Abstract
CD30 is highly expressed on Hodgkins lymphoma and anaplastic large cell lymphoma, making it an attractive target for therapy. We describe the generation of serum-stabilized ssDNA aptamers that bind CD30 via a hybrid SELEX methodology. The selected aptamer bound CD30 with high affinity and specificity. Further optimization of the aptamer led to a short, truncated variant with a 50-fold higher affinity than its longer counterpart. The multivalent aptamer was able to induce oligomerization of CD30 receptors and, in effect, activate downstream signaling, which lead to apoptosis of ALCL cells. Immunotherapy using aptamer-based co-stimulation provides an alternative to antibodies, and has potential to transform cancer treatment.
Keywords: ssDNA aptamer, CD30, lymphoma, ALCL, cHL, cancer immunotherapy
Introduction
Aptamers are small oligonuleotide molecule ligands, RNA or single-stranded DNA (ssDNA), and have high affinity for target binding [1–3]. In contrast to protein antibodies, aptamers can be easily generated through chemical synthesis and manufactured at much less cost. In addition, as a short oligonucleotide biomaterial, the aptamers show little or no activation of the immune response in vivo. These features of aptamers allow numerous possibilities for their use in medical applications. While clinical applications of aptamers have not yet been well investigated, they have demonstrated an ability to specifically target certain biomarkers on cancer cells, including CD30 protein, which has been detected in some hematological malignancies [4, 5]. Expression of CD30 has been considered as a specific diagnostic biomarker of anaplastic large cell lymphoma (ALCL) and classical Hodgkin lymphoma (cHL)[6–11]. CD30 is also a biomarker used for targeted therapy by an antibody-drug conjugate, brentuximab, which was recently approved by the FDA[12–14]. Moreover, studies have shown that trimerization of CD30 receptors induced by the CD30 ligands can activate cellular signaling, and subsequently regulate functions of the targeted cells [15]. Although CD30 receptors lack the death domain, the ligand-induced activation could induce apoptosis of the ALCL tumor cells [16]. These observations suggest a potential immunotherapy approach to treat ALCL by selectively targeting and inducing trimerization of CD30, activating the cellular signaling pathway and triggering tumor cell apoptosis through a natural biological process. To advance aptamer technology for clinical use, we previously tested a reported RNA-based aptamer sequence for both flow cytometry analysis of CD30-expressing lymphoma cells and immunostaining of formalin-fixed and paraffin-embedded tumor tissues [17, 18]. Although the RNA-based aptamers are widely studied, their clinical applications are largely limited due to poor stability under biological and physiological conditions [19]. Modified RNA nucleotides have been incorporated into the aptamers to enhance their stability, however, chemical modification of RNA nucleotides have demonstrated minimal improvement in prolonging the half- life of aptamers in vivo [19–21]. Another potential solution to overcome this technical obstacle is to exploit the inherent stability of ssDNA (compared to RNA) in biological environments, and develop ssDNA-based aptamers. In this study, a ssDNA-based aptamer specific for CD30 was developed and its physical and biological properties were investigated.
Materials and Methods
Cell lines and reagents
Karpas 299 (K299, T-cell lymphoma), Jurkat, Molt-4, SupT1 (T-cell leukemia), U937 (histiocytic lymphoma), HDLM2, KM-H2, (Hodgkin lymphoma), K562 (chronic myeloid leukemia), HL60 (acute promyelocytic leukemia), HEL (erythroleukemia), Jeko-1 (B-cell lymphoma), Maver-1 (mantle cell lymphoma), CA46 (Burkitts lymphoma), SKBR3 (breast adenocarcinoma), and LNCAP (prostate carcinoma) cell-lines were used and obtained from American Type Culture Collection (Manassas, VA). All cell lines were cultured in recommended medium supplemented with heat-inactivated Fetal Bovine Serum (FBS) (GIBCO, Grand Island, NY), and 100 IU/mL penicillin-streptomycin. The washing buffer used during aptamer enrichment contained 4.5 g/l glucose and 5 mM MgCl2 in Dulbecco’s PBS (Sigma, St. Louis, MO). One mg/mL BSA (Fisher, Waltham, MA) with 0.1 mg/mL t-RNA was added to reduce nonspecific background binding, and to make binding buffer from the wash buffer. Trypsin was purchased from Fisher, and PCR reagents and Taq polymerase were purchased from Takara Bio (Mountain View, CA).
Development of ssDNA aptamers using hybrid systematic evolution of ligands by exponential enrichment (hybrid SELEX) approach
The library for SELEX contained a random core of 30 mer with an 18 mer primer binding region on both sides. Biotin reverse primer was used to generate single-stranded DNA, and a forward primer labeled with either FITC or Cy5 was used to monitor aptamer selection. OligAnalyzer® software from IDT Technologies was used to optimize primers. The aptamer pools were PCR amplified with Fwd Primer: 5′-TAC CAG TGC GAT GCT CAG -3′ and Rev Primer: 5′-GTC AAC CGA ATG CGT CAG -3′
For SELEX, approximately two million CD30-positive K299 cells were washed with PBS and centrifuged at 270 g. The cells were incubated with a DNA library which was rapidly cooled on ice after heating at 95°C for 5 min. Selection was initiated with a 20 nmol ssDNA library and gradually reduced as the selection progressed. The selection stringency was also increased by reducing the incubation time from 60 min in the first round to 20 min at the end of selection. Unbound DNA was removed by centrifugation, and the target-bound DNA eluted by heating the cells at 95°C for 5 min. The eluted DNA was PCR amplifi ed by Taq DNA polymerase, and PCR conditions were optimized to yield a clear, single band after each round of SELEX. Single-stranded DNA was generated from the PCR product using high-affinity streptavidin-sepharose beads which acted as binding sites for the biotin-labeled anti-sense strand. The sense strand with the flurophore was eluted using 200 mM NaOH. This ssDNA was used for the next round and the process was repeated iteratively until significant affinity towards target CD30+ cells was observed using a flow cytometer. To reduce the number of probable aptamers, a counter selection with Jurkat cells was performed after 10 rounds of SELEX, and then performed for every alternate round of SELEX.
In addition, protein selection with purified His-tagged CD30 protein was carried out similarly, with minor changes: 1) the binding buffer did not contain glucose and included 2 mg/ml BSA; 2) His-tagged CD30 protein was conjugated to TALON magnetic beads and incubated with DNA aptamer pools; 3) bound and unbound DNA was separated on a magnetic stand; and 4) aptamer pools were further refined by performing a negative selection specifically against the TALON beads.
The identification of ssDNA aptamers with higher nuclease stability and in-vivo use were generated by modifying the cell-SELEX protocol, and terming the protocol “hybrid SELEX.” Hybrid SELEX involves the use of cell surface markers to isolate aptamers binding the target of interest (e.g., CD30) expressed on tumor cells via cell-SELEX and through the use of purified protein. We started the selection with ~1014 unique DNA sequences contained in 20 nmoles of a ssDNA library containing a 30 mer random region. The stringency of selection was increased by reducing the concentration of the amplified ssDNA pool and the time of incubation, and increasing the washing time and volume. Also, a negative selection was performed with Jurkat cells, which do not express the CD30 protein, to remove aptamers binding the commonly expressed proteins and other molecular markers on the surface of cells. After 15 rounds of selection, the resulting aptamer pool not only possesses binding specificity for the target CD30 biomarkers, but for any other unique surface makers expressed on the target CD30-expressing K299 cell line. This results in a family of aptamers which may also include a few off-target aptamers specific to the target cells. We then used purified His-tagged CD30 protein to further focus the aptamer pool specifically towards CD30 and remove aptamers targeting other markers. The His-tagged CD30 was then immobilized on TALON magnetic beads. Three rounds of selection were performed with increasing stringency in which we reduced the concentration of the DNA pool by 100-fold, and increased the washing time from 10 min to 30 min, and the washing volume from 100 ul to 1000 ul. The pool was then incubated with TALON beads to remove the sequences binding the beads. We tested the selection progress with flow cytometry using Cy5-labeled primer to generate a fluorescently-labeled pool with CD30-positive and -negative cells. The selection was stopped when no further progress was observed for 3 rounds of selection.
The enriched pool from the last round of hybrid SELEX was cloned using a T7 vector and sequenced at the sequencing core of the Baylor College of Medicine, Houston. Pools 20–23 were tagged with multiplex identifier primers for next-generation sequencing, and sequenced using the Ion-Torrent sequencing platform. Analysis of NGS data was performed using MAFFT alignment software [22, 23].
Flow cytometric analysis
One-half million cells were washed with PBS and then incubated with 100 ul of a 250 nM Cy5- or FITC- labeled DNA pool, then re-suspended in 250 μL binding buffer. Ten thousand cells were counted on a BD LSR II flow cytometer (Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ). The fluorescence readouts represented the affinity of the fluorophore-labeled pools towards the CD30-positive cells.
After the generation of aptamers, FITC-labeled aptamers were used for characterization using CD30-positive and CD30-negative cells. The apparent dissociation constants (Kds) were measured by flow cytometry using a series dilution of the aptamers that was incubated and analyzed on the flow cytometer.
Competition experiments
Competition was performed by incubating a fluorophore-labeled aptamer with an unlabeled aptamer sequentially, in reverse order, and simultaneously. The experiments were performed using a 1:10 ratio of labeled aptamer to unlabeled aptamers.
Biostability assays
One microgram of DNA and RNA CD30 aptamer was incubated with human serum for 24 hr, and samples were collected at different times using phenol-chloroform extraction. The amount of digested aptamer was visualized on a 5% agarose gel.
Aptamers in complex media
Target K299 cells were mixed with non-target U937 cells and incubated with 10 nM Cy3-labeled aptamer for 30 min, washed with 5× washing buffer, and tested on a flow cytometer. Non-specific binding of the aptamer was tested by addition of K299 cells with leukocytes after lysing red blood cells from the whole blood. Similarly, K299 cells were also added directly to whole blood and tested for specific binding of aptamers. Flow cytometry was performed with PerCP CD45 and FAM CD30 antibody, along with the Cy-3-CD30 aptamer.
Fluorescence microscopy
Imaging was performed using an Olympus epifluorescence microscope (Olympus America, Center Valley, PA). The mixture of CellTrace CFSE-stained U937 control cells and unstained K299 cells was incubated with 100 nM of the Cy3-labelled aptamer C2NP for 30 min, then washed with 5× washing buffer.
Biotherapy assay
K299 and HDLM2 cells were incubated with the CD30 aptamer and the ratio of dead/live cells was measured using a dual-staining assay after 24, 48, 72, and 96 hr. The dual-staining assay used propidium iodide, which stains for dead cells, and Hoechst 33362 dye, which stains for live cells. Absolute cell counts were measured using flow count beads in the assay. For immunotherapy, the experiments were performed with biotin-labeled 1 μM DNA and 250 nM streptavidin to form a multimeric aptamer, and CD30 signaling was initiated by trimerization of CD30 antigens. A sample control with streptavidin was also included in the experiments.
Statistics
The binding affinity and immunotherapy co-stimulation experiments were performed in duplicate and repeated two times. The average of the readings was used, and the error bars represent the standard deviation. The graphs were plotted using MS-Excel 2010.
Results
Development of ssDNA aptamers specific for CD30
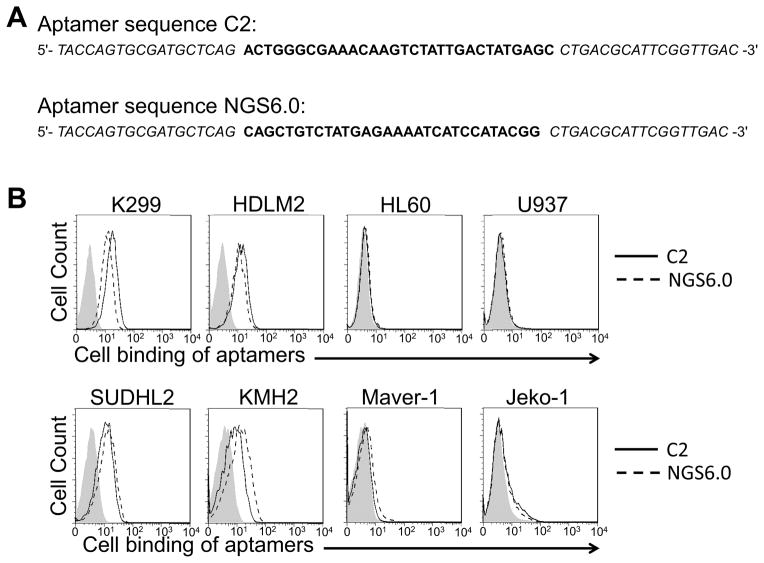
The resultant ssDNA pool was cloned after 23 rounds of enrichment and selection using the hybrid SELEX approach (Supplemental Figure S1). Among the sequenced clones, sequence C2 (Figure 1A) showed the highest frequency and was selected for further analysis. Simultaneously, next-generation sequencing analysis using multiplex identifiers was also performed on the enriched ssDNA pools from different rounds of SELEX (rounds 20–23). Sequence NGS6.0 (Figure 1A) was detected in multiple enriched ssDNA pools, with an increasing percentage as the selection progressed.
Fig. 1.
Selection and characterization of CD30-binding aptamers. A) Resulting individual aptamer sequences from the sequencing of pools of aptamers that demonstrating high affinity for target cells. B) Aptamers tested against CD30-positive and CD30-negative cells. C) Binding curves of aptamers (or apparent Kds) against CD30-positive and CD30-negative cells.
It was expected that employing the hybrid SELEX approach would allow us to develop an aptamer that was specific for the target biomarker, and also able to bind to live tumor cells. To validate cell binding capacity, the ssDNA aptamer sequences C2 and NGS6.0 were synthesized and conjugated with a Cy5 fluorescent reporter. The aptamer probes were incubated with cultured CD30-expressing tumor cells (K299, HDLM2, SUDHL2, and KMH2) and control cells (HL60, U937, Maver-1, and Jeko-1) known to be CD30 negative. After treatment for 30 min at room temperature, resultant cell binding of aptamers was quantified by flow cytometry analysis. As shown in Figure 1B, both aptamer sequences specifically bound to CD30-expressing cells, but did not react to control cells. In addition, cell-binding affinity of aptamers was analyzed using a series dilution. Flow cytometry assays revealed that aptamer C2 had a slightly higher binding affinity than that of aptamer NGS6.0 (50 nM vs. 100 nM, respectively) (Figure 1C).
To optimize aptamers C2 and NGS6.0, a series of trimmed sequences were screened in an attempt to achieve a minimum sequence length that improved binding affinity and specificity. The secondary structure of naive and sequence-trimmed aptamers was visualized using mfold [24–26], and is shown in Figure 2A. Modeling analysis of secondary structures showed trimming of either primer on each site altered the secondary structure, yet both aptamers retained a part of the parent stem-loop structure. Core sequences of each aptamer without primer sequences on both sites were then synthesized, and the resulting truncated aptamers were designated C2NP and NGS6.0NP (Figure 2B). Flow cytometric analysis confirmed that both C2NP and NGS6.0NP specifically bound to CD30-expressing cells (K299 and HDLM2), but not to CD30-negative cells (Maver-1 and U937) (Figure 2C). Although aptamers C2NP and NGS6.0NP have different sequences and secondary structures, both of them are specific for CD30. It would be helpful to know whether these aptamers target the same epitope of the CD30 receptors on the cell surface. A competition assay was carried out by simultaneously incubating K299 cells with unlabeled aptamer NGS6.0NP and Cy5-labeled aptamer C2NP at a ratio of 10:1. Flow cytometry analysis revealed that the presence of unlabeled aptamer NGS6.0NP almost completely blocked cell binding of C2NP (Figure 2D), indicating that both aptamers targeted the same epitope of the CD30 receptor.
Fig. 2.
Truncation of selected CD30 aptamers and testing of optimized short aptamers. Truncation of aptamers C2 and NGS6.0 was performed to retain a stem-loop structure similar to the parent aptamers potential binding site. A) mfold software (State University of New York, Albany, NY) was used to predict the secondary structure of the DNA aptamers C2 (panel i) and NGS6.0 (panel ii). The above example shows that truncation of an aptamer, by removing either primer site, changes the secondary structure significantly. Surprisingly, a stem-loop structure highly similar to a part of the parent sequence is maintained when both primer sites are removed. B) The truncated aptamer sequences C2NP and NGS6.0NP. C) Testing of the truncated aptamers with positive and negative cell lines showed that the specificity of the aptamers was maintained, indicating that the primer regions were not involved in recognition of the binding sites on the target cells. D) A competition experiment with a 10× excess of unlabeled aptamer (NGS6.0NP) was used in the presence of a labeled aptamer (C2NP). A loss of shift was observed indicating that the aptamers C2NP and NGS6.0NP were competing with each other for a single aptamer binding site. E) Apparent Kds of truncated aptamer C2NP with CD30-positive and -negative cells.
Characterization of ssDNA aptamers specific for CD30
Since aptamer C2 showed slightly higher binding affinity (Figure 1C), it was used for further investigation. To rule out the potential adverse effects of sequence shortening, specificity of aptamer C2NP was further validated by cell-binding analysis. This analysis confirmed that C2NP did not react to CD30-negative cells (Supplementary Figure S2 and Table 1). More importantly, a dose-response assay demonstrated that aptamer C2NP had a significantly high affinity to CD30-expressing cells, reaching maximal binding at as low as 2 nM final concentration (Figure 2E), nearly 50-fold higher than the full-length aptamer C2.
Our previous study demonstrated the utility of a RNA-based aptamer to detect CD30-positive cells [17, 18]. To compare characterize the binding performance of the aptamer C2NP to that of the reported RNA-based CD30 aptamer, specific cell binding of ssDNA and RNA aptamers was analyzed following trypsin digestion of cell surface proteins. Digestion resulted in an equal proportional loss of cell binding capacity by the RNA aptamer and aptamer C2NP (Figure 3A), confirming the cell surface binding nature of aptamers. To further characterize the target-binding properties of the aptamer C2NP, we performed a series of competition studies utilizing the RNA-based aptamer, the aptamer C2NP, and an anti-CD30 antibody. The aptamer C2NP showed reduced binding ability to CD30-expressing cells in the presence of excess RNA-based aptamers (Figure 3B, left). Similarly, the presence of excess C2NP inhibited cell binding of RNA-based aptamers (Figure 3B, right), indicating that C2NP and the RNA-based aptamer may target the same epitope of the CD30 receptors on the cell surface. In contrast, even though the presence of anti-CD30 antibody had no effect on the cell biding of C2NP aptamers (Figure 3C, right), the presence of C2NP slightly inhibited the binding of anti-CD30 antibody (Figure 3C, left). This suggests that either the antibody or the aptamer may target different epitopes of the CD30 receptors expressed in close proximity on the surface of the cells. This can be partly attributed to the relative small size of the aptamer, which allows it to access its binding site even in the presence of antibody. However, the larger antibody loses some of its binding ability in the presence of the aptamer.
Fig. 3.
Comparison of DNA and RNA aptamers, and competition between the DNA aptamer, RNA aptamer, and CD30 antibody. A) CD30-positive K299 cells are treated with trypsin for 2 min and incubated with the DNA aptamer (left panel) and the RNA aptamer (right panel). Both aptamers show a similar pattern of aptamer binding, and the loss of binding after trypsin treatment represents a similar protein target. B) Subsequently, competition experiments with the CD30 RNA aptamer, C2NP DNA aptamer, and CD30 antibody in different configurations were performed. In the left panel, the fluorescence peak due to a Cy5-labeled DNA aptamer moves left, indicating a loss of binding ability in the presence of 1:10 unlabeled CD30 RNA aptamers. The loss of binding ability implies that the RNA and DNA aptamers bind the same site. The right panel shows the loss of ability in reverse configuration, wherein the RNA aptamer was labeled and the unlabeled DNA aptamer was present in 10× excess. C) Moreover, competition between the CD30 antibody and the DNA aptamer present a more complex picture. The binding of the antibody is affected due to the presence of the aptamer, but not vice-versa. The left panel shows that addition of antibody after binding of 1 μM of aptamer partially inhibits the binding of the antibody by occupying the CD30 binding sites. In the right panel, no effect of prior binding with the CD30 antibody is seen on the binding of the C2NP aptamer. The results indicate that the aptamer and antibody bind CD30 in close proximity, wherein the binding of the antibody is partially affected due to the prior aptamer binding, which limits the antibody’s access to its target sites. However, the aptamer, due to its small size, is able to bind its target site even in the presence of prior antibody binding.
Biostability analysis of ssDNA aptamer
High susceptibility of RNA sequences to environmental nucleases limits their potential clinical application. Thus, in this study, we developed an ssDNA aptamer to be significantly more stable than its RNA counterpart. To compare biostability, the aptamer C2NP or the RNA aptamer were added into human serum to mimic an in vivo physiologic condition. After incubation for 24 hr at 37°C, the CD30-expressing K299 tumor cells were added into aptamer serum and changes in aptamer function were evaluated by cell binding assay. Flow cytometry analysis demonstrated that C2NP remained at a near 100% binding capacity to K299 cells (Figure 4A, left). In contrast, under the same condition, the RNA aptamer had a rapid 50% loss of binding ability to tumor cells within 30 min (Figure 4A, right). In addition, a time-course analysis of gel electrophoresis was also performed. The C2NP and RNA aptamers were incubated in serum as described above, and subsequently recovered from serum and examined on 5% agarose gel. As shown in Figure 4B, the aptamer C2NP had minimal change from 0 to 24 hr incubation in serum (top panel), and the RNA aptamer was completely digested within 1 hr (bottom panel). These findings indicate that the developed ssDNA aptamers are stable in human serum at 37°C, a biological and physiological condition, and therefore may be suitable for in vivo use.
Fig. 4.
Biostability of the DNA aptamer in comparison with the RNA aptamer. A) The RNA aptamer starts losing is binding affinity due to its low biostability within 1 hour; the nucleases present in serum digest the RNA aptamer which loses 50% of its binding ability, whereas the DNA aptamer is stable even after 8 hr of incubation in serum. Of note, we observed 50% of the DNA aptamer intact after 24 hr of incubation with serum. Flow cytometry graphs show minimal loss of binding ability of the DNA aptamer (panel1) after 24 hr, but the RNA aptamer (panel 1) starts losing its binding ability after 1 hour. B) Agarose gels showing stability of DNA (panel1) and the RNA (panel2) aptamer over the time course of 24 hr.
Aptamers in complex media
To validate potential use of the aptamer C2NP, a cell mixture was made by diluting CD30-expressing K299 cells into CD30-negative U937 cells at a ratio of 1:10. The cell mixture was then treated with aptamer C2NP and anti-CD15 antibody for 30 min at room temperature. Flow cytometry analysis showed that C2NP detected a population of cells in the cell mixture (Figure 5A, left) with an identical staining pattern to that observed by anti-CD30 antibody in the control group (Figure 5A, center). Furthermore, double-staining of the cell mixture with both C2NP and anti-CD30 antibody confirmed that aptamer C2NP and anti-CD30 antibody targeted the same cell population (Figure 5A, right). To assess potential clinical use, cultured K299 cells were diluted into human leukocytes freshly isolated from normal donors at an approximate ratio of 1:30. The tumor cell/leukocyte mixture was double-stained with anti-CD45 antibody and aptamer C2NP or anti-CD30 antibody as a standard control. As shown in Figure 5B, the diluted tumor cells were highlighted by aptamer C2NP or anti-CD30 antibody with similar staining patterns when compared to the control group stained by CD45 antibody alone. Importantly, double-staining by both C2NP and anti-CD30 antibody detected the same population of tumor cells (Figure 5B, far right). Furthermore, cultured K299 cells were diluted into whole blood at an approximate ratio of 1:100, and the tumor cell/blood was stained with antibodies and aptamer C2NP as described above. Again, aptamer C2NP detected the same population of tumor cells in the whole blood mixture as was detected by antibody (Figure 5C), suggesting that the aptamer C2NP may be suitable for clinical use. Selective cell staining with aptamer C2NP of CD30-positive cells in a mixture of K299 and U937 cells was also observed by fluorescent microscope (Figure 5D).
Fig. 5.
The aptamer recognizes and binds its target with high affinity in a complex biological environment without any background noise and non-specific binding. A) The aptamer specifically stains only CD30-positive K299 cells at 10 nM concentration in a mixture of K299 and U937 cells co-stained with CD30 antibody-binding K299 cells and CD15 antibody-recognizing U937 cells. B) K299 cells with leukocytes from blood and whole blood (C) were co-stained with Cy5-labeled CD30 aptamers, FITC-labeled CD30 antibody, and PerCP-labeled CD45 antibody. The aptamer binds specifically to CD30-positive K299 cells without any non-specific binding to leukocytes or any other elements present in the whole blood. D) U937 cells stained with CellTrace CFSE dye (visualized in FITC channel) were mixed with K299 cells, and the mixture was incubated with 50 nm of Cy3-C2NP aptamer. The aptamer specifically stains only the K299 cells.
Biotherapeutic effect of ssDNA aptamer on ALCL tumor cells
After confirmation of its biostability, we investigated whether the developed aptamer C2NP can be used in a therapeutic capacity against CD30-expressing lymphoma tumors. It has been reported that triggering polymerization of CD30 receptors with antibodies or ligands induces apoptosis of ALCL tumor cells [4, 5, 27, 28]. For this purpose, the aptamer C2NP was biotinylated and its polymer forms were generated with incorporation of streptavidin. For the immunotherapy assay, cultured K299 cells of ALCL were treated with the aptamer C2NP monomers, polymer forms, or streptavidin as a control (Figure 6B). After treatment for 3 days, cells were collected and percentages of viable and dead cells were counted by flow cytometry with propidium iodide staining of dead cells. As shown in Figure 6C, exposure of ALCL tumor cells to polymers of C2NP significantly stimulated cell death 6-fold higher than that observed in the control group treated with streptavidin alone. Treatment by the aptamer C2NP alone also triggered more than a 1-fold increase in dead cells. In contrast, under the same treatment conditions, the aptamer C2NP did not have any effect on CD30-negative HL60 tumor cells, indicating the observed immunotherapy effect was cell-specific and mediated by CD30 receptors.
Fig. 6.
Aptamer-based biotherapy of ALCL cells. A) Schema showing receptor oligomerization inducing downstream signaling. CD30-associated signaling is activated by its ligand through trimerization of the receptor, leading to varied outcomes that range from apoptosis to proliferation. B) CD30-positive and -negative cells were incubated without any treatment; in presence of control streptavidin, monomeric aptamer C2NP, and multimeric aptamer C2NP, for 72 hr to detect aptamer-mediated CD30 signal transduction. A multivalent CD30 aptamer was made using biotinylated C2NP (3×) with streptavidin (1×). C) We observed that the multivalent CD30 aptamer induced signaling, resulting in a higher percentage of dead cells in CD30-positive ALCL (K299 cells), and had no effect on cell death in CD30-negative (HL60) cells. The ratio of dead/live cells was calculated by using Hoechst 33342 to stain live cells and propidium iodide to stain dead cells. Experiments were performed twice in duplicate, and the error bars indicate the standard deviation.
Discussion
Elevated CD30 expression is a defining feature of ALCL and, therefore, the CD30 biomarker could serve as a highly promising target for therapies aimed at modulating tumor cells. Thus, interest in generating aptamers to target cell surface molecules, such as CD30, for diagnostic and therapeutic applications is growing. This is due in large part to the inherent advantages of aptamers over antibodies, such as chemical synthesis and stability [3, 13, 29, 30]. We previously characterized the ability of RNA aptamers to bind specifically to CD30, and showed their diagnostic utility in detecting CD30-expressing lymphoma cells and as an immunostain for formalin-fixed and paraffin-embedded tissues [17, 18]. However, RNA-based aptamers are not stable for in-vivo applications due to rapid digestion by serum nucleases. Therefore, there is a need to develop biostable aptamers by either chemical modification of RNA oligonucleotides or, alternatively, by developing DNA-based aptamers, which would be an easier, less costly process and result in a more stable product [31, 32]. We therefore set out to develop ssDNA-based aptamers that target CD30, show high biostability, and can be utilized for both in-vivo diagnostics and therapy.
Aptamers are developed for variety of targets using an in vitro selection process known as SELEX [33–38]. Cell-SELEX is a modification of this process that generates aptamers for live cells [30, 39–43]. Aptamers generated with purified proteins may not recognize their target in its natural environment and, similarly, it is difficult to generate high-affinity aptamers via cell-SELEX for certain targets [44, 45]. In this study, we have modified the cell-SELEX protocol [46] to include selection with a purified protein in order to focus the DNA library towards the target of interest, while isolating aptamers unique for the surface biomarkers expressed on the target cells (Supplementary Figure S1). This hybrid SELEX modification allows for the generation of aptamers that recognize their target with high affinity in the target’s natural environment, while simultaneously eliminating binding to non-target cell surface markers. Utilizing this hybrid SELEX technique, we isolated two aptamers, C2 and NGS6.0, that specifically bound with high affinity only to CD30-expressing cells (Figure 1). In order to make the aptamers suitable for clinical applications, increase bioavailability, and decrease potential off-target effects, the secondary structure analysis of truncated forms of the isolated aptamers led to two minimized aptamer sequences, C2NP and NGS6.0NP, that retained the core 2-dimensional structure; the likely binding motif (Figure 2 A, B). These truncated aptamers demonstrated increased affinity, when compared to their longer counterparts, and retained specificity; binding only the CD30-positive cells (Figure 2 C, E).
Initial comparison of the DNA and RNA aptamers (Figure 3) showed that they compete with each other, and the alteration from RNA to DNA aptamer did not affect the target binding profile. We intended to generate the ssDNA aptamer to provide nuclease resistance, which is of critical importance for clinical applications. In order to demonstrate the superior biostability of DNA-based over RNA-based aptamers for in vivo use, each aptamer was tested in a 24-hour time-course analysis with serum (nuclease) treatment (Figure 4). Unlike the RNA aptamer, which began degrading almost immediately and was digested in less than an hour, the ssDNA aptamer was stable in serum for more than 24 hours at 37°C. For clinical applications, in addition to stability, the selected aptamer needs to function properly in complex biological fluids. The aptamers and the antibody both stained the CD30-positive cells in cell mixtures that included non-target cells (Figure 5A), leukocytes (Figure 5B), and whole blood (Figure 5C) spiked with CD30-positive tumor cells. This further strengthened our confidence in the utility of this biostable ssDNA aptamer for in-vivo use).
CD30 is a diagnostic, as well as therapeutic, target for ALCL, and its activation results in pleiotropic signal transduction [28]. This signal transduction is induced by trimerization of the CD30 receptors and results in activation of multiple signaling pathways [47, 48]. Previous studies have shown that antibody can bind to CD30 receptors on ALCL cells, and induce cell death [49, 50]. However, aptamer-mediated activation of downstream signaling is limited to one aptamer, in contrast with numerous examples of aptamer-directed inhibition of function [51–55]. We detail a process of oligomerization of CD30 aptamers, and the potential of using such “functional aptamers” as an immunotherapy to induce downstream signaling upon binding to a cell surface target (Figure 6A). As a chemical antibody, ssDNA aptamers are easily conjugated to form polymers. In an attempt to artificially activate CD30 signal transduction, we generated a streptavidin-bound, biotinylated aptamer that resulted in multimer (dimer, trimer, and tetramer) formation. Using this aptamer multimer, we attempted to induce aptamer-mediated signaling by promoting CD30 oligomerization, resulting in the regulation of cell function of ALCL cells. Our experiments using the CD30 aptamer multimer showed a concentration-dependent promotion of apoptosis in the CD30-positive ALCL cells, but not the CD30-negative control cells (Figure 6C). The ratio of dead to live cells after flow cytometry analysis indicated successful trimerization of the CD30 receptors in ALCL cells, and specific induction of cell signaling that resulted in apoptosis. In contrast, the control cells were unaffected. These in-vitro studies provide substantial preliminary evidence supporting the use of ssDNA aptamers against CD30-expressing cancer cells for in-vivo, aptamer-based immune modulation or immunotherapy. These ssDNA aptamer-based biomaterials could constitute an important tool to control and stimulate response to disease with specificity, and represent an in vivo approach that has not been possible via antibody-based or non aptamer-mediated techniques. In addition, the aptamer can be easily modified to enhance the immunotherapy via conjugation of chemotherapeutic drugs. Multimeric C2NP can host slightly modified aptamers that could provide simultaneous immunotherapy, chemotherapy, and imaging via conjugated drugs and imaging reporters.
Conclusion
In this paper, we used a hybrid SELEX method to generate a ssDNA aptamer (C2NP) that targeted CD30 biomarkers expressed on cell surfaces. The selected aptamer recognized its target with nanomolar affinity and did not exhibit any non-specific binding in complex media, including whole blood. Furthermore, C2NP was highly stable when exposed to environmental nucleases, thus providing a highly effective potential biomaterial for targeted diagnostic and therapeutic in vivo applications. Importantly, multimeric C2NP was able to trimerize CD30 and activate downstream signaling, resulting in apoptosis of ALCL cells. In summary, these results suggest that aptamer-based immunotherapy could provide a new platform for cancer treatment.
Supplementary Material
Fig. S1. Scheme for hybrid selection of aptamers. Hybrid SELEX is a two-step process that combines cell-based SELEX with purified target SELEX to simultaneously eliminate non-specific binding and select high-affinity aptamers. A) CD30-positive cells were used to initiate selection of aptamers using a synthetic DNA library with primers on both ends. Negative selection by incubation with CD30-negative cells reduced non-specific binding by eliminating sequences binding commonly expressed proteins. PCR amplification of this provided a pool with affinity for CD30-positive cells; this selection was repeated iteratively for 20 rounds. B) The affinity of the enriched pool was enhanced by performing a selection on a protein affinity column using purified His-tagged CD30 protein bound to TALON magnetic beads. Sequences binding the beads were subtracted and the process repeated 3 times. The final aptamer pool was amplified and sequenced, revealing the sequences of CD30-specific aptamers.
FigureS2: Specificity of truncated aptamer C2NP depicted by histograms showing the cell binding of aptamer C2NP to different CD30-negative cells.
Supplementary Table 1: Different tumor cell lines with or without CD30 expression were tested for specificity of ssDNA aptamer C2NP.
Acknowledgments
This study was supported in part by NIH grants R01CA151955, R33CA173382, and 5P50CA126752 to Y.Z.
Footnotes
This address may also be used for all co-authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Famulok M, Mayer Gn, Blind M. Nucleic acid aptamers from selection in vitro to applications in vivo. Acc Chem Res. 2000;33:591–9. doi: 10.1021/ar960167q. [DOI] [PubMed] [Google Scholar]
- 2.Gold L, Brody E, Heilig J, Singer B. One, two, infinity: genomes filled with aptamers. Chem & Biol. 2002;9:1259–64. doi: 10.1016/s1074-5521(02)00286-7. [DOI] [PubMed] [Google Scholar]
- 3.Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628–50. [PubMed] [Google Scholar]
- 4.Horie R, Watanabe T. CD30: expression and function in health and disease. Sem Immunol. 1998;10:457–70. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 5.Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999;90:157–64. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 6.Nawrocki JF, Kirsten ES, Fisher RI. Biochemical and structural properties of a Hodgkin’s disease-related membrane protein. J Immunol. 1988;141:672–80. [PubMed] [Google Scholar]
- 7.Stein H, Mason D, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 8.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2008;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 9.Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–95. [PubMed] [Google Scholar]
- 10.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2010;24:585–95. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros LJ, Elenitoba-Johnson KSJ. Anaplastic large cell lymphoma. Am J Clin Path. 2007;127:707–22. doi: 10.1309/r2q9ccuvtlrycf3h. [DOI] [PubMed] [Google Scholar]
- 12.Vaklavas C, Forero-Torres A. Safety and efficacy of brentuximab vedotin in patients with Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Ther Adv Hematol. 2012 doi: 10.1177/2040620712443076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab Vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 14.Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of Brentuximab Vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18:248–55. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MK, Willis CR, Armitage RJ. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology. 2006;118:143–52. doi: 10.1111/j.1365-2567.2006.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Prete G, De Carli M, Almerigogna F, Daniel C, D’elios M, Zancuoghi G, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB. 1995;9:81–6. [PubMed] [Google Scholar]
- 17.Zhang P, Zhao N, Zeng Z, Feng Y, Tung CH, Chang CC, et al. Using an RNA aptamer probe for flow cytometry detection of CD30-expressing lymphoma cells. Lab Invest. 2009;89:1423–32. doi: 10.1038/labinvest.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z, Zhang P, Zhao N, Sheehan AM, Tung CH, Chang CC, et al. Using oligonucleotide aptamer probes for immunostaining of formalin-fixed and paraffin-embedded tissues. Mod Pathol. 2010;23:1553–8. doi: 10.1038/modpathol.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keefe AD, Cload ST. SELEX with modified nucleotides. Curr Opin Chem Biol. 2008;12:448–56. doi: 10.1016/j.cbpa.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Bell NM, Micklefield J. Chemical modification of oligonucleotides for therapeutic, bioanalytical and other applications. ChemBioChem. 2009;10:2691–703. doi: 10.1002/cbic.200900341. [DOI] [PubMed] [Google Scholar]
- 21.Micklefield J. Backbone modification of nucleic acids: synthesis, structure and therapeutic applications. Curr Med Chem. 2001;8:1157–79. doi: 10.2174/0929867013372391. [DOI] [PubMed] [Google Scholar]
- 22.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh K, Misawa K, Kuma Ki, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuker M, Mathews DH, Turner DH. RNA Biochemistry and Biotechnology. Springer; 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide; pp. 11–43. [Google Scholar]
- 25.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuker M. Calculating nucleic acid secondary structure. Curr Opin Struct Biol. 2000;10:303–10. doi: 10.1016/s0959-440x(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 27.Podack ER, Strbo N, Sotosec V, Muta H. CD30—governor of memory T cells? Ann NY Acad Sci. 2002;975:101–13. doi: 10.1111/j.1749-6632.2002.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 28.Schneider C, Hübinger G. Pleiotropic signal transduction mediated by human CD30: a member of the tumor necrosis factor receptor (TNFR) family. Leuk Lymphoma. 2002;43:1355–66. doi: 10.1080/10428190290033288. [DOI] [PubMed] [Google Scholar]
- 29.Schnell R, Dietlein M, Staak JO, Borchmann P, Schomaecker K, Fischer T, et al. Treatment of refractory Hodgkin’s lymphoma patients with an Iodine-131–labeled murine anti-CD30 monoclonal antibody. J Clin Oncol. 2005;23:4669–78. doi: 10.1200/JCO.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Tan W. Aptamers generated from cell-SELEX for molecular medicine: A chemical biology approach. Acc Chem Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell MA, Wengel J. Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. Chem Soc Rev. 2011;40:5680–9. doi: 10.1039/c1cs15048k. [DOI] [PubMed] [Google Scholar]
- 32.Joachimi A, Benz A, Hartig S., Jr A comparison of DNA and RNA quadruplex structures and stabilities. Bioorg Med Chem. 2009;17:6811–5. doi: 10.1016/j.bmc.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 33.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 34.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 35.Mallikaratchy P, Stahelin RV, Cao Z, Cho W, Tan W. Selection of DNA ligands for protein kinase C-δ. Chem Commun. 2006:3229–31. doi: 10.1039/b604778e. [DOI] [PubMed] [Google Scholar]
- 36.Martin JA, Parekh P, Kim Y, Morey TE, Sefah K, Gravenstein N, et al. Selection of an aptamer antidote to the anticoagulant drug Bivalirudin. PloS One. 2013;8:e57341. doi: 10.1371/journal.pone.0057341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaherian S, Musheev MU, Kanoatov M, Berezovski MV, Krylov SN. Selection of aptamers for a protein target in cell lysate and their application to protein purification. Nucleic Acids Res. 2009;37:e62-e. doi: 10.1093/nar/gkp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopinath SCB. Methods developed for SELEX. Anal and Bioanal Chem. 2007;387:171–82. doi: 10.1007/s00216-006-0826-2. [DOI] [PubMed] [Google Scholar]
- 39.Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, et al. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci U S A. 2006;103:11838–43. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci U S A. 2003;100:15416–21. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayrac AT, Sefah K, Parekh P, Bayrac C, Gulbakan B, Oktem HA, et al. In vitro selection of DNA aptamers to glioblastoma multiforme. ACS Chemical Neuroscience. 2011;2:175–81. doi: 10.1021/cn100114k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parekh P, Tang Z, Turner PC, Moyer RW, Tan W. Aptamers recognize glycosylated hemagglutinin expressed on the surface of vaccinia virus-infected cells. Anal Chem. 2010;82:8642. doi: 10.1021/ac101801j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sefah K, Tang Z, Shangguan D, Chen H, Lopez-Colon D, Li Y, et al. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia. 2009;23:235–44. doi: 10.1038/leu.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li N, Ebright JN, Stovall GM, Chen X, Nguyen HH, Singh A, et al. Technical and biological issues relevant to cell typing with aptamers. J Proteome Res. 2009;8:2438–48. doi: 10.1021/pr801048z. [DOI] [PubMed] [Google Scholar]
- 45.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PloS One. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sefah K, Shangguan D, Xiong X, O’Donoghue MB, Tan W. Development of DNA aptamers using cell-SELEX. Nat Protoco. 2010;5:1169–85. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- 47.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF 2 are involved in CD30-mediated NFκB activation. J Biol Chem. 1997;272:2042–5. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 48.Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–30. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 49.Hirsch B, Hummel M, Bentink S, Fouladi F, Spang R, Zollinger R, et al. CD30-induced signaling is absent in Hodgkin’s cells but present in anaplastic large cell lymphoma cells. Am J Pathol. 2008;172:510–20. doi: 10.2353/ajpath.2008.070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mir SS, Richter BWM, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000;96:4307–12. [PubMed] [Google Scholar]
- 51.Famulok M, Mayer G. Aptamers as tools in molecular biology and immunology. Curr Top in Microbiol Immunol. 1999;243:123–36. doi: 10.1007/978-3-642-60142-2_7. [DOI] [PubMed] [Google Scholar]
- 52.Hicke BJ, Watson SR, Koenig A, Lynott CK, Bargatze RF, Chang Y-F, et al. DNA aptamers block L-selectin function in vivo. Inhibition of human lymphocyte trafficking in SCID mice. J Clin Invest. 1996;98:2688. doi: 10.1172/JCI119092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou J, Li H, Li S, Zaia J, Rossi JJ. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol Ther. 2008;16:1481–9. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, et al. 2| - Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165) inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–67. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Scheme for hybrid selection of aptamers. Hybrid SELEX is a two-step process that combines cell-based SELEX with purified target SELEX to simultaneously eliminate non-specific binding and select high-affinity aptamers. A) CD30-positive cells were used to initiate selection of aptamers using a synthetic DNA library with primers on both ends. Negative selection by incubation with CD30-negative cells reduced non-specific binding by eliminating sequences binding commonly expressed proteins. PCR amplification of this provided a pool with affinity for CD30-positive cells; this selection was repeated iteratively for 20 rounds. B) The affinity of the enriched pool was enhanced by performing a selection on a protein affinity column using purified His-tagged CD30 protein bound to TALON magnetic beads. Sequences binding the beads were subtracted and the process repeated 3 times. The final aptamer pool was amplified and sequenced, revealing the sequences of CD30-specific aptamers.
FigureS2: Specificity of truncated aptamer C2NP depicted by histograms showing the cell binding of aptamer C2NP to different CD30-negative cells.
Supplementary Table 1: Different tumor cell lines with or without CD30 expression were tested for specificity of ssDNA aptamer C2NP.