Abstract
Epidemiological studies have found that low 25-hydroxyvitamin D levels may be associated with coronary risk factors and adverse cardiovascular outcomes. Additionally, vitamin D deficiency causes an increase in parathyroid hormone, which increases insulin resistance and is associated with diabetes, hypertension, inflammation, and increased cardiovascular risk. In this review, we analyze the association between vitamin D supplementation and the reduction in cardiovascular disease. The role of vitamin D deficiency in cardiovascular morbidity and mortality is still controversial, and larger scale, randomized placebo controlled trials are needed to investigate whether oral vitamin D supplementation can reduce cardiovascular risk. Given the low cost, safety, and demonstrated benefit of higher 25-hydroxyvitamin D levels, vitamin D supplementation should become a public health priority for combating common and costly chronic cardiovascular diseases.
Keywords: Cardiovascular disease, Morbidity, Mortality, Review, Vitamin D
Core tip: We performed an extensive review to determine whether vitamin D supplementation reduces cardiovascular risk. Only double-blind, placebo- and randomized-controlled trials were included. The role of vitamin D deficiency in cardiovascular morbidity and mortality is still controversial, and larger scale, randomized placebo controlled trials are underway to address this issue. These results from these studies will likely not be available for another 3-5 years. At this stage, we propose recommendations for preventing of vitamin D deficiency and conclude that there is a benefit to vitamin D supplementation.
INTRODUCTION
Vitamin D is likely one of the oldest hormones, having existed for at least 750 million years[1]. Studies have demonstrated that low levels of vitamin D represent a problem of global dimensions[2-13]. A recent Workshop Consensus for Vitamin D Nutritional Guidelines estimated that approximately 50% and 60% of the elderly in North America and the rest of the world, respectively, do not have satisfactory vitamin D levels[14]. The situation is similar in younger subjects. Reasons for this widespread deficiency remain unclear but are likely related to factors such as urbanization, demographic shifts, decreased outdoor activity, air pollution and global dimming, as well as decreases in the cutaneous production of vitamin D with age. Epidemiological pooled analysis of prospective observational studies of diverse populations demonstrates that hypovitaminosis D is associated with a modest risk of cardiovascular events[15-20]. The amount of vitamin D obtained from dietary sources is generally viewed as too low in many regions of the world to have an effect on the vitamin D status at the population level[14]. This review introduces the general concept of vitamin D, defines vitamin D deficiency, evaluates the relationship between vitamin D deficiency and cardiovascular disease, proposes a recommendation for preventing vitamin D deficiency and offers conclusions.
NATURE OF VITAMIN D
There are 2 major forms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is found in plants and can be consumed in fortified foods or as a supplement. Vitamin D3 is obtained from either dietary sources or through the conversion of 7-dehydrocholesterol in the skin upon exposure to ultraviolet B (UVB) radiation[10,21]. Vitamin D3 from the skin is bound to the vitamin D-binding protein, whereas vitamin D2 and vitamin D3 from diet are bound to vitamin D-binding protein and lipoproteins. Both forms are hydroxylated in the liver to 25-hydroxyvitamin D [25(OH)D; D represents D2 or D3]. However, 25(OH)D is inactive and requires hydroxylation in the kidney to form 1,25-dihydroxyvitamin D[1,25(OH)2D, calcitriol]. Calcitriol [1,25(OH)2D] maintains calcium in the blood and has an array of effects on the body’s organs. Calcitriol acts in an endocrine manner to regulate calcium metabolism by enhancing intestinal calcium absorption and mobilizing calcium from the skeleton[10,19,22,23]. Although 1,25(OH)2D is considered to be the active form of vitamin D, its levels in the serum do not correlate with overall vitamin D status, whereas the 25(OH)D levels is a more clinically relevant marker[24]. Vitamin D activity is measured in μg of 25(OH)D (1 μg = 40 International Units, IU). The minimum desirable serum level of 25(OH)D has been suggested to be 20-30 ng/mL according to the consensus conference[14].
Dietary sources of vitamin D are limited to fatty fish (wild or farm salmon, mackerel, tuna fish, sardines, and cod liver oil) and products fortified with vitamin D, which include dairy products, cereals, margarine, flour, and orange juice[24,25].
DEFINITION OF VITAMIN D DEFICIENCY
Several measures have been used to define vitamin D deficiency, insufficiency, and adequacy. A 25(OH)D of < 20 ng/mL is associated with suppressible levels of parathyroid hormone when challenged with pharmacologic dosages of vitamin D[26]. Parathyroid hormone levels begin to reach their nadir when the 25(OH)D levels are > 30 ng/mL[27,28]. Intestinal calcium absorption in adults is maximized when 25(OH)D is > 30 ng/mL[29]. Thus, many experts define vitamin D deficiency, insufficiency, and sufficiency as levels of < 20, 21 to 29, and > 30 ng/mL, respectively. To achieve these levels, a minimum of 1000 IU of vitamin D2 or vitamin D3 is needed daily when sun exposure is either unavailable or inadequate for producing vitamin D3, such as during the winter or when a sunscreen is used[30,31].
In the United States, Europe, India, Asia, Middle East, New Zealand, and Australia, vitamin D deficiency is common in pregnant women, newborns, young and adolescent children, and the elderly[32-37]. Serum vitamin D levels are lower in European young adults than in North American young adults during winter[37]. Vitamin D deficiency is especially common in people of color or who avoid sunlight[38].
RELATIONSHIP BETWEEN VITAMIN D DEFICIENCY AND CARDIOVASCULAR DISEASE
Numerous studies have found high rates of CV diseases among patients with lower levels of vitamin D. More recently, low levels of 25(OH)D have been linked to the presence of cardiovascular disease, hypertension, and the metabolic syndrome[39-42]. It is still unclear whether supplementation with vitamin D is beneficial to cardiovascular health. To this end, we have performed an extensive survey of published studies. Only double-blinded and randomized controlled trials (RCT) were included. The databases searched include MEDLINE, EMBASE, and PUBMED from January 1966 to May 2013. We selected search terms that capture generic and specific words relevant to the exposure and outcome on the basis of Medical Subject Heading terms and text words from a priori identified key articles. The terms selected for vitamin D were the following: “vitamin D intake, vitamin D supplement, calcidiol, calcitriol, cholecalciferol, and ergocalciferol”. The terms selected for cardiovascular disease (CVD) were the following: “cardiovascular disease, ischemic heart disease, coronary artery disease, cardiovascular mortality, myocardial infarction, and stroke”. We restricted the search to articles published in English and studies of humans that double-blinded and RCT. We applied the same search strategy to each database. Because of the limitations in assessing cause-effect relationships, we excluded ecological, cross-sectional, and retrospective case-control studies. By screening abstracts, we also excluded case reports, studies of vitamin D combination treatment (e.g., combined vitamin D + calcium supplementation), and studies that did not assess the use of vitamin D supplementations. We retrieved articles that passed the abstract screening test for a full-text review, and we further excluded review articles, editorials, or letters to editors as well as studies lacking a comparison between participants who received vitamin D supplementation and non-recipients.
After the abstract screening and full-text review, we selected 19 eligible articles. Ten articles favored beneficial cardiovascular effects after supplementation with vitamin D (Table 1)[43-52]. A trial in the United States randomly assigned 283 African American subjects into a 4-arm, double-blind trial of placebo, 1000, 2000, or 4000 IU of oral cholecalciferol per day. At baseline and 3 mo, the systolic and diastolic pressure and 25(OH)D were measured. This study found that although cholecalciferol supplementation did not affect the diastolic pressure (P = 0.37), the difference in systolic pressure between baseline and 3 mo was +1.7 mmHg for those receiving placebo, -0.66 mmHg for 1000 U/d, -3.4 mmHg for 2000 U/d, and -4.0 mmHg for 4000 U/d of cholecalciferol (-1.4 mmHg for each additional 1000 U/d of cholecalciferol; P = 0.04). For each 1-ng/mL increase in the plasma 25(OH)D, there was a significant 0.2-mmHg reduction in the systolic pressure (P = 0.02)[43]. Larsen et al[45] investigated the effect of 3000 IU vitamin D per day for 20 wk in a randomized, placebo-controlled, double-blind study in 130 hypertensive patients residing in Denmark. Vitamin D supplementation reduced the systolic pressure significantly. In a post-hoc subgroup analysis of 92 subjects with baseline p-25(OH)D levels < 32 ng/mL, significant decreases in the 24-h systolic and diastolic BP were observed in response to cholecalciferol supplementation[45]. Similar reports[44,46-52] relevant to “vitamin D supplementation produces beneficial cardiovascular effects” are summarized in Table 1.
Table 1.
Double-blind, placebo- and randomized-controlled trials that favor supplement with vitamin D may have a beneficial cardiovascular effects
| Ref. | Country | Participants | Intervention | Duration of follow-up | Results |
| Forman et al[43] | United States | 283 African-American | Oral vitamin D3 (cholecalciferol, 1000, 2000, or 4000 IU), or placebo per day for 3 mo | 6 mo | Reduction in systolic pressure. |
| subjects | |||||
| Harris et al[44] | United States | 45 African-American adults | 60000 IU monthly oral vitamin D(3) or placebo for 16 wk | 16 wk | Effective at improving vascular endothelial function |
| Larsen et al[45] | Denmark | 112 Hypertensive patients | 75 µg (3000 IU) cholecalciferol per day or placebo for 20 wk | 20 wk | Significant decreases in systolic blood pressure |
| Lind et al[46] | Sweden | 65 subjects with impaired glucose tolerance | Alphacalcidol (0.75 microgram daily) or placebo over 12 wk | 12 wk | Significant reduction of blood pressure |
| Lind et al[47] | Sweden | 65 Hypertensive patients with primary hyperparathyroidism | Alphacalcidol, (1 microgram daily) or placebo over 6 mo | 6 mo | Significant reduction of blood pressure |
| Longenecker et al[48] | United States | 45 HIV-infected individuals with vitamin D deficiency | Vitamin D3 4000 IU daily or placebo for 12 wk | 12 wk | Modestly improved cholesterol |
| Salehpour et al[49] | Iran. | 77 healthy premenopausal overweight and obese women | Vitamin D (25 μg/d as cholecalciferol) or the placebo group for 12 wk | 12 wk. | Significantly improvement of HDL-cholesterol, apoA-I |
| concentrations and LDL-cholesterol:apoB-100 ratio. | |||||
| Shedeed[50] | Egypt | 80 infants with CHF | Vitamin D(3) oral drops or placebo oral drops for 12 wk | 12 wk | Significant improvement of HF score, LV end-diastolic diameter, LV end-systolic diameter, LV ejection fraction%, and myocardial performance index. |
| Witham et al[51] | United Kingdom | 58 stroke patients | 100000 units of a single oral dose of vitamin D2 or placebo | 16 wk | Short-term improvement in endothelial function (Flow mediated dilatation was significantly higher in the intervention group at 8 wk) |
| Zittermann et al[52] | Germany | 200 healthy overweight subjects in a weight-reduction program | Vitamin D (83 microg/d) or placebo for 12 mo | 12 mo | Significant improvement of cardiovascular disease risk markers |
LV: Left-ventricular; CHF: Congestive heart failure; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; IU: International units.
In contrast, the remaining nine articles did not find a cardioprotective effect of vitamin D supplementation (Table 2)[53-61]. In Ireland, 202 healthy adults (20-40 years old) and 192 healthy elders (≥ 64 years old) were recruited and received vitamin D supplementation at a dosage of 0, 200, 400, or 600 IU for 22 wk. Serum 25(OH)D, intact parathyroid hormone, systolic and diastolic blood pressure, fasting lipids, glucose and insulin, high-sensitivity CRP, matrix metalloproteinase-9, and its inhibitor (tissue inhibitor metalloproteinase-1) were measured at baseline and 22 wk later, which was the endpoint. This study revealed that there were no significant effects of supplementation on the CVD risk biomarkers in either age group[56]. Wood et al[60] conducted a parallel-group, double-blind, placebo- and randomized-controlled trial in 305 healthy postmenopausal women to test whether daily doses of vitamin D3 at 400 or 1000 IU/d for 1 year affected the conventional markers of cardiovascular disease risk. The serum lipid profile (total, high-density lipoprotein, and low-density lipoprotein cholesterol; triglycerides; and apolipoproteins A-1 and B100), insulin resistance (homeostatic model assessment), inflammatory biomarkers (high-sensitivity C-reactive protein, IL-6, and soluble intracellular adhesion molecule-1), and blood pressure were studied. They found that dietary vitamin D supplementation is unlikely to reduce CVD risk factors, such as serum lipid profile, insulin resistance, inflammatory biomarkers, and blood pressure[60]. Additional reports that did not find a cardioprotective effect of vitamin D supplementation are summarized in Table 2[53-55,57-59,61].
Table 2.
Double-blind, placebo- and randomized-controlled trials that do not favor supplementation with vitamin D
| Ref. | Country | Participants | Intervention | Duration of follow-up | Results |
| Gepner et al[53] | United States | 114 post-menopausal women | Vitamin D3 2500 IU or placebo, daily for 4 mo | 4 mo | No significant effects of vitamin D supplementation to reduce cardiovascular disease risk |
| Jorde et al[54] | Norway | 330 overweight or obese subjects | Vitamin D [cholecalciferol, vitamin D(3)] 40000 IU, vitamin D 20000 IU, or placebo per week for 1 yr | 1 yr | No significant effect of vitamin D on glucose tolerance, blood pressure or serum lipids |
| Marckmann et al[55] | Denmark | 52 chronic kidney disease patients with vitamin D deficiency | 40000 IU of cholecalciferol orally per week for 8-wk | 8 wk | No significant impact on functional markers and plasma concentrations of biomarkers related to cardiovascular disease |
| Muldowney et al[56] | Ireland | 394 healthy participants | Cholecalciferol at doses of 0, 5, 10 , or 15 μg/d (0-600 IU) for 22 wk | 22 wk | No significant effects of supplementation on CVD risk biomarkers |
| Scragg et al[57] | United Kingdom | 95 elderly adults | A single oral dose of 2.5 mg cholecalciferol or placebo | 5 wk | No significant effect of vitamin D supplementation to change blood pressure or serum cholesterol |
| Stricker et al[58] | Switzerland | 62 peripheral arterial disease patients with vitamin D deficiency | A single, oral supplementation of 100000 IU vitamin D3 or placebo | 1 mo | Unlikely to influence endothelial function, arterial stiffness, coagulation and inflammation |
| Thadhani et al[59] | United States | 227 patients with chronic kidney disease | Paricalcitol or placebo over 48 wk | 48 wk | Unlikely to alter left ventricular mass index or improve certain measures of diastolic dysfunction |
| Wood et al[60] | United Kingdom | 305 healthy postmenopausal women | A daily capsule of 400 or 1000 IU vitamin D(3) or placebo for 12 mo | 12 mo | Unlikely to reduce CVD risk factors |
| Yiu et al[61] | Hong Kong | 100 patients with type 2 DM | Oral vitamin D (5000 IU/d) or placebo per day for 12 wk | 12 wk | No significant effect on vascular function or serum biomarkers of inflammation and oxidative stress |
CVD: Cardiovascular disease; IU: International units; DM: Diabetes mellitus.
MECHANISMS FOR THE CARDIOVASCULAR EFFECTS OF VITAMIN D DEFICIENCY
The results of recent nationwide investigations showed an association between low 25(OH)D levels and important cardiovascular risk factors[40,62], and further supported the findings of preclinical and clinical investigations that demonstrated positive effects of vitamin D and its analogues on fibrinolysis, blood lipids, thrombogenicity, endothelial regeneration, and smooth muscle cell growth[63-69]. Together, these findings strongly suggest that 25(OH)D has beneficial effects, some involving the cardiovascular system, that are independent of calcium metabolism. Several mechanisms might be responsible for the protective effect of calcitriol on atherosclerotic lesions and vascular calcification (Figure 1). First, vascular smooth cells express vitamin D receptors. Calcitriol inhibits proliferation of these cells with an acute influx of calcium into the cells[69]. Second, a lack of calcitriol results in an increase in the serum parathyroid hormone (PTH) levels. Excess PTH levels may at least in part promote cardiovascular disease by increased the cardiac contractility and myocardial calcification[70]. Third, experimental studies have shown that calcitriol suppresses the release of the inflammatory cytokines such as tissue necrosis factor-α (TNF-α), IL-6, and IL-10. There is now increasing evidence that inflammatory processes play an important role in the development of a vascular insult[71-88]. Fourth, calcitriol is a negative endocrine regulator of the rennin-angiotensin-aldosterone system (RAAS) The RAAS plays a central role in the regulation of blood pressure, electrolytes, and volume hemostasis. Calcitriol treatment reduces blood pressure, plasma rennin activity and angiotensin IIlevels[89]. Fifth, vascular smooth muscle cell proliferation and migration, as well as the osteogeneic processes may contribute to the vascular calcification, which may eventually cause the thrombogenesis[90]. Sixth, vitamin D plays a role in the insulin sensitivity, which has a role in diabetes and in metabolic syndrome[78,90].
Figure 1.
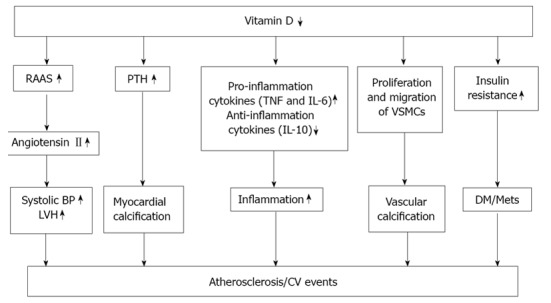
Potential mechanisms for cardiovascular effects of vitamin D deficiency. The data were modified from References 51, 52, and 54. RAAS: Renin-angiotensin-aldosterone system; PTH: Parathyroid hormone; BP: Blood pressure; LVH: Left ventricular hypertrophy; TNF: Tumor necrosis factor; IL-6: Interleukin-6; VSMCs: Vascular smooth muscle cells; DM: Diabetes mellitus; MetS: Metabolic syndrome; CV: Cardiovascular.
Essential hypertension is related to several disturbances in the systemic and cellular calcium metabolism. Extracellular ionized or ultrafiltrable calcium levels are decreased while intracellular cytosolic calcium concentrations are increased. Dietary calcium intake is often lower and renal calcium loss is higher in hypertensive than in normotensive subjects. Epidemiologic studies have demonstrated an inverse association between serum 25(OH)D levels and diastolic blood pressure[91]. Moreover, Afro-Americans have a significantly higher prevalence of diastolic hypertension and have lower 25(OH)D levels compared with white Americans[88,92,93]. In clinical trials, the daily administration of 5 μg of vitamin D showed no effects on blood pressure in normotensive subjects. However, some studies have demonstrated a blood pressure lowering effect with 0.75 or 1.0 μg vitamin D/d in hypertensive patients[94]. Short-term supplementation with 20 μg of vitamin D/d significantly reduced diastolic blood pressure. A reduction in the diastolic and systolic blood pressure was observed in mildly hypertensive patients after 6 wk of UV-B exposure[88,94]. A normalization of the enhanced intracellular calcium levels seems to be an important measure for reducing blood pressure, which can explain the therapeutic effects of calcium-channel blockers in hypertensive patients[95,96]. Low adenylate cyclase activity can result in a decreased calcium re-uptake into the sarcoplasmic reticulum and can contribute to an accumulation of intracellular free calcium and to an increase in vascular reactivity and blood pressure[97]. Activity of the intracellular adenylate cyclase is calcitriol-dependent and improvement of the activity of this enzyme may thus reduce free cellular calcium concentrations.
Hyperlipidemia, diabetic mellitus, and an increase in blood coagulation factors, blood viscosity, and leukocyte counts are important risk factors for the development of arteriosclerosis. There is now increasing evidence that arteriosclerosis is a low-grade systemic inflammatory disease. An increase in serum C-reactive protein levels is an important indicator of inflammatory reactions and also of the risk of developing arteriosclerosis[98]. The synthesis of C-reactive protein is regulated by IL-6 and IL-10 as well as TNF-α[73,99]. Animal studies have demonstrated that IL-6 and IL-10 accelerate arteriosclerosis[100]. Calcitriol can suppress the secretion of TNF-α and IL-6 in vitro in a dose-dependent manner[101]. A recent study identified an inverse association between TNF-α and 25(OH)D levels in human subjects[102].
PREVENTION OF VITAMIN D INSUFFICIENCY
Preventive measures must take into account that there is a high risk of vitamin D insufficiency in the whole population during winter and that the elderly population, especially institutionalized subjects, are at an increased risk for vitamin D insufficiency or even deficiency. There are two prevention models available: increased exposure to ultraviolet light and increased oral vitamin D intake. Sunlight provides the most potent source of vitamin D, with approximately 3000 IU vitamin D3 for 5 to 10 min of mid-day, mid-year exposure of the arms and legs for a light-skinned Caucasian[10]. Adequate daily oral vitamin D intake could be an easy and effective measure for maintaining a physiological vitamin D status. In November 2010, the Institute of Medicine of the National Academies of United States provided an update to the recommended intakes of calcium and vitamin D. For vitamin D intake, the committee assumed that North Americans need on average 400 IU of vitamin D daily; people 71 years old and older may require as much as 800 IU per day[103]. However, nutrition experts have suggested that vitamin D intake of 800 to 2000 IU daily may be needed. These doses are quite difficult to obtain without routine supplementation, particularly in areas with extreme winter climates and higher latitudes[104]. The United States Food and Drug Administration reported that a dose of 2000 IU daily is safe[21]. The Institute of Medicine of the National Academies has recently suggested a new tolerable upper intake level of only 4000 IU of vitamin D per day for the general adult population[103] because of the concern about potential toxicity at higher levels of 25(OH)D[103-106]. However, currently there is no recommended daily intake dose for vitamin D. For a practical approach, maintenance therapy can be continued by routine sunlight exposure or by administering vitamin D supplements, 800 to 2000 IU vitamin D3 daily or 50000 IU of either D2 or D3 every 2 wk[10,21,107].
RECOMMENDATIONS
It is still unproven whether supplementation with vitamin D reduces the cardiovascular risks. Autier et al[108] analyzed 18 independent randomized controlled trials of more than 57000 participants with mean follow-up of 5.7 years. Although there was considerable variability in the dose of vitamin D administered (from 300 to 2000 IU daily), the summary relative risk for all-cause mortality was reduced by 7% with vitamin D therapy[109]. Wang et al[106] performed a meta-analysis of 8 randomized trials, showing a slight, but statistically nonsignificant, 10% reduction in CV disease risk with vitamin D supplementation at moderate to high doses (approximately 1000 IU daily). Another meta-analysis evaluated the relationship between vitamin D levels and cardiovascular risk and reported that vitamin D was associated with nonsignificant effects on the patients’ death, myocardial infarction and stroke rates[109]. However, this study did not focus on the effect of vitamin D supplementation in the reduction of cardiovascular risks. At the present stage, we still feel confident that the benefits of vitamin D will likely outweigh the risks. A large double-blind randomized placebo-controlled trial (Vitamin D and Omega-3 Trial, VITAL) sponsored by the National Institutes of Health and run by Harvard Medical School and the Brigham and Women’s Hospital is underway[110]. This study should help to determine whether increasing low vitamin D levels will reduce the risk of CV events, depression, and death. O’Keefe et al[111] have claimed that several large scale trials have just started but the results of these trials will not be available for another 3-5 years or more; in the meantime, they recommend a daily intake of 1500 to 2000 IU of vitamin D3 for most American adults.
CONCLUSION
On the basis of this review, hypovitaminosis D has been observed worldwide, and many studies have demonstrated a strong association between vitamin D status and cardiovascular disease risk factors, including hypertension, diabetes, metabolic syndrome and inflammation. In the meantime, health professionals should be aware of the potential negative implications of vitamin D insufficiency and make recommendations for their patients to improve their vitamin D status. We suggest that to maintain health in younger and older adults and prevent hypertension, chronic heart diseases, and cardiovascular events, an increase in the current recommended intake of vitamin D is warranted. However, definitive randomized controlled trials are still needed to determine whether vitamin D therapy is beneficial to preventing cardiovascular disease. Given the low cost, safety, and demonstrated benefits of higher 25(OH)D concentration, vitamin D supplementation should become a public health priority to combat these common and costly chronic cardiovascular diseases.
Footnotes
Supported by (in part) the Kaohsiung Veterans General Hospital, No. VGHKS100-032
P- Reviewers Georgescu A, Su H S- Editor Song XX L- Editor A E- Editor Lu YJ
References
- 1.Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3:1548–1554. doi: 10.2215/CJN.01350308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11:847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 5.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 7.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 13.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 14.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleck A. Latitude and ischaemic heart disease. Lancet. 1989;1:613. doi: 10.1016/s0140-6736(89)91634-6. [DOI] [PubMed] [Google Scholar]
- 16.Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- 17.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 18.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 19.Sokol SI, Tsang P, Aggarwal V, Melamed ML, Srinivas VS. Vitamin D status and risk of cardiovascular events: lessons learned via systematic review and meta-analysis. Cardiol Rev. 2011;19:192–201. doi: 10.1097/CRD.0b013e31821da9a5. [DOI] [PubMed] [Google Scholar]
- 20.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 21.Lüderitz B, Holmes DR, Harold J. The history of the German Cardiac Society and the American College of Cardiology and their two founders. J Am Coll Cardiol. 2013;61:802–807. doi: 10.1016/j.jacc.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 24.Mosekilde L. Vitamin D requirement and setting recommendation levels: long-term perspectives. Nutr Rev. 2008;66:S170–S177. doi: 10.1111/j.1753-4887.2008.00103.x. [DOI] [PubMed] [Google Scholar]
- 25.Holden JM, Lemar LE. Assessing vitamin D contents in foods and supplements: challenges and needs. Am J Clin Nutr. 2008;88:551S–553S. doi: 10.1093/ajcn/88.2.551S. [DOI] [PubMed] [Google Scholar]
- 26.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 27.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 29.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 30.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 32.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 33.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie MA, Singh S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 34.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int. 2006;17:441–446. doi: 10.1007/s00198-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 36.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 37.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 38.Sedrani SH. Low 25-hydroxyvitamin D and normal serum calcium concentrations in Saudi Arabia: Riyadh region. Ann Nutr Metab. 1984;28:181–185. doi: 10.1159/000176801. [DOI] [PubMed] [Google Scholar]
- 39.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 41.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 43.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens. 2012;25:1215–1222. doi: 10.1038/ajh.2012.111. [DOI] [PubMed] [Google Scholar]
- 46.Lind L, Lithell H, Skarfors E, Wide L, Ljunghall S. Reduction of blood pressure by treatment with alphacalcidol. A double-blind, placebo-controlled study in subjects with impaired glucose tolerance. Acta Med Scand. 1988;223:211–217. [PubMed] [Google Scholar]
- 47.Lind L, Wengle B, Wide L, Sörensen OH, Ljunghall S. Hypertension in primary hyperparathyroidism--reduction of blood pressure by long-term treatment with vitamin D (alphacalcidol). A double-blind, placebo-controlled study. Am J Hypertens. 1988;1:397–402. doi: 10.1093/ajh/1.4.397. [DOI] [PubMed] [Google Scholar]
- 48.Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, Labbato DE, Storer N, Tangpricha V, McComsey GA. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17:613–621. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Hoshiarrad A, Gohari M. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. Br J Nutr. 2012;108:1866–1873. doi: 10.1017/S0007114512000098. [DOI] [PubMed] [Google Scholar]
- 50.Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol. 2012;33:713–719. doi: 10.1007/s00246-012-0199-6. [DOI] [PubMed] [Google Scholar]
- 51.Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:864–870. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 53.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267:462–472. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 55.Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg EM, Sidelmann JJ, Jespersen J, Nybo M, Rasmussen LM, Hansen D, Scholze A. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant. 2012;27:3523–3531. doi: 10.1093/ndt/gfs138. [DOI] [PubMed] [Google Scholar]
- 56.Muldowney S, Lucey AJ, Hill TR, Seamans KM, Taylor N, Wallace JM, Horigan G, Barnes MS, Bonham MP, Duffy EM, et al. Incremental cholecalciferol supplementation up to 15 μg/d throughout winter at 51-55° N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. J Nutr. 2012;142:1519–1525. doi: 10.3945/jn.111.154005. [DOI] [PubMed] [Google Scholar]
- 57.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 58.Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg. 2012;44:307–312. doi: 10.1016/j.ejvs.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 60.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–3568. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 61.Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Lau CP, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227:140–146. doi: 10.1016/j.atherosclerosis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 63.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 64.Pacifico L, Anania C, Osborn JF, Ferraro F, Bonci E, Olivero E, Chiesa C. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165:603–611. doi: 10.1530/EJE-11-0545. [DOI] [PubMed] [Google Scholar]
- 65.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 66.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 67.Mitsuhashi T, Morris RC, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87:1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 69.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186:20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 70.Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 71.de Boer OJ, Hirsch F, van der Wal AC, van der Loos CM, Das PK, Becker AE. Costimulatory molecules in human atherosclerotic plaques: an indication of antigen specific T lymphocyte activation. Atherosclerosis. 1997;133:227–234. doi: 10.1016/s0021-9150(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 72.Breithaupt-Faloppa AC, Vitoretti LB, Cavriani G, Lino-dos-Santos-Franco A, Sudo-Hayashi LS, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Intestinal lymph-borne factors induce lung release of inflammatory mediators and expression of adhesion molecules after an intestinal ischemic insult. J Surg Res. 2012;176:195–201. doi: 10.1016/j.jss.2011.06.074. [DOI] [PubMed] [Google Scholar]
- 73.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–357. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 74.Dirksen MT, van der Wal AC, van den Berg FM, van der Loos CM, Becker AE. Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation. 1998;98:2000–2003. doi: 10.1161/01.cir.98.19.2000. [DOI] [PubMed] [Google Scholar]
- 75.Fukuo K, Nakahashi T, Nomura S, Hata S, Suhara T, Shimizu M, Tamatani M, Morimoto S, Kitamura Y, Ogihara T. Possible participation of Fas-mediated apoptosis in the mechanism of atherosclerosis. Gerontology. 1997;43 Suppl 1:35–42. doi: 10.1159/000213884. [DOI] [PubMed] [Google Scholar]
- 76.Goon PK, Boos CJ, Lip GY. Circulating endothelial cells: markers of vascular dysfunction. Clin Lab. 2005;51:531–538. [PubMed] [Google Scholar]
- 77.Kohchi K, Takebayashi S, Hiroki T, Nobuyoshi M. Significance of adventitial inflammation of the coronary artery in patients with unstable angina: results at autopsy. Circulation. 1985;71:709–716. doi: 10.1161/01.cir.71.4.709. [DOI] [PubMed] [Google Scholar]
- 78.Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease will it live up to its hype? J Am Coll Cardiol. 2011;58:1547–1556. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 79.Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- 80.Mach F, Schönbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magnus T, Wiendl H, Kleinschnitz C. Immune mechanisms of stroke. Curr Opin Neurol. 2012;25:334–340. doi: 10.1097/WCO.0b013e328352ede6. [DOI] [PubMed] [Google Scholar]
- 82.Pasterkamp G, Schoneveld AH, van der Wal AC, Hijnen DJ, van Wolveren WJ, Plomp S, Teepen HL, Borst C. Inflammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unruptured plaques of femoral and coronary arteries. Arterioscler Thromb Vasc Biol. 1999;19:54–58. doi: 10.1161/01.atv.19.1.54. [DOI] [PubMed] [Google Scholar]
- 83.Polverini PJ, Cotran PS, Gimbrone MA, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 84.Signore A, Annovazzi A, Corsetti F, Capriotti G, Chianelli M, De Winter F, Scopinaro F. Biological imaging for the diagnosis of inflammatory conditions. BioDrugs. 2002;16:241–259. doi: 10.2165/00063030-200216040-00002. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan GW, Sarembock IJ, Linden J. The role of inflammation in vascular diseases. J Leukoc Biol. 2000;67:591–602. doi: 10.1002/jlb.67.5.591. [DOI] [PubMed] [Google Scholar]
- 86.Tenaglia AN, Buda AJ, Wilkins RG, Barron MK, Jeffords PR, Vo K, Jordan MO, Kusnick BA, Lefer DJ. Levels of expression of P-selectin, E-selectin, and intercellular adhesion molecule-1 in coronary atherectomy specimens from patients with stable and unstable angina pectoris. Am J Cardiol. 1997;79:742–747. doi: 10.1016/s0002-9149(96)00861-2. [DOI] [PubMed] [Google Scholar]
- 87.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 89.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9:337–347. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 91.McCarron DA, Morris CD, Bukoski R. The calcium paradox of essential hypertension. Am J Med. 1987;82:27–33. doi: 10.1016/0002-9343(87)90268-3. [DOI] [PubMed] [Google Scholar]
- 92.MacGregor GA, Cappuccio FP. The kidney and essential hypertension: a link to osteoporosis? J Hypertens. 1993;11:781–785. doi: 10.1097/00004872-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Strauzzullo P. The renal calcium leak in primary hypertension: pathophysiological aspects and clinical implications. Nutr Meta Cardiovasc Dis. 1991;1:98–103. [Google Scholar]
- 94.Scragg R, Holdaway I, Jackson R, Lim T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol. 1992;2:697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 95.Dustan HP. Obesity and hypertension in blacks. Cardiovasc Drugs Ther. 1990;4 Suppl 2:395–402. doi: 10.1007/BF02603183. [DOI] [PubMed] [Google Scholar]
- 96.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 97.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 98.Van Lente F. Markers of inflammation as predictors in cardiovascular disease. Clin Chim Acta. 2000;293:31–52. doi: 10.1016/s0009-8981(99)00236-3. [DOI] [PubMed] [Google Scholar]
- 99.Mendall MA, Patel P, Asante M, Ballam L, Morris J, Strachan DP, Camm AJ, Northfield TC. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart. 1997;78:273–277. doi: 10.1136/hrt.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 101.Müller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–512. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 102.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 106.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Report of the Institute of Medicine of the National Academies, Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 107.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 108.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 109.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 110.Vitamin D and Omega-3 TriaL (VITAL) ClinicalTrials.gov ID: NCT01169259. Cited 2013-08-21. Available from: http://clinicaltrials.gov/show/NCT01169259.
- 111.O’Keefe JH, Patil HR, Lavie CJ. Can vitamin D deficiency break your heart? Mayo Clin Proc. 2012;87:412–413; author reply 413. doi: 10.1016/j.mayocp.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


