To the Editor:
Munafò et al. (1) conducted a meta-analysis of a subset of studies that examined the interaction between the serotonin transporter (5-HTTLPR) gene and stressful life events (SLEs) in conferring risk for depression. 5-HTTLPR has two common alleles of varying length, which are designated “s” for short and “l” for long. They concluded that the prior “positive results for the 5-HTTLPR × SLE interactions in logistic regression models are compatible with chance findings” and stated further that “the 5-HTTLPR and SLE interaction effect is negligible.” These conclusions, however, were based on assumptions in the simulation model that are inconsistent with prior research findings and a bias in sampling strategy that call into question the authors’ conclusions.
In the simulation reported in the Munafò article, the environmental effect was specified as having two levels—unexposed (E1) and exposed (E2)—corresponding to zero and one + SLE. As can be seen in Figure 1, in the original report by Caspi et al. (2) the presence of only one SLE conferred no risk for depression, regardless of genotype. Risk for depression in association with the “s” allele of 5-HTTLPR only began to increase significantly in the presence of three or more SLEs and was most pronounced in individuals with four or more SLEs. Collapsing subjects across the various levels of exposure to SLEs added significant noise in the model and compromised efforts to detect the 5-HTTLPR × SLE interaction in the meta-analysis.
Figure 1.
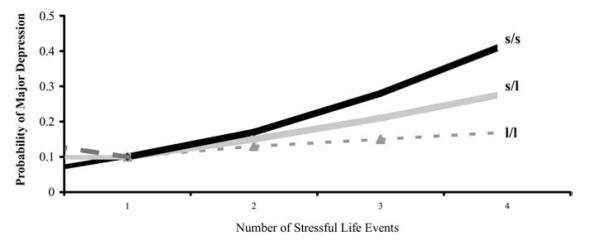
Results of multiple regression analyses estimating the association between number of stressful life events (between ages 21 years and 26 years) and the probability of major depressive disorder by age 26 as a function of serotonin transporter protein genotype. Among the 146 s/s homozygotes, 43 (29%), 37 (25%), 28 (19%), 15 (10%), and 23 (16%) study members experienced zero, one, two, three, and four or more stressful events, respectively. Among the 435 s/l heterozygotes, 141 (32%), 101 (23%), 76 (17%), 49 (11%), and 68 (16%) experienced zero, one, two, three, and four or more stressful events. Among the 264 l/l homozygotes, 79 (29%), 73 (28%), 57 (21%), 26 (10%), and 29 (11%) experienced zero, one, two, three, and four or more stressful events. The main effect of the serotonin transporter gene was not significant (b=−.15, SE = .14, z = 1.07, p = .29), the main effect of life events was significant (b=.37, SE=.06, z=5.99, p=.001), and the gene × environment interaction was in the predicted direction (b=−.19, SE=.10, z=1.91, p=.056). Life events predicted a diagnosis of major depression among s carriers (b=.52, SE=.16, z=3.28, p=.001 among s/s homozygotes, and b=.39, SE=.09, z=4.24, p=.001 among s/l heterozygotes) but not among l/l homozygotes (b=.16, SE=.13, z=1.18, p=.24). From Caspi et al., Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386–389. Reprinted with permission from AAAS.
The nonrepresentative nature of the sample of studies included in the meta-analysis is also a limitation of this report. Munafò et al. identified 14 studies of 33 published reports that met their criteria for inclusion in the meta-analysis but were unfortunately only able to obtain data from 5 of the 14 studies. The meta-analysis was conducted with data from less than one-half the studies that met the stated inclusion criteria, with a trend for negative studies to be over-represented in the meta-analysis. Of the 14 studies meeting the inclusion criteria for the meta-analysis, 75% (3/4) of the negative studies and only 20% (2/10) of the positive studies were included in the analysis (Fisher exact test: p < .10).
There has been a proliferation of studies examining this gene × environment interaction over the past 2 years and, to date, no comprehensive meta-analysis of all studies. Since the publication of the article by Munafò et al., a second meta-analysis was published that similarly concluded that there is no evidence that the serotonin transporter genotype alone or in interaction with SLEs is associated with an elevated risk for depression (3). Although a larger number of studies were included in the second meta-analysis, 12 studies were published after the dataset for this second meta-analysis was closed, with only 3 of the 12 studies including adequate data in the published reports to be examined in the second meta-analysis (K.R. Merikangas, written communication, July 2009). Seven of the nine studies excluded because they were published after the dataset was closed were full or partial replications (4–10), as were four other earlier studies that were not included in the second meta-analysis because the data were either not received or received in an incompatible format (11–14). Like the publication by Munafò et al., the second meta-analysis also included an excess representation of nonreplications. It was conducted with data from one-half of the 26 studies that met the inclusion criteria for the meta-analysis (3), with a trend again for negative studies to be over-represented. Of the 26 studies meeting the inclusion criteria for the second meta-analysis, 78% (7/9) of the negative studies and only 35% (6/17) of the positive studies were included in the analysis (Fisher exact test: p < .10).
Most studies in the literature (17/26) have replicated in part or fully the finding that individuals with the “s” allele of 5-HTTLPR are at higher risk for depression after exposure to multiple SLEs or experiences of child maltreatment, but to date a comprehensive meta-analysis of published results has not been completed. The serotonin transporter is a protein critical to the regulation of serotonin levels in the brain, on the basis of its ability to remove serotonin from the synapse and terminate its action. Serotonin transporter protein (5-HTT) knockout mice show stress-induced anxious- and depressive-like behaviors and increased dendritic spine density in the amygdala (15). This latter finding is interesting in light of the fact that the “s” allele of 5-HTTLPR has been associated with increased amygdala activation to negative stimuli in multiple imaging genomic studies (16). In experimental stress-induction paradigms, the “s” allele of 5-HTTLPR has also been associated with increased hypothalamic pituitary adrenal axis activity in both humans (17) and nonhuman primates (18) and heightened brain activity in stress-responsive brain regions (19).
A comprehensive meta-analysis is required to determine the strength of the 5-HTTLPR × SLE interaction in conferring risk for depression. In addition, we believe more work is needed to unravel the mechanisms by which stress might confer vulnerability to depression in individuals with the “s” allele of 5-HTTLPR, to further characterize allelic variants of this gene, and identify additional genetic (e.g., BDNF) and environmental (e.g., social supports) factors that can modify the magnitude of the 5-HTTLPR × SLE interaction.
Acknowledgments
Dr. Kaufman has served as a consultant for Bristol Myers Squibb, Pfizer, Wyeth-Ayerst, Forest Laboratories, Johnson and Johnson Research Pharmaceutical Institute, Shire, and Otsuka Pharmaceutical. Drs. Caspi and Moffitt, through the Wisconsin Alumni Research Foundation, have applied for a patent: USPTO Number 10/889,450, A method for assessing behavioral predisposition: Depression in a human subject who has experienced a stressful life event, determining whether the subject carries one or more copies of a short promoter allele of a 5-HTT gene. The authors of this letter receive support from the National Institute of Mental Health MH65519 (JK); MH077874 (AC and TM); National Institute on Drug Abuse: DA15105 (JG); the National Center for Posttraumatic Stress Disorder, Veterans Administration, West Haven, Connecticut, and the VA Depression Research Enhancement Award Program (VA CT REAP (JG, JK), and Yale University General Clinical Research Center Grant (M01RR06022).
Footnotes
Drs. Gelernter and Kaffman report no biomedical financial or potential conflicts of interest.
Contributor Information
Avshalom Caspi, Department of Psychology and Neuroscience Duke University Durham, North Carolina.
Terrie Moffitt, Institute of Psychiatry King’s College London London, United Kingdom; Department of Psychology and Neuroscience Duke University Durham, North Carolina.
References
- 1.Munafò MR, Durrant C, Lewis G, Flint J. Gene × environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: New evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- 5.Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsuyama H, Tomita M, Hidaka K, Fushimi S, Okuyama T, Watanabe Y, et al. Association between serotonin transporter gene polymorphisms and depressed mood caused by job stress in Japanese workers. Int J Mol Med. 2008;21:499–505. [PubMed] [Google Scholar]
- 7.Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, Bagdy G. New evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biol Psychiatry. 2008;64:498–504. doi: 10.1016/j.biopsych.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Scheid JM, Holzman CB, Jones N, Friderici KH, Nummy KA, Symonds LL, et al. Depressive symptoms in mid-pregnancy, lifetime stressors and the 5-HTTLPR genotype. Genes Brain Behav. 2007;6:453–464. doi: 10.1111/j.1601-183X.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- 9.Veletza S, Samakouri M, Emmanouil G, Trypsianis G, Kourmouli N, Livaditis M. Psychological vulnerability differences in students—carriers or not of the serotonin transporter promoter allele S: Effect of adverse experiences. Synapse. 2009;63:193–200. doi: 10.1002/syn.20598. [DOI] [PubMed] [Google Scholar]
- 10.Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van Os J. The BDNF Val(66)Met × 5-HTTLPR × child adversity interaction and depressive symptoms: An attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:120–3. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 14.Sjöberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindström L, Oreland L. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 15.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


