2058. [DOI] [PubMed] [Google Scholar]
- 72.Leslie DL, Kozma L, Martin A, et al. Neuropsychiatric disorders associated with streptococcal infection: a case-control study among privately insured children. J Am Acad Child Adolesc Psychiatry. 2008;47:1166–1172. doi: 10.1097/CHI.0b013e3181825a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schrag A, Gilbert R, Giovannoni G, Robertson MM, Metcalfe C, Ben-Shlomo Y. Streptococcal infection, Tourette syndrome, and OCD: is there a connection? Neurology. 2009;73:1256–1263. doi: 10.1212/WNL.0b013e3181bd10fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy TK, Sajid M, Soto O, et al. Detecting pediatric autoimmune neuropsychiatric disorders associated with streptococcus in children with obsessive-compulsive disorder and tics. Biol Psychiatry. 2004;55:61–68. doi: 10.1016/S0006-3223(03)00704-2. [DOI] [PubMed] [Google Scholar]
- 75.Lin H, Williams KA, Katsovich L, et al. Streptococcal upper respiratory tract infections and psychosocial stress predict future tic and obsessive-compulsive symptom severity in children and adolescents with Tourette syndrome and obsessive-compulsive disorder. Biol Psychiatry. 2010;67:684–691. doi: 10.1016/j.biopsych.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lit L, Gilbert DL, Walker W, Sharp FR. A subgroup of Tourette’s patients overexpress specific natural killer cell genes in blood: a preliminary report. Am J Med Genet B. 2007;144B:958–963. doi: 10.1002/ajmg.b.30550. [DOI] [PubMed] [Google Scholar]
- 77.Lit L, Enstrom A, Sharp FR, Gilbert DL. Age-related gene expression in Tourette syndrome. J Psychiatr Res. 2009;43:319–330. doi: 10.1016/j.jpsychires.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matz J, Krause DL, Dehning S, et al. Altered monocyte activation markers in Tourette’s syndrome: a case-control study. BMC Psychiatry. 2012;12:29. doi: 10.1186/1471-244X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krause D, Matz J, Weidinger E, et al. Association between intracellular infectious agents and Tourette’s syndrome. Eur Arch Psychiatry Clin Neurosci. 2010;260:359–363. doi: 10.1007/s00406-009-0084-3. [DOI] [PubMed] [Google Scholar]
- 80.Luo F, Leckman JF, Katsovich L, et al. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics. 2004;113:e578–585. doi: 10.1542/peds.113.6.e578. [DOI] [PubMed] [Google Scholar]
- 81.Hoekstra PJ, Anderson GM, Troost PW, et al. Plasma kynurenine and related measures in tic disorder patients. Eur Child Adolesc Psychiatry. 2007;16(Suppl. 1):71–77. doi: 10.1007/s00787-007-1009-1. [DOI] [PubMed] [Google Scholar]
- 82.Leckman JF, Katsovich L, Kawikova I, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol Psychiatry. 2005;57:667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Hoekstra PJ, Bijzet J, Limburg PC, et al. Elevated binding of D8/17-specific monoclonal antibody to B lymphocytes in tic disorder patients. Am J Psychiatry. 2004;161:1501–1502. doi: 10.1176/appi.ajp.161.8.1501-a. [DOI] [PubMed] [Google Scholar]
- 84.Moller JC, Tackenberg B, Heinzel-Gutenbrunner M, et al. Immunophenotyping in Tourette syndrome—a pilot study. Eur J Neurol. 2008;15:749–753. doi: 10.1111/j.1468-1331.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 85.Kawikova I, Leckman JF, Kronig H, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with tourette syndrome: a preliminary study. Biol Psychiatry. 2007;61:273–278. doi: 10.1016/j.biopsych.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Bos-Veneman NG, Olieman R, Tobiasova Z, et al. Altered immunoglobulin profiles in children with Tourette syndrome. Brain Behav Immun. 2011;25:532–538. doi: 10.1016/j.bbi.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawikova I, Grady BP, Tobiasova Z, et al. Children with Tourette’s syndrome may suffer immunoglobulin A dysgammaglobulinemia: preliminary report. Biol Psychiatry. 2010;67:679–683. doi: 10.1016/j.biopsych.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy TK, Storch EA, Turner A, Reid JM, Tan J, Lewin AB. Maternal history of autoimmune disease in children presenting with tics and/or obsessive-compulsive disorder. J Neuroimmunol. 2010;229:243–247. doi: 10.1016/j.jneuroim.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang YT, Li YF, Muo CH, et al. Correlation of Tourette syndrome and allergic disease: nationwide population-based case-control study. J Dev Behav Pediatr. 2011;32:98–102. doi: 10.1097/DBP.0b013e318208f561. [DOI] [PubMed] [Google Scholar]
- 90.Wenzel C, Wurster U, Muller-Vahl KR. Oligoclonal bands in cerebrospinal fluid in patients with Tourette’s syndrome. Mov Disord. 2011;26:343–346. doi: 10.1002/mds.23403. [DOI] [PubMed] [Google Scholar]