Summary
Cancer is influenced by its microenvironment, yet broader, environmental effects also play a role but remain poorly defined. We report here that mice living in an enriched housing environment show reduced tumor growth and increased remission. We found this effect in melanoma and colon cancer models, and that it was not caused by physical activity alone. Serum from animals held in an enriched environment (EE) inhibited cancer proliferation in vitro and was markedly lower in leptin. Hypothalamic BDNF was selectively upregulated by EE, its genetic overexpression reduced tumor burden, whereas BDNF knockdown blocked the effect of EE. Mechanistically, we show that hypothalamic BDNF downregulated leptin production in adipocytes via sympathoneural β-adrenergic signaling. These results suggest that genetic or environmental activation of this BDNF/leptin axis may have therapeutic significance for cancer.
Introduction
The growth of most cancers is dependent in part on their microenvironment – the balance between factors which act to facilitate growth, induce angiogenesis and cell survival and those that inhibit cell proliferation and lead to apoptosis (Hanahan and Weinberg, 2000). This local microenvironment is influenced by systemic factors, and the cancer itself induces both local and distant changes through paracrine signaling (Aaronson, 1991) and interactions with the immune and nervous systems (Darnell and Posner, 2006). The effect of the macroenvironment on systemic cancer, specifically an individual’s interaction with its physical living and social environment is much less well defined. There is significant interest in neuroscience in the interaction between genes and the environment, and specifically how living in complex housing with increased space, physical activity and social interactions influences brain structure and function (van Praag et al., 2000). What is remarkable is just how robust and powerful the physical and social environment can have on brain function. We previously published that such an enriched environment (EE) leads to changes in expression of growth factors and survival of cells within the brain (Young et al., 1999; Cao et al., 2004). Moreover, EE has considerable impact on the phenotype of a variety of toxin- and genetically-induced models of human neurological disease (Nithianantharajah and Hannan, 2006). In cancer research, environmental effects have largely focused on diet and exposure to mutagens and carcinogens. Here, we wished to investigate whether the social and physical components of an animal’s environment could impact on cancer growth, and if so, define potential mediators. Specifically, we were intrigued with the hypothesis, that an enriched environment, one optimized for cerebral health, as defined by improved learning and memory, increased neurogenesis and reduced apoptosis and resistance to external cerebral insults, could also lead to an anti-cancer phenotype – in other words a mens sana associated with a corpore sano. We therefore asked could simply placing animals in a more complex living environment induce effects that were profound enough to significantly influence the growth of a highly malignant cancer? We wanted to test this in both syngeneic models in which cancer cells continue to proliferate following transplantation, leading to highly reproducible solid tumors and ultimately death (Dranoff et al., 1993), as well as in APCmin/+ mice, a spontaneous model with a germline mutation in APC similar to humans with familial adenomatous polyposis, and a gene in which somatic mutations occur in 80% of human colon cancers. We show here that EE leads to a remarkable suppression of cancer proliferation in all three models tested, even when delayed until the tumor was well established. We teased out the molecular pathways, and showed selective activation of a hypothalamic-sympathoneural-adipocyte (HSA) axis. Notably, in a comprehensive set of experiments using transgenic animals, somatic gene transfer, controlled-release and drug infusion, we were able to dissect out a mechanism whereby the EE paradigm induced hypothalamic BDNF expression as an effector immediate early gene, leading to preferential activation of sympathetic innervation of white adipose tissue, which in turn via ß-adrenergic receptors led to suppression of leptin expression and release. This marked drop in serum leptin levels mediated the anti-proliferative phenotype.
Results
Housing in an enriched environment reduces tumor growth
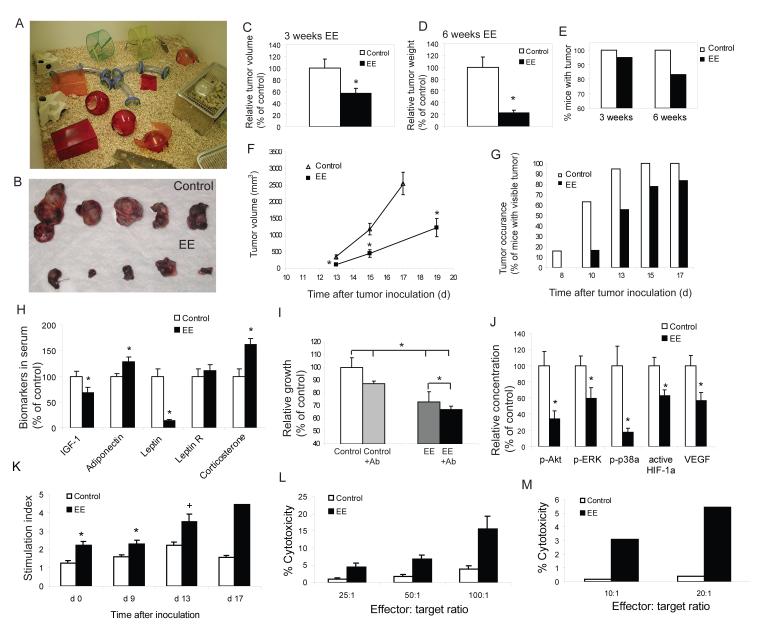
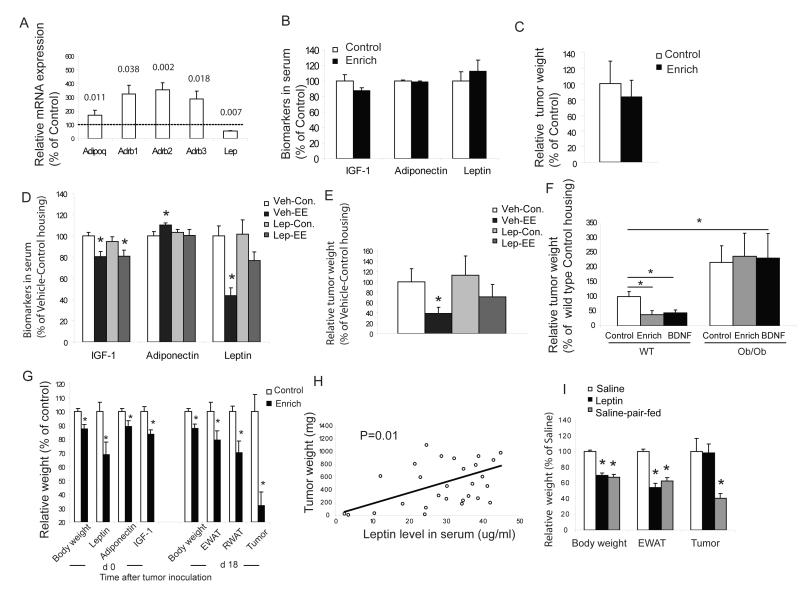
Immediately weaned (three week old) C57BL/6 mice were randomized to live in either EE (n=18-20; Figure 1A), or in grouped control housing (5 mice per cage) for 3 or 6 weeks. Both EE and control mice then received subcutaneous injections of B16 melanoma cells (105 cells per mouse) and were returned to their respective homes. At 17-19 days post inoculation, tumor size was determined (Figure 1B). In the mice housed in EE for 3 weeks prior to tumor implantation, the mean volume of the tumor was 43% smaller than those in the control housing (P<0.05, Figure 1C). For the 6 week groups, the tumor mass in EE mice was reduced by 77.2% (Figure 1D, P<0.001). Notably, all mice in the control groups developed solid tumors, whereas 5% of mice with 3 weeks of EE had no visible tumors, and this tumor resistant group reached 17% with 6 weeks EE (Figure 1E). Moreover, the rate of tumor growth over time was both delayed and more linear in the EE mice without the exponential growth curve observed in the control mice (Figure 1F). The occurrence of visible tumors were delayed in EE mice (Figure 1G) with all control mice showing visible tumors by day 15 after inoculation while 15% of the EE mice showed no visible tumor at day 19 when the experiment ended (Figure 1G). Reduced tumor size in EE mice was associated with a decrease in cell proliferation as shown by PCNA immunohistochemistry (Figure S1A) as well as an increase in apoptosis as shown by both TUNEL (Figure S1B) and active caspase 3 immunoreactivity (Figure S1C).
Figure 1. Enriched environment reduces tumor growth and affects biomarkers in serum, B16 melanoma cell proliferation in vitro, signaling pathways in the tumors and immune functions.
(A) EE cage. (B) Representative B16 melanoma dissected d 17 after inoculation of 105 cells per mouse. Mice were housed in the EE or control cages for 6 w prior to tumor inoculation. (C) Three weeks of EE decreased tumor volume d 19 after inoculation (n=20 in each group, * P<0.05). (D) Six weeks of EE further reduced tumor weight d 17 after inoculation (n=18 in each group, * P<0.05). (E) EE induced complete tumor resistance in a subset of mice. All control mice showed visible tumors. (F) Four weeks of EE decreased tumor growth rate (n=20 in each group, * P<0.05). (G) Four weeks of EE delayed the occurrence of tumor. (H) EE effects on biomarkers in serum. Sera were collected before tumor inoculation in the 6 week EE (n=20 in each group, * P<0.05). (I) B16 cells grew more slowly when cultured with serum from EE mice compared to control mice while pretreatment with leptin neutralizing antibody inhibited the effect of serum on B16 cell growth (n=4 in each group, * P<0.05 between groups as indicated). (J) Phospho-Akt1 (S473), ERK1 (T202/Y204)/ERK2 (T185/Y187), Phosphop38α (T180/Y182), active HIF-1α activity and VEGF concentration were significantly reduced in tumors from EE mice compared to control mice (n=7 in each group, * P<0.05). (K) The proliferative response of splenic lymphocytes to ConA was increased in EE mice before and after tumor inoculation (n=5 in each group at each time point, * P<0.05, + P=0.054). (L) NK cytotoxicity was higher in EE mice (n=5 in each group, P<0.05) before tumor inoculation. (M) CD8 T cell cytotoxicity was higher than control mice (bar represents a pool of 4 mice in each group, P<0.05).Values are means ± SEM. See also Figure S1.
Enriched environment induces systemic metabolic changes
We found that EE mice weighed 6% less than control mice although being fed ad libitum on identical diets (Figure S1D). To investigate potential systemic metabolic changes associated with EE induced tumor resistance, peripheral blood was taken from the mice housed for 6 weeks in either control or EE. Hormone and metabolite levels were measured using ELISA kits. IGF-1 levels have been consistently associated with cancer risk and progression including melanoma (Jenkins and Bustin, 2004). Serum IGF-1 was significantly decreased in the EE mice (Figure 1H) while its major binding protein, IGFBP3, did not change (data not shown). The adipocyte hormone, adiponectin, showed a significant increase (Figure 1H). This abundant protein is of particular interest in diabetes due to its role in regulating insulin sensitivity but recently it has been shown to have pleiotropic properties including suppressing carcinogenesis and inhibiting angiogenesis in cancer models (Fujisawa et al., 2008). As previously described for EE (Benaroya-Milshtein et al., 2004), the adrenal glucocorticoid and stress hormone, corticosterone was elevated (Figure 1H). In contrast, serum leptin levels in the EE group was markedly reduced to 13% of controls (P<0.01; Figure 1H). Leptin is not only the major adipocyte hormone that conveys metabolic information to the brain but is also involved in other pathways affecting many peripheral organs as a mitogen, metabolic regulator, survival or angiogenic factor depending on the tissue type (Wauters et al., 2000). Recent clinical reports show that elevated serum leptin levels are linked to an increased risk of certain cancers including prostate (Garofalo and Surmacz, 2006), breast cancer (Cirillo et al., 2008) and melanoma (Gogas et al., 2008).
As the serum levels of factors associated with survival and proliferation of cancer cells were influenced by EE, we investigated whether B16 melanoma cells incubated with serum obtained from either EE or control mice would impact on the cell growth in vitro using the Promega Cell Proliferation Assay. Sera from EE mice significantly slowed the B16 cells growth compared to control sera (Figure 1I). Since leptin showed the most marked change among the serum factors, we further examined the direct role of leptin on tumor cell proliferation using a leptin-neutralizing antibody. Pretreatment of sera with leptin-neutralizing antibody inhibited tumor cell growth significantly (Figure 1I).
Enriched environment influences signaling pathways in the tumor
Melanoma cells have been associated with activation of a large number of signal transduction enzymes, which can influence the growth of the cancer (Miller and Mihm, 2006). For example a genome-wide screen for oncogenes reported that 66% of melanoma patients carry an activating mutation in the BRAF gene that leads to constitutive activation of the MAPK pathway whose aberrant activation can result in unharnessed cell proliferation (Davies, 2002). These signal transduction pathways are also regulated in part by extracellular mediators acting via cell surface receptors, and since EE altered circulating levels of growth factors, it was of interest to determine whether downstream signal transduction pathways would be altered in the tumors growing in EE mice. Signal transduction assays were run on nuclear extracts using commercial kits. The tumors from EE mice had highly significant decreases in multiple signal transduction pathway mediators including phospho-Akt, phospho-ERK1/ERK2 and phospho-p38a (Figure 1J). In addition both active HIF-1α and vascular endothelial growth factor (VEGF) were also decreased, consistent with a reduction in angiogenesis within the tumor as shown by immunostaining of vascular marker CD31 (Figure 1J, Figure S1E).
Immunocompetence is enhanced in an enriched environment
In addition to the effects on learning and memory, EE may influence immunity directly or indirectly via an interaction between brain, neuroendocrine and immune systems (Benaroya-Milshtein et al., 2004). Spleens isolated from EE animals were significantly enlarged compared to control animals after tumor cells were implanted (Figure S1F). Furthermore the splenic lymphocytes of EE mice showed a near 2-fold increase in proliferation in response to the T cell mitogen Concavalin A both before (Day 0) and following tumor inoculation (Days 9, 13 & 17) (Figure 1K). In addition, natural killer cell (NK) activity was greater in EE mice before tumor inoculation (Figure 1L), and CD8+ T cell cytotoxicity was also increased (Figure 1M), demonstrating a significant effect on cancer cell specific immunological responses, again consistent with cancer regression.
Enriched environment changes hypothalamic gene expression
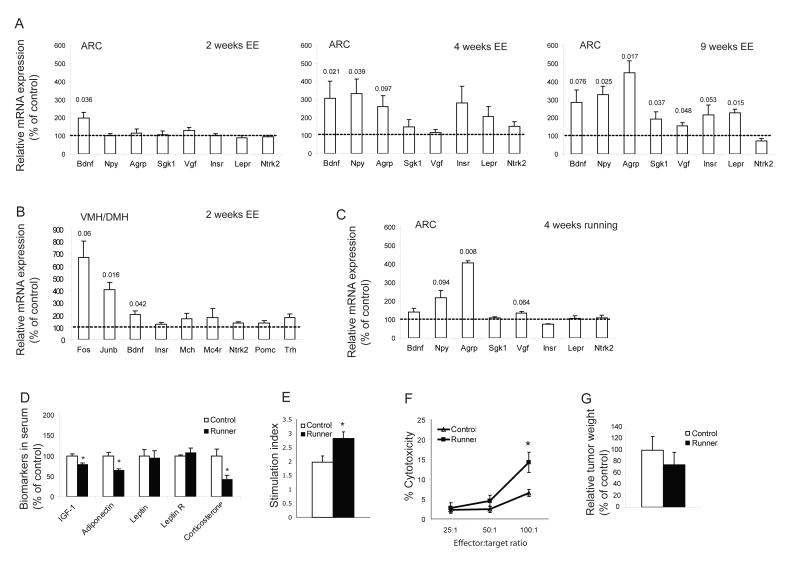
In order to elucidate the mechanism of this EE-associated tumor resistance, we chose to target the hypothalamus, an area of the brain that is critical in the regulation of both energy balance and neuroendocrine-immune interaction through the hypothalamic-pituitary-adrenal (HPA) axis (Dhillo, 2007; McEwen, 2007). The hypothalamus contains a number of discrete nuclei including the arcuate (ARC), paraventricular (PVN), ventromedial (VMH), dorsomedial (DMH) and lateral hypothalamic area (LHA). The ARC is thought to receive information regarding metabolic status from peripheral circulating factors including leptin, insulin, glucose and the gut peptides (Dhillo, 2007). The neuronal projections from the ARC to other brain areas are thought to mediate the effects of the ARC on energy balance. We screened a number of genes known to be involved in metabolic regulation and neuronal-immune crosstalk to evaluate the potential mediators of EE-associated metabolic and immune changes. We housed mice in either EE or control housing for 2, 4 or 9 weeks. The ARC was microdissected using laser capture and mRNA expression was examined by quantitative RT-PCR. At the early time point of 2 weeks EE, BDNF was the only gene screened with a significant change, a 2-fold increase (Figure 2A, left panel). It remained upregulated at 4 weeks. Neuropeptide Y (Npy) and agouti related peptide (Agrp) expression were increased in mice with 4 weeks EE and this upregulation was further increased at 9 weeks suggesting a secondary response to metabolic changes when exposed to long-term EE (Figure 2A mid and right panels). Meanwhile other genes regulating food intake and energy expenditure (serum/glucocorticoid regulated kinase, Sgk1 and nerve growth factor inducible, Vgf) were upregulated only after 9 weeks of EE. In addition, leptin receptor (Lepr) was significantly increased while insulin receptor (Insr) showed a strong trend towards upregulation (Figure 2A right panel), indicating enhanced sensitivity of ARC neurons to peripheral hormonal signals. In contrast, anorexigenic peptides POMC and CART were not changed in the ARC (data not shown).
Figure 2. Enriched environment induces gene expression changes in the hypothalamus distinctive to voluntary running.
(A) Arcuate nucleus at 2, 4 and 9 weeks of EE. (B) VMH/DMH. (n=5 per group). (C) Running induced gene expression changes in the arcuate nucleus. (n=5 per group). P values of significance or strong trends are shown above the bars. (D) Running led to some changes in serum biomarkers which were distinctive to those observed in EE (n=16 in runners, n=13 in control mice, *P<0.05). (E) The proliferative response of splenic lymphocytes to the T-cell mitogen Con A was increased in runner (n=4 in each group, * P<0.05). (F) NK cytotoxicity was higher in runner (n=4 in each group, P<0.05) before tumor inoculation. (G) Running did not reduce tumor growth. (n=11 in runner, n=10 in control mice, P>0.05).Values are means ± SEM.
Previous reports suggest that BDNF is most abundant in the VMH and its secretion from the VMH and/or DMH is required for the suppression of appetite (Rios et al., 2001; Xu et al., 2003). We sought to examine whether BDNF expression in VMH/DMH could also respond to EE. We microdissected VMH/DMH using laser capture from mice living in EE for 2 weeks and measured a number of genes known to be highly expressed in VMH/DMH including BDNF, Junb and Fos which have been widely used as markers of neuronal activity. Similar to the pattern observed in the ARC, BDNF showed a significant 2-fold upregulation (Figure 2B). Interestingly, BDNF expression responds to environmental stimulation in a pattern similar to neuronal activation markers Junb and Fos (Figure 2B), and is consistent with BDNF as a plasticity-related and effector immediate early gene (Nedivi et al., 1993; Hughes et al., 1993).
Voluntary wheel running has not the same effect as an enriched environment
Physical exercise is known to enhance immune function (Suzuki et al., 2005), decrease body fat, and has recently been shown to inhibit ultraviolet B light-induced carcinogenesis (Lu et al., 2006). To investigate whether physical exercise could alone account for the EE-induced melanoma resistance, we subjected mice to voluntary wheel running for 4 weeks followed by tumor implantation. Mice living in cages with free access to running wheels ran approximately 2 km per day. Running led to physiological changes including lower body weight, reduced fat and increased lean mass similar to that observed in the EE mice (Figure S1D). Moreover, runners also showed altered biomarkers in serum (Figure 2D), but with a pattern quite distinct to that of EE mice (Figure 1H). In runners, IGF-1 was significantly reduced similar to that in the EE mice. However leptin was not changed while both adiponectin and corticosterone were significantly decreased, in contrast to the increase in the EE mice. Although an enhanced immune response was observed in runners (Figure 2E, F), exercise did not significantly reduce tumor weight (Figure 2G). These data suggest that physical exercise alone is insufficient to account for EE-induced tumor resistance although it likely contributes. Since EE consists of a much larger environment, with access to running wheels and various toys and objects to explore, we quantified the total physical activity of EE animals including wheel-running and general motor activity. The EE mice traveled a mean total distance of 0.64 km per day which is approximately 66% less than the runners and further suggests physical activity per se is unlikely the major contributor to the EE-associated tumor reduction.
Of particular interest, running influenced gene expression in the ARC with a pattern that was qualitatively different than EE. In contrast to the EE mice whose BDNF was increased 3-fold at 4 weeks, running did not upregulate BDNF significantly while the two orexigenic peptides NPY and AgRP were increased (Figure 2C). Hence among the genes being screened, BDNF appeared to be the most selectively responsive to EE and could therefore serve as a potential mediator of EE-induced tumor resistance while the change in expression of other genes appeared secondary to systemic physiological changes associated with EE.
Hypothalamic overexpression of BDNF mimics the effects of an enriched environment
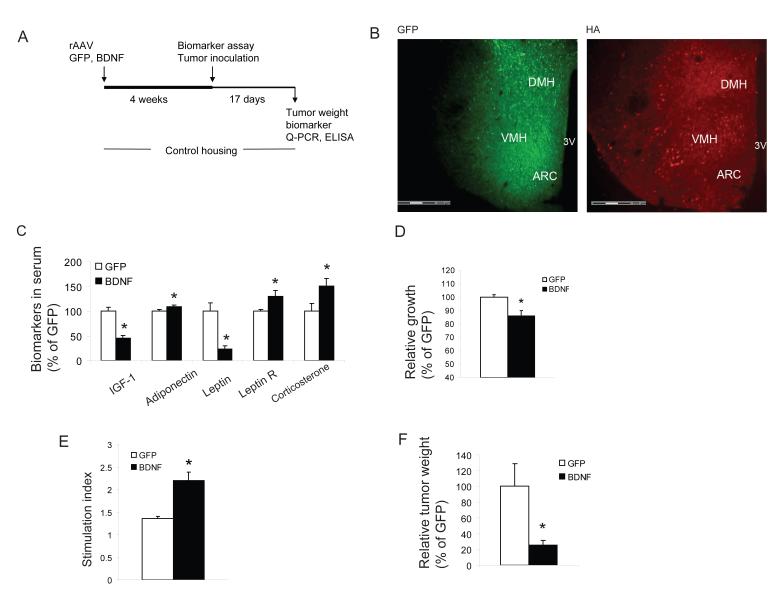
BDNF has diverse functions in brain development and plasticity (Lu et al., 2005) and its expression is highly responsive to activity and environment (Young et al., 1999) and is considered to be an effector immediate early gene (Hughes et al., 1993). In addition, BDNF is an important component of the hypothalamic pathway that controls energy homeostasis (Wisse and Scwartz, 2003). Its expression in VMH/DMH is rapidly induced by glucose administration consistent with a role in satiety regulation (Unger et al., 2007). Both peripheral and central administration of BDNF decreases food intake, increases energy expenditure and leads to weight loss (Bariohay et al., 2005; Pelleymounter, 1995). Obesity phenotypes have been observed in BDNF heterozygous mice (Lyons et al., 1999), in a conditional knockout model (Xu et al., 2003) and viral-mediated selective deletion in VMH/DMH of adult mice (Unger et al., 2007). We used rAAV vectors to deliver an HA-tagged human BDNF gene to the hypothalamus bilaterally with a GFP vector as a control. Four weeks after injection when transgene expression reached stable levels (Figure 3A, B), mice receiving the BDNF vector showed significantly lower body weight gain than GFP control mice (1.25±0.37 g vs. 4.31±0.44 g, P<0.05). The biomarkers in serum of BDNF mice showed the same pattern of changes (Figure 3C) as that observed in the EE mice (Figure 1H), namely a decrease in IGF-1 and leptin, and an increase in adiponectin and corticosterone. Leptin circulates in a free form and bound to soluble leptin receptor (Hahn et al., 2006). Only free leptin is biological active. The soluble leptin receptor level was increased in BDNF mice (Figure 3C) leading to a further reduction in the free leptin index. Consistent with the biomarker changes, B16 melanoma cell growth in vitro was slower when cultured with sera from BDNF mice compared to GFP mice (Figure 3D). In addition BDNF mice showed an enhanced immune response (Figure 3E). Also similar to EE mice, tumor weight was decreased by 75% in BDNF compared to GFP mice (Figure 3F, P=0.026).
Figure 3. Hypothalamic gene delivery of BDNF mimics enriched environment associated metabolic changes and melanoma resistance.
(A) Experimental design of BDNF overexpression in the hypothalamus. (B) Transgene expression in hypothalamus: GFP fluorescence and immunofluorescence of HA tag (human BDNF has HA tag at 3′ terminal). (C) BDNF overexpression led to serum biomarker changes similar to that found in EE (n=10 in BDNF mice, n=16 in GFP mice, *P<0.05). (D) B16 cells grew more slowly when cultured with serum from BDNF mice compared to GFP mice 4 weeks after AAV injection (n=5 in each group, * P<0.05). (E) The proliferative response of splenic lymphocytes to the T-cell mitogen Con A was increased in BDNF mice (n=3 in each group, * P<0.05). (F) BDNF overexpression reduced tumor weight d 17 after inoculation (n=10 in BDNF mice, n=16 in GFP mice, * P<0.05). Values are means ± SEM.
BDNF depletion inhibits tumor resistance induced by an enriched environment
To determine whether hypothalamic BDNF expression might be an upstream mediator of the EE-induced metabolic profile and anti-cancer phenotype, we generated a vector expressing a microRNA targeting mouse BDNF (miR-Bdnf). In vitro experiments demonstrated that this microRNA vector knocked down BDNF mRNA by 65% and protein levels by 80%. We also generated a control microRNA vector targeting a scrambled sequence (miR-scr) against no known genes.
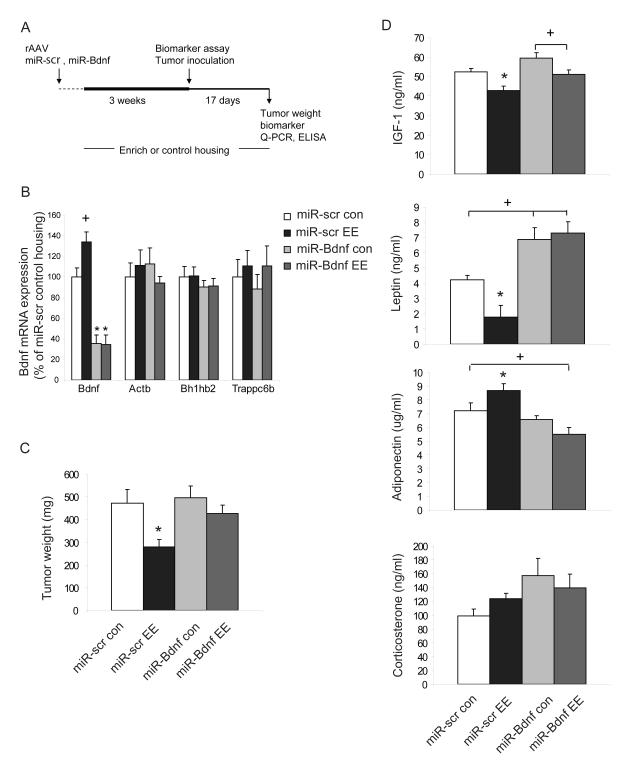
We injected rAAV vectors of miR-Bdnf or miR-scr bilaterally into the hypothalamus and then assigned the mice to EE or control housing (Figure 4A). We measured RNAi efficiency in the hypothalamus at both mRNA and protein levels by quantitative RT-PCR and ELISA, respectively. Both BDNF mRNA (Figure 4B) and BDNF protein levels (Figure S4) were reduced significantly in mice receiving miR-Bdnf living in control housing as well as EE housing compared to mice receiving miR-scr. We assessed the specificity of miR-Bdnf by quantifying the levels of other mRNAs and observed no significant difference in expression of house keeping gene Actb, or the genes with sequences most homologous to the targeting sequence of miR-Bdnf: Bh1hb2 or Trappc6b (Figure 4B). The changes of biomarkers in serum associated with EE was largely preserved in mice receiving miR-scr (Figure 4D). In contrast, the EE associated drop in leptin levels and the increase in adiponectin was diminished in mice receiving miR-Bdnf (Figure 4d, P=0.64 leptin level miR-Bdnf EE vs. miR-Bdnf con.; P=0.136 adiponectin level miR-Bdnf EE vs. miR-Bdnf con.). Although not influenced by environment, the leptin levels in miR-Bdnf mice were higher than those in the miR-scr mice, which was consistent with the accelerated weight gain in miR-Bdnf mice (191.5% of miR-scr) due to BDNF knockdown. After housing the mice in EE or control housing for 3 weeks, we implanted B16 melanoma cells. Consistent with the results we observed with naive wild-type mice, the tumor weight was significantly reduced in miR-scr mice living in EE compared to control housing (Figure 4C, P<0.05 miR-scr EE vs. miR-scr con.), whereas in mice receiving miR-Bdnf this EE effect on tumor mass was blocked (Figure 4C, P=0.249 miR-Bdnf EE vs. miR-Bdnf con.; P<0.05 miR-Bdnf EE vs. miR-scr EE).
Figure 4. Hypothalamic BDNF knockdown inhibits enriched environment induced tumor resistance.
(A) Experimental design of RNAi knockdown of hypothalamic BDNF expression. (B) Quantitative RT-PCR. The miR-Bdnf vector significantly reduced hypothalamic BDNF mRNA levels in mice housed in both control and EE condition (n=7-17 per group, * P<0.01 miR-Bdnf compared to miR-scr in both of control housing and EE, + P=0.061 miR-scr EE compared to miR-scr control housing). (C) miR-Bdnf blocks EE-induced tumor resistance. * P<0.05, miR-scr EE vs. all other groups, con: control housing, EE: EE housing. (D) Biomarkers in serum 4 weeks after AAV injection and 3 weeks EE (* P<0.05 miR-scr EE vs. all other groups, + P<0.05 between groups as indicated). Values are means ± SEM. See also Figure S2.
In order to further elucidate the role of BDNF in the tumor resistance, we investigated melanoma growth in BDNF heterozygous (BDNF+/−) mice whose BDNF protein levels in the hypothalamus is ~40% lower than wild type mice (Lyons et al., 1999). Both BDNF+/− mice and their wild type littermates in standard housing were injected with melanoma cells. BDNF+/− mice were heavier (26.0±0.82 g vs. 23.8±0.63 g) and had elevated serum leptin levels (2902±662 pg/ml vs. 1178±143 pg/ml, P<0.05) with no difference in IGF-1 levels. Tumor weight was significantly increased in BDNF+/−mice (896.5±224.9 mg vs. 443.4±102.9 mg, P<0.05).
Sympathetic modulation of leptin expression via β-adrenergic receptors mediates the anti-proliferative phenotype associated with an enriched environment
Our data showing 1) hypothalamic overexpression of BDNF leads to a phenotype mimicking that of EE; and 2) knockdown of BDNF expression blocks EE effects on tumor growth, suggested that hypothalamic BDNF is a mediator linking the environment to cancer growth. We then investigated potential peripheral effectors in this regulatory network. The most robust and consistent changes among serum biomarkers associated with EE and its impact on tumor growth were a profound decrease in leptin and an increase in adiponectin circulating levels. Both adipokines are secreted predominantly by white adipose tissue (WAT) and serum leptin levels reflect synthesis and secretion in WAT (Evans and Summers, 1999) suggesting that WAT is responsive to EE. We examined WAT gene expression in mice living in EE. Leptin expression was decreased approximately 50% while adiponectin expression was increased by 70% in EE mice consistent with the changes observed in serum (Figure 5a). There is considerable evidence that leptin expression is suppressed by sympathetic tone via β-ARs (Evans and Summers, 1999; Bartness and Song, 2007). All of the three β-AR genes Adrb1, 2, 3 were upregulated by approximately 3-fold in WAT (Figure 5a) of EE mice but not in muscle (data not shown). The EE mice showed a trend towards higher norepinephrine (NE) levels in serum (2.563±0.187 ng/ml vs. 1.811±0.228 ng/ml, P=0.066) and a significant increase in NE level in WAT lysates (Figure S3A) indicating elevated sympathetic drive to WAT. Epinephrine levels were not changed in either serum (data not shown) or WAT (Figure S5A). Hypothalamic overexpression of BDNF also led to an increase in WAT NE levels similar to EE (Figure S3B) while both NE and epinephrine were significantly decreased in WAT of BDNF+/− mice (Figure S3C) suggesting a role of BDNF in sympathetic modulation of WAT.
Figure 5. Sympathetic regulation of WAT adipokine expression via β-ARs serves as a peripheral pathway of the enriched environment -associated anti-cancer phenotype.
(A) EE induced gene expression changes in WAT of mice living in EE for 9 weeks (n=5 per group). P values of significance were shown above bars. (B, C) Propranolol completely blocked EE effects on serum biomarkers and melanoma growth (n=20 per group). (D, E) Administration of leptin-releasing liposomes attenuated the EE-associated leptin drop in circulation and inhibited the EE effect on tumor growth (n=10 per group). * P<0.05 compared to mice receiving vehicle and living in control housing. (F) EE or hypothalamic overexpression of BDNF failed to inhibit melanoma growth in ob/ob mice (n=10 per group) as observed in wild type mice (* P<0.05 between groups as indicated). (G) EE inhibited tumor growth in obese DIO mice (n=15 per group, * P<0.05 compared to control housing). (H) Correlation of tumor weight of individual mouse in the DIO experiment with basal serum leptin level before tumor implantation (n=28). (I) Leptin replacement enhanced melanoma growth in ob/ob mice compared to pair-fed saline infused mice. (n=10 per group, * P<0.01 compared to saline infused mice). Values are means ± SEM. See also Figure S3.
We then sought to examine the potential role of the sympathetic drive in mediating EE effects by using the β-blocker, propranolol. Mice receiving propranolol (0.5 g/L) in drinking water were randomly assigned to live in EE or control housing for 3 weeks. The changes in serum biomarkers associated with EE, particularly the decrease in leptin and increase in adiponectin levels (Figure 1H), were completely blocked when propranolol was administered (Figure 5B). Furthermore, EE failed to reduce tumor growth when β-adrenergic signaling was inhibited (P=0.644, Figure 5C).
We further investigated the relevance of leptin using both pharmacological and genetic approaches. Firstly we implanted mice with leptin-releasing liposomes before exposing them to the EE. The leptin-encapsulated multilamellar liposome vesicles (leptin-MLV, releasing 1.8 ng leptin/ l/24 hrs in vitro) attenuated the leptin drop associated with EE (Figure 5D) and blocked the tumor-inhibiting effect of EE (P=0.41 leptin-MLV EE vs. vehicle control housing Figure 5E). Secondly, leptin-deficient ob/ob mice were randomly assigned to live in control or EE housing and a third ob/ob group receiving hypothalamic rAAV-BDNF and placed in control housing. At 3 weeks, all mice were implanted with melanoma cells. Tumors were larger in ob/ob mice than wild-type mice (Figure 5F), likely due to the general effects of obesity (Brandon et al., 2009). In contrast to naïve wild-type mice, neither EE nor hypothalamic overexpression of BDNF showed inhibition of tumor in the leptin-deficient ob/ob mice (Figure 5F) although both groups showed lower body weight (Control housing: 39.1±0.8 g, EE housing: 36.6±0.9 g, rAAV-BDNF: 36.2±1.2 g).
In order to rule out the possibility of obesity per se preventing EE effects, we generated a diet-induced obesity (DIO) model by feeding mice with a high fat diet (45% fat, caloric density 4.73 kcal/g) for 10 weeks. When the body weights of DIO mice were similar to those of ob/ob mice, we randomly assigned the DIO mice to EE or control housing. Three week EE lowered body weight and visceral fat mass including the epididymal fat pad and retroperitoneal fat pad as well as serum levels of leptin and IGF-1(Figure 5G). In contrast to ob/ob mice, EE led to a 68% reduction of tumor mass (Figure 5G, P<0.001). In addition, regression analysis showed a significant positive correlation between the basal serum leptin level before tumor implantation and subsequent tumor weight (Figure 5H, P=0.01).
To further define the specific role of leptin on tumor growth from general effects associated with obesity, we carried out a leptin replacement experiment in ob/ob mice using osmotic minipumps to deliver leptin (0.3 mg/kg/d for 14 days). The leptin replacement supplied physiological levels of leptin (1.97±0.31 ng/ml) and decreased food intake by approximately 50% compared to ob/ob mice receiving saline. We pair fed a third group of mice receiving saline to the leptin replacement group. All of the mice were injected with melanoma cells 4 hrs after minipump implantation. The leptin infused mice showed similar body weight and fat mass as the pair-fed saline infused mice (Figure 5I). However the tumor weight was 140% greater in leptin infused mice than pair-fed saline mice (Figure 5I) further supporting leptin’s role in melanoma growth.
Importantly, these experiments showed a consistent inverse relationship between circulating leptin and tumor mass, independent of body weight. Moreover, data in ob/ob mice as well as wild type mice living in control or EE housing showed no correlation between body weight change and subsequent tumor mass following transplantation.
Enriched environmental housing improves survival in a colon cancer model after the tumor is established
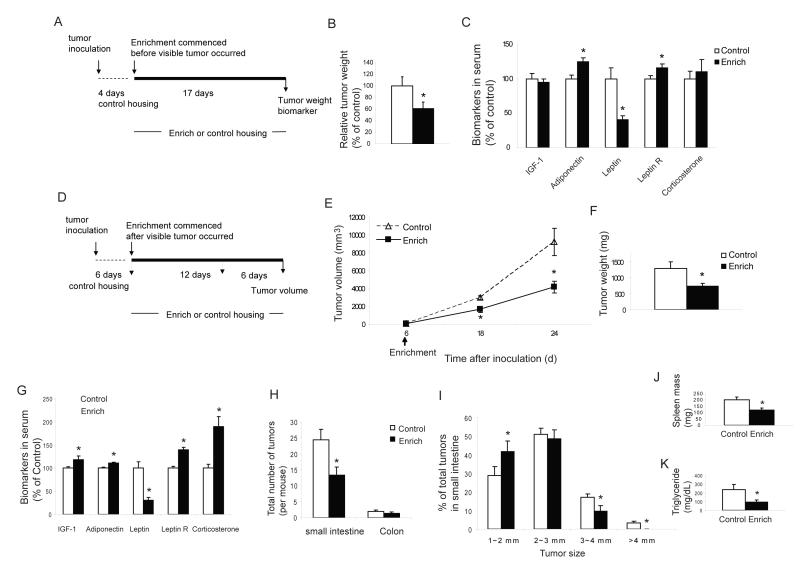
To determine whether our results could be generalized, we studied another well defined murine cancer model, the MC38 colon cancer. Moreover, we also investigated whether this intervention could influence tumor growth after the cancer had been established. We used two cancer models: firstly a minimal disease model in which EE was initiated 4 days after tumor inoculation before the presence of any visible tumors, and secondly, an established tumor model in which EE was initiated following the development of visible tumors. In the minimal disease model, naïve mice received subcutaneous implantation of MC38 colon cancer cells (105 cells per mouse) and were housed in identical standard conditions for 4 days. Half of the mice were then randomly assigned to live in the EE with the other half remaining in control housing. When large tumors were observed in control mice 17 days later, the experiment was terminated (Figure 6A). Tumor weight was significantly reduced in EE mice by 40±11% compared to control mice (Figure 6b) with 10% of EE mice bearing a barely palpable tumor of 25 mg or less. The serum biomarkers in EE mice when carrying the colon cancer showed changes similar to those observed in EE mice before tumor inoculation (Figure 1H) namely an increase in adiponectin and a reduction in leptin (Figure 6C). Consistent with the B16 melanoma experiment, MC38 colon cancer cells also grew more slowly in vitro when cultured with sera from EE mice compared to control mice (data not shown). In the established tumor model, mice received 3 ×104 tumor cells and were maintained in identical housing for 6 days until visible tumors were observed and then randomized to either EE or control housing (Figure 6D). The growth rate of the established tumor was markedly reduced even in this paradigm in which EE was delayed. Tumor size was reduced by 44% at 12 days and 55% at 18 days (Figure 6E) with tumor weight decreased by a comparable degree (Figure 6F).
Figure 6. Enriched environment inhibits colon cancer growth when commenced after MC38 implantation and suppresses intestinal tumorigenesis in ApcMin/+ mice.
(A) Experimental design of the minimal disease model. (B) In the minimal disease MC 38 model, EE significantly reduced tumor weight (n=20 per group, * P<0.05). (C) EE affects biomarkers in the minimal disease model (n=20 per group, * P<0.05). (D) Experimental design of the established MC38 model. (E) In the established tumor model, EE commenced after visible tumor occurred reduced tumor growth rate (n=6 per group, * P<0.05). (F) EE decreased tumor weight d 24 after tumor inoculation (n=6 per group, * P<0.05). (G) Serum biomarkers in ApcMin/+ mice after 3 weeks EE (n=15 per group, *P<0.05). (H) Total number of visible tumors larger than 1 mm in diameter (n=15 EE, n=14 control, P=0.01). (I) EE reduced the size of polyps in small intestine (*P<0.05). (J) EE reduced splenomegaly (n=15 EE, n=14 control, P<0.01). (K) EE reduced triglyceride level (n=15 EE, n=14 control, P<0.01).Values are means ± SEM.
Suppression of intestinal tumorigenesis in ApcMin/+ mice in an enriched environment
To determine whether the EE-induced tumor suppression in tumor implant models could be effective in spontaneous tumorigenesis models more relevant to human disease, we chose to use the ApcMin/+ model of intestinal cancer. The ApcMin/+ mouse contains a germline mutation in the adenomatous polyposis coli (APC) tumor suppressor gene leading to mice highly susceptible to spontaneous intestinal adenoma formation (Su et al., 1992). Mutation of APC is common to most human colon cancers being found in 80% of all colorectal adenomas and carcinomas and is also one of the earliest mutations in colon cancer progression (Goss and Groden, 2000). Therefore the ApcMin/+ mouse has been extensively used as an animal model in understanding colon cancer biology and progression as well as in the evaluation of potential therapeutic interventions. We randomly assigned male ApcMin/+ mouse seven weeks of age to live in EE or control housing. After 3 weeks of EE housing, we measured biomarkers in serum and found similar EE-associated changes (Figure 6G) as those observed in wild type mice (Figure 1H), namely a sharp drop in leptin and increases in adiponectin and corticosterone levels. One of the mice in control housing died at 11 weeks of age while all EE mice survived to the end of the experiment at 13 weeks of age. We blindly examined the entire intestine and scored all visible polyps larger than 1 mm. EE significantly reduced the total number of polyps within the small intestine by 46% (Figure 6H, P=0.01). EE also substantially reduced the size of polyps with a ~50% reduction in all polyps greater than 3 mm in diameter, and no polyps larger than 4 mm (Figure 6I). Splenomegaly occurs in ApcMin/+ mice and spleen size is directly related to the polyp burden (Mehl et al., 2005). Consistent with the reduction in polyp burden, EE significantly reduced splenomegaly by 40% (Figure 6J). Hyperlipidemia is also associated with adenoma formation in ApcMin/+ mouse (Su et al., 1992) with EE decreasing triglyceride level by 61% (Figure 6K).
Discussion
Our results demonstrate that EE significantly reduces cancer burden in both a syngeneic melanoma as well as a colon cancer model. Moreover, the intervention was further generalizable to a spontaneous cancer model, the Apcmin/+ mouse, whose mutation is a common and early event of human colon cancer progression. Moreover, a significant subset of EE mice remained tumor free while all the controls had tumors. In addition EE was effective even though it was initiated after the establishment of the peripheral tumor suggesting potential therapeutic relevance.
The relative tumor resistance in the EE mice was associated with changes in the endocrine axis as well as enhanced immune responses. EE consists of more complex housing with sensory, cognitive, motor and social stimulation (Nithianantharajah and Hannan, 2006). This complexity is further manifest in the interaction between the central nervous, endocrine and immune systems. The pathways involved serve as components of a larger regulatory network that impact on a host’s response to cancer. It is unlikely that a single variable accounts for all the effects of EE although it is plausible that changes in the brain play a central role with the peripheral pathways as secondary effectors. The enriched living condition with increased dynamic social interactions, frequent exposure to novel objects and enhanced physical activity leads to a small (within the physiological range), but statistically significant increase in serum corticosterone. In contrast, runners had a decrease in resting corticosterone. This corticosterone elevation in EE mice is consistent with a mild stress and activation of the HPA. It seems paradoxical that chronic, mild activation of the HPA axis could be associated with a tumor-resistant phenotype. However, classical stress literature would consider the EE “eustress” (positive stress) as opposed to maladaptive “distress” (negative stress), which is associated with exposure to more severe aversive or hostile environments (Selye, 1974; Milsum, 1985). More recently, the concept of allostasis has been used to describe adaptive responses to external challenges (Sterling and Eyer, 1988). Hence, aversive stress may lead to allostatic overload resulting in a compromised axis, poorly equipped to respond to external stressors, and many maladaptive changes including organ dysfunction and suppression of the immune system (McEwen, 1998). In contrast, the allostasis associated with exposure to the mild, non-aversive challenges of EE leads to a more adaptive HPA axis and may therefore buffer the reaction to subsequent major external stressors (Benaroya-Milshtein et al., 2004; Larsson et al., 2002).
The central components of the stress system within the CNS include corticotrophin-releasing hormone (CRH) and the noradrenergic/sympathetic neurons of the hypothalamus and brainstem, which regulate the peripheral activity of the HPA axis and the sympathetic nervous system (SNS). These two arms act in a coordinated manner resulting in adaptations in an animal’s physiological and behavioral state integrated at many levels including the hypothalamus (McDougall et al., 2005), a brain structure critical to the cross-talk between the CNS, endocrine and immune systems. Here we observed that the EE paradigm activated the hypothalamus with induction of the immediate early genes, Fos, Junb and BDNF. This data is consistent with reports showing that acute immobilization stress induces a rapid increase in BDNF mRNA expression in the hypothalamus preceding the activation of CRH neurons, suggesting that hypothalamic BDNF is involved early in the regulation of the HPA axis (Rage et al., 2002; Naert et al., 2006).
In our study, genetic overexpression of BDNF in the hypothalamus increased CRH expression (Cao et al., 2009) and HPA activity, which is consistent with other studies using central administration of BDNF protein (Naert et al., 2006). Our results suggest that the EE effect on sympathetic tone is likely one of the mechanisms underlying the tumor resistance, with hypothalamic BDNF a critical mediator and WAT as the principal peripheral organ responsive to this central regulation (Figure 7). WAT receives sympathetic innervation from cell groups that are part of the general SNS outflow from the brain including hypothalamic PVN, VMH and ARC (Bartness and Song, 2007). Our data suggest hypothalamic BDNF expression signals to increase SNS outflow to WAT. In turn, the sympathetic nerves innervating WAT release NE which activates β-ARs and subsequently leads to lipid mobilization, higher energy expenditure and changes in adipokine production – in particular, an increase in adiponectin and a decrease in leptin and their respective circulating concentrations. Notably, the β-AR blocker propranolol fully inhibited the EE-associated changes in leptin and adiponectin and completely abolished the inhibition of tumor growth, suggesting a link between β-AR activity in WAT, circulating leptin/adiponectin levels and tumor growth. The relationship of leptin/adiponectin with oncogenesis and cancer proliferation is not fully elucidated. However accumulating clinical and experimental evidence supports a role of these adipokines in the development and progression of several cancers including melanoma and colon cancer (Gogas et al., 2008; Barb et al., 2007) via either a direct influence on cellular proliferation and/or indirect effects on inflammation and angiogenesis (Fujisawa et al., 2008; Brandon et al., 2009). We also observed that leptin significantly enhanced both B16 melanoma and MC38 colon cancer cell growth in culture while in contrast, adiponectin significantly inhibited tumor cell growth in vitro (data not shown). The relevance of leptin in EE-induced tumor inhibition was further investigated using both pharmacological and genetic approaches. Firstly, when exogenous leptin was delivered via controlled release liposomes to offset the EE-induced decrease in endogenous leptin levels, the EE-induced tumor reduction was inhibited. Secondly, both EE and hypothalamic overexpression of BDNF failed to reduce tumor growth in leptin deficient ob/ob mice despite a reduction in body weight. In contrast, the equally obese DIO mice responded to EE with a marked reduction in tumor growth indicating obesity per se does not prevent the effect of EE on tumor growth. Our data showed that obesity increases melanoma growth in both genetically-obese ob/ob and diet-induced obese mice. This data is consistent with a recent report that shows B16 melanoma growth is accelerated in both obese ob/ob mice as well as obese MC4R knockout mice. However prevention of obesity by pair feeding ob/ob mice dramatically reduces tumor weight to a level significantly lower than in wild type mice of the same weight (Brandon et al., 2009). Our leptin replacement data also showed that leptin increased melanoma mass in ob/ob mice by 140% compared to pair-fed saline infused mice with identical body weight and fat mass. Moreover, although leptin levels are typically used as a surrogate marker of fat mass and body weight, our data showed that whereas baseline leptin levels consistently and strongly correlated with tumor mass, no such relationship existed for body weight per se, both in the setting of ob/ob mice and non-obese wild type mice. Taken together, leptin is likely a critical peripheral effector linking EE with tumor inhibition.
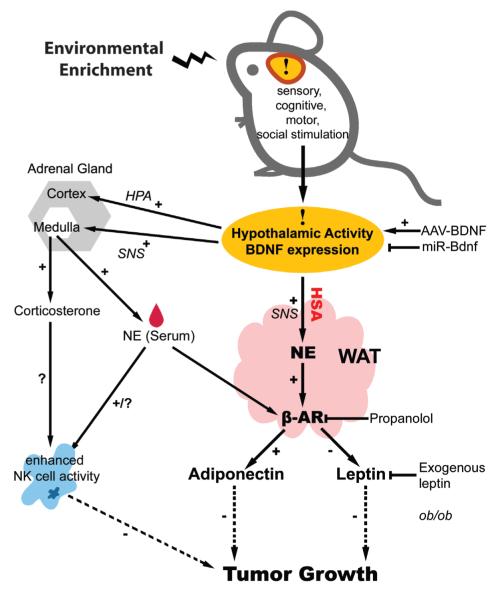
Figure 7. Mechanism of enriched environment -induced tumor resistance.
See Discussion for details.
It is known that physical activity enhances natural immunity and the number and activity of NK cells in the circulation fluctuate greatly during and after exercise and in response to other stressors (Jonsdottir, 2000). NK cells are mainly driven from the spleen into the circulation depending on sympathetic outflow and ambient catecholamine levels. Hence, the observed NK activity change may also be a result of altered NK distribution. The enhanced NK activity in enriched mice can be partly explained by increased sympathetic activity similar to that shown for “mirthful laughter” (Nagatomi, 2006). Given the role of NK cells in tumor immunity, modulation of the immune system is likely a contributor to the tumor resistance phenotype we observed. However voluntary running did not lead to significant decrease of tumor weight, although its effect on immune function was comparable to that of EE, suggesting only a partial role of immune modulation in the observed tumor suppression. Therefore our data is consistent with a model in which WAT adipokines, driven by hypothalamic BDNF induced sympathetic outflow, serve as the major downstream effectors of the complex regulatory network leading to an anti-proliferative phenotype.
In summary, our results demonstrate that living in an enriched environment leads to significant inhibition of cancer growth. Here we propose one mechanism, the activation of the HSA axis. Specifically, the induction of hypothalamic BDNF expression in response to environmental stimuli leads to sympathoneural activation. The elevated sympathetic drive activates adipocyte β-ARs inhibiting leptin expression and release, and increasing adiponectin expression and circulating concentrations. These adipokines have both direct mitogenic (leptin) and antimitogenic (adiponectin) activity, and in addition can influence peripheral tumor growth indirectly including effects on angiogenesis. At a clinical level, our data shows that direct gene transfer of BDNF can mimic the anti-proliferative effects of EE, and suggests that either environmental or direct molecular approaches to induce hypothalamic BDNF expression may have therapeutic potential.
Experimental Procedures
Environmental enrichment protocol
Male 3 week old C57/BL6 mice were housed in groups (18-20 mice per cage) in EE cage as detailed in supplementary data. We carried out all mice experiments in compliance with the regulations of the Institutional Animal Ethics Committees.
Microdissection with laser capture
We randomized mice 3 weeks of age to live in standard housing or EE for 2, 4 and 9 weeks. At each time point, brains were isolated and stored at −80 °C until microdissection as detailed in supplementary data.
AAV mediated BDNF overexpression
We generated AAV serotype 2 vectors of human BDNF as detailed in supplementary data. We randomly assigned 26 C57BL/6 mice, male, 8 weeks of age, to receive AAV-BDNF (n=10) or AAV-GFP (n=16). rAAV vectors (1×1010 genomic particles per site) were injected bilaterally into the hypothalamus at the stereotaxic coordinates −1.2AP, ±0.5ML, −6.2DV (mm from bregma) using a microinfusion pump. Four weeks after AAV injection, we withdrew blood and inoculated B16 melanoma cells.
ob/ob mice EE and hypothalamic BDNF overexpression experiment
We randomly assigned 30 male ob/ob mice (Jackson Laboratory), 4 weeks of age, to live in EE, control housing, or receive AAV-BDNF as described above and living in control housing, n=10 per group. We inoculated melanoma cells 3 weeks after EE or AAV injection.
ApcMin/+ mice experiment
Male C57BL/6J-ApcMin/+ mice were purchased from the Jackson Laboratory. 7-week old male ApcMin/+ mice were randomly assigned to live in the EE or standard housing for 6 weeks (n=15 per group). The location, number and size of visible tumors in the entire intestine were determined under a dissection scope.
Statistical analysis
Values are expressed as mean±s.e.m. We used one-way ANOVA to analyze tumor volume, tumor weight, ELISA and tumor cell proliferation in culture. We used multivariate ANOVA to analyze quantitative RT-PCR data. For the immune cell proliferation and cytotoxicity, we determined the overall significance using repeated measures ANOVA.
Supplementary Material
Acknowledgements
We thank Dr. F. Lee of Weill Medical College of Cornell University for providing the BDNF+/− mice and Professors C. Croce, J. Groden, M. Caligiuri for helpful comments and discussion on the manuscript. We appreciate the technical assistance of Michael Cahill, Adam Martin. This work was supported in part by the NIH.
Footnotes
Competing Interests statement The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Barb D, Williams C, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am. J. Clin. Nutr. 2007;86:858s–866s. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor pays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol. Behav. 2007;91:343–351. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decrease anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Brandon EL, Gu JW, Cantwell L, He Z, Wallace G, Hall JE. Obesity promotes melanoma tumor growth: role of leptin. Cancer Biol. Ther. 2009;8:1879–1887. doi: 10.4161/cbt.8.19.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cao L, Lin ED, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat. Med. 2009;15:447–454. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo D, Rachiglio D, A.M., la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J. Cell. Biochem. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Darnell RB, Posner B. Paraneoplastic syndromes affecting the nervous system. Semin. Oncol. 2006;33:270–298. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine GM-CSF stimulates potent, specific, and long-lasting antitumor immunity. Proc. Natl. Acad. Sci. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Agar L, Summers RJ. The role of the sympathetic nervous system in the regulation of leptin synthesis in C57BL/6 mice. FEBS. Lett. 1999;444:149–154. doi: 10.1016/s0014-5793(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, Inamori M, Nakajima N, Watanabe, Kubota M, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;51:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C, Surmacz E. Leptin and cancer. J. Cell. Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- Gogas H, Trakatelli M, Dessypris N, Terzidis A, Katsambas A, Chrousos GP, Petridou ET. Melanoma risk in association with serum leptin levels and lifestyle parameters: a case-control study. Ann. Oncol. 2008;19:384–389. doi: 10.1093/annonc/mdm464. [DOI] [PubMed] [Google Scholar]
- Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J. Clin. Oncol. 2000;18:1967–1979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- Hahn S, Haselhorst U, Quadbeck B, Tan S, Kimmig R, Mann K, Janssen OE. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2006;154:287–294. doi: 10.1530/eje.1.02078. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hughes P, Beilharz E, Gluckman P, Dragunow M. Brain-derived neurotrophic factor is induced as an immediate early gene following N-methyl-D-aspartate receptor activation. Neuroscience. 1993;57:319–328. doi: 10.1016/0306-4522(93)90065-n. [DOI] [PubMed] [Google Scholar]
- Jenkins PJ, Bustin SA. Evidence for a link between IGF-1 and cancer. Eur. J. Endocrinol. 2004;151(suppl 1):s17–22. doi: 10.1530/eje.0.151s017. [DOI] [PubMed] [Google Scholar]
- Jonsdottir IH. Exercise immunology: neuroendocrine regulation of NK-cells. Int. J. Sports. Med. 2000;21:s20–23. doi: 10.1055/s-2000-1447. [DOI] [PubMed] [Google Scholar]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol. Biochem. Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Nolan B, Peng QY, Xie JG, Wagner GC, Conney AH. Stimulatory effect of voluntary exercise or fat removal (partial lipetomy) on apoptosis in the skin of UVB light-irradiated mice. Proc. Natl. Acad. Sci. USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE, Lawrence AJ. Central autonomic integration of psychological stressors: focus on cardiovascular modulation. Auton. Neurosci. 2005;123:1–11. doi: 10.1016/j.autneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in Apcmin/+ mice. J. Appl. Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Mihm MC., Jr. Melanoma. N. Engl. J. Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Milsum JH. A model of the eustress system for health/illness. Behav. Sci. 1985;30:179–186. doi: 10.1002/bs.3830300402. [DOI] [PubMed] [Google Scholar]
- Naert G, Ixart G, Taoua-Arancibia L, Givalois L. Continuous I.C.V. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neurosci. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Nagatomi R. The implication of alterations in leukocyte subset counts on immune function. Exerc. Immunol. Rev. 2006;12:54–71. [PubMed] [Google Scholar]
- Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of nervous system. Nat. Rev. Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Rage F, Givalois L, Marmigere F, Tapia-Arancibia L, Arancibia S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neurosci. 2002;112:309–318. doi: 10.1016/s0306-4522(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress without distress. McClelland and Stewart Ltd.; Toronto: 1974. [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. John Wiley; New York: 1988. pp. 629–649. [Google Scholar]
- Su LK, Kinzler KW, Vogelsterin B, Preisinger AG, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tagami K. Voluntary wheel-running exercise enhances antigen-specific antibody-producing splenic B cell response and prolongs IgG half-life in the blood. Eur. J. Appl. Physiol. 2005;94:514–519. doi: 10.1007/s00421-005-1378-4. [DOI] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteve M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Wauters M, Considine RV, Van Gool LF. Human leptin: From an adipocyte hormone to an endocrine mediator. Eur. J. Endocrionol. 2000;143:293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- Wisse BE, Scwartz MW. The skinny on neurotrophins. Nat. Neurosci. 2003;6:655–666. doi: 10.1038/nn0703-655. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat. Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.