Abstract
Background
Response to antidepressant medication is higher in comparator vs. placebo-controlled randomized controlled trials (RCTs). Patient expectancy is an important influence on clinical outcome in the treatment of depression and may explain this finding. Results are reported from a pilot RCT studying expectancy and depression outcome in placebo-controlled vs. comparator treatment conditions.
Methods
Outpatients aged 18-65 years with Major Depressive Disorder were enrolled in this 8 week RCT. Subjects were randomized to placebo-controlled (escitalopram or placebo), or comparator (escitalopram or citalopram) administration of antidepressant medication. Subjects reported their expected likelihood and magnitude of depression improvement before and after randomization using questions from the Credibility and Expectancy Scale. A regressed change model of post-randomization expectancy of improvement was fit to the data to determine whether subjects in the comparator group reported greater expectancies of improvement than subjects in the placebo-controlled group.
Results
20 subjects with mean age 56.5 ± 11.7, baseline HRSD 24.2 ± 5.3, baseline Beck Depression Inventory (BDI) 24.9 ± 6.4, and baseline Clinical Global Impressions (CGI) Severity 4.0 ± 0.3 were enrolled in the study. Adjusting for other factors, the effect of group assignment on expected magnitude of improvement was significant and large (effect size 1.5). No group differences in expected likelihood of improvement were found.
Conclusions
Randomization to comparator vs. placebo-controlled administration of antidepressant medication produced greater expectancies of how much patients would improve during the trial. This expectancy difference may explain the higher response and remission rates that are observed in comparator vs. placebo-controlled trials.
Keywords: depression, patient expectancy, placebo effect, clinical trial design, placebo-controlled, comparator
Introduction
Meta analyses of antidepressant response rates in comparator (i.e., medication vs. medication) and placebo-controlled randomized controlled trials (RCTs) consistently indicate that response rates to medication are higher in comparator trials. Rutherford et al. (2009) analyzed 48 placebo-controlled and 42 comparator trials of antidepressants for Major Depressive Disorder (MDD) in adults aged 18-65. The odds of being classified as a responder to antidepressant medication in comparator trials were 1.8 times the odds of being classified as a responder in placebo-controlled trials (95% CI = 1.45 - 2.17, p < 0.001). The odds of being classified as a remitter to medication in comparator trials were 1.5 times the odds of being classified a remitter in placebo-controlled trials (95% CI = 1.11 - 2.11, p < 0.001). Similar results have been found in a population of patients with late life depression (Sneed et al. 2008).
One salient difference between these different study designs is that subjects in a comparator trial know they are receiving active medication, while subjects in placebo-controlled trials are aware they may be receiving placebo. Experiments in many medical conditions have confirmed that open treatments, where subjects know a treatment is being administered and expect it to have a therapeutic effect, are more effective than hidden treatments, where subjects are unaware a treatment is being administered (Pollo et al. 2002, Benedetti et al. 2003). This suggests that what subjects are told about a treatment may modify their expectancies about its effects and influence depression outcome.
Studies that have specifically investigated the effects of patient expectancy in clinical trials for depression support its role in depression improvement (Noble et al. 2001). In the NIMH Treatment of Depression Collaborative Study (TDCRP), which enrolled 239 outpatients with MDD, higher expectancy of improvement predicted greater likelihood of depression response and lower final depression scores in all four treatment conditions (cognitive behavior therapy, interpersonal therapy, imipramine, and placebo-clinical management) (Sotsky et al. 1991). Among the 156 subjects completing a trial of psychotherapy or medication, 48% with expectancy scores above the median exhibited a complete response to treatment compared to 33% with scores below the median. In a single-blind trial of reboxetine for 25 subjects with MDD, subjects with a higher pre-treatment expectancy of medication effectiveness had a greater likelihood of response: 90% of patients with high expectancy of improvement responded compared to 33% of patients with lower expectancy (X2= 7.819, p<0.005) (Krell et al. 2004).
Patient expectancy is also hypothesized to be a major mechanism of the placebo effect, which may be responsible for most of the change observed in patients receiving antidepressants for MDD (Kirsch 1997, Haour 2005). An analysis of clinical trials of antidepressants submitted to the Food and Drug Administration (FDA) prior to 1998 reported that the placebo groups in these trials averaged 1.5 standard deviation units of improvement in their baseline depression rating scores, which was 75% of the improvement shown in the antidepressant groups (Kirsch and Sapirstein 1998). In a subsequent report that included new studies published up to 2008, the same investigators found that placebo treatment resulted in similar improvement in depressive symptoms for all patients with depression except the most severely ill (i.e., those with HRSD scores above 28) (Kirsch et al. 2008). These results are consistent with another meta-analysis of 75 placebo-controlled antidepressant RCTs published between 1981 and 2000, which found a mean medication response rate of 50%, compared to a mean placebo response rate of 30% (i.e., over half of the drug response) (Walsh et al. 2002).
Given their significant influence on clinical outcome in antidepressant trials, it is surprising that expectancy effects have not been studied more extensively. MDD affects approximately 121 million people worldwide (including nearly 19 million American adults each year) and is a leading cause of disability due to illness (Kessler et al. 2003, World Health Organization 2004, Kessler 2005). Even with maximal treatment, many patients will not experience sustained remission of their depression. The cumulative percentage of patients achieving remission after four sequential antidepressant trials in the National Institute of Mental Health (NIMH) sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study was only 51% (Rush et al. 2006). Residual depressive symptoms place patients at increased risk of suicide, cardiovascular morbidity and mortality, and other problems (Paykel et al. 1995, Jiang et al. 2002, Kennedy and Paykel 2004). Until novel treatments with proven efficacy for depression can be developed, methods of optimizing response to currently available antidepressant treatments are urgently needed. Enhancing patient expectancy may be a safe and effective way of optimizing treatment for depression.
In order to determine whether it has a causal relationship with depression outcome, expectancy must be experimentally manipulated and prospectively studied. There has been no such study of depressed patients, possibly due to the absence of an effective and ethical means of manipulating expectancy in this patient population. The goal of this pilot study was to determine whether clinical trial design might be an effective method of manipulating patient expectancy and lead to differences in the outcome of antidepressant pharmacotherapy. Adult outpatients with MDD were randomized to treatment in a comparator group (subjects randomly assigned to escitalopram or citalopram) or a placebo-controlled group (subjects randomly assigned to escitalopram or placebo). Two dimensions of patient expectancy, expected likelihood of improvement and expected magnitude of improvement, were measured before and after randomization using questions from a standardized scale. It was hypothesized that randomization to the comparator group would result in higher expectancy scores and higher depression response rates than randomization to the placebo-controlled group.
Method
Subjects
Adult outpatients were recruited through physician referral as well as radio and newspaper advertisements to the Adult and Late Life Depression Clinic of the New York State Psychiatric Institute. Inclusion criteria were (1) men or women aged 18-65 years, (2) Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) unipolar MDD, (3) 24-item Hamilton Rating Scale for Depression (HRSD) score ≥ 16 (Hamilton 1960), and (4) capable of providing informed consent. Exclusion criteria are (1) pregnant or lactating women, (2) current psychosis or history of a psychotic disorder, (3) substance dependence other than nicotine, (4) >2 on HRSD suicide item, (5) acute severe or unstable medical illness, (6) non-response to treatment with escitalopram 10 mg per day or citalopram 20mg per day given for at least 4 weeks during the current episode, and (7) Clinical Global Impressions-Severity score of 7 at baseline (Guy1961).
Assessments
At the initial screening visit, a psychiatrist conducted a medical and psychiatric evaluation. Patients with a clinical diagnosis of mood disorder were interviewed by a research rater, who completed the Structured Clinical Interview for the DSM-IV axis I disorders—Patient edition (SCID-P) (Ventura et al. 1998) and an HRSD 24-item questionnaire. In addition, a physical examination, routine blood tests (complete blood count and SMAC-20), and an electrocardiogram were completed for eligible candidates. A final DSM-IV diagnosis was made at a weekly research meeting based on the psychiatrist's clinical assessment and the SCID-P interview.
Following enrollment in the study, subjects returned for weekly visits, at which observer-rated (HRSD, CGI) and self-report (Beck Depression Inventory, California Pharmacotherapy Alliance Scale) were completed (Beck et al. 1961, Marmar and Gaston 1998). Experts in the topic of expectancy suggest that it comprises two conceptually distinct dimensions: expected likelihood of therapeutic improvement and expected magnitude of therapeutic improvement (Kirsch 1997). The Credibility and Expectancy Scale (CES) includes questions that measure both of these dimensions (Borkovec and Nau 1972). Specifically, question 2 pertains to likelihood and states “This treatment will be successful in reducing my symptoms of depression” (rated Disagree to Agree on a 9 point Likert scale). Question 4 pertains to the magnitude of improvement and asks “How much improvement in your symptoms of depression do you think will occur?” (rated from 0-100%).
Psychometric testing in multiple patient populations has shown the CES is internally consistent and has good test-retest reliability (Devilly and Borkovec 2000). Versions of the CES have been used to measure treatment credibility and patient expectancy in several psychotherapy and pharmacotherapy studies (Borkovec and Costello 1993). Subjects in this study completed the CES at the initial screening visit (prior to randomization in the study) and at the first study visit following randomization.
Procedures
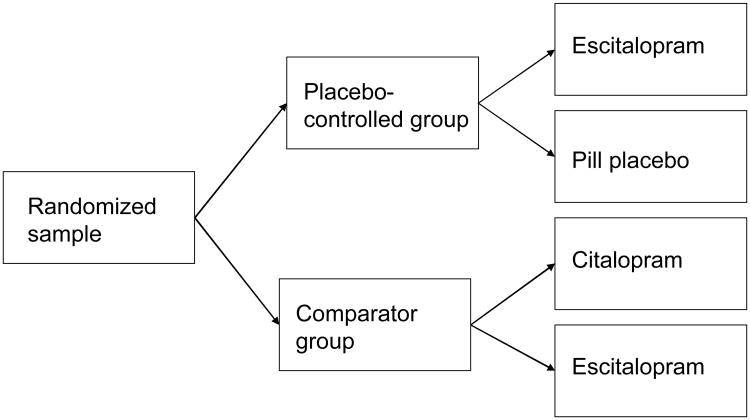
Eligible subjects signing informed consent were randomized to a placebo-controlled group or a comparator group (see Figure 1). Subjects were informed of their group assignment but were blinded to their specific treatment assignment. Subjects in the placebo-controlled group were informed: “You have been randomly assigned to the placebo-controlled group of the study. This means that there is a 50% chance you will receive the antidepressant medication citalopram for the duration of the study. Citalopram has been proven effective for the treatment of depression in patients like you. There is also a 50% chance you will receive placebo for the duration of the study. A placebo is a sugar pill that is not specifically effective for depression. Neither you, nor your doctors, will know whether you are receiving citalopram or placebo.” Subjects in the comparator group were informed: “You have been randomly assigned to the comparator group of the study. This means that there is a 50% chance you will receive the antidepressant medication citalopram and a 50% chance you will receive the antidepressant medication escitalopram for the duration of the study. Citalopram and escitalopram have been proven effective for the treatment of depression in patients like you. You will not be receiving any placebo pills for the duration of the study.”
Figure 1.
Treatment allocation of subjects.
At the time of their entry into the study, subjects were started on citalopram 20mg per day, escitalopram 10mg per day, or pill placebo. After 4 weeks if subjects did not meet remission criteria (HRSD ≤ 7), citalopram dose was increased to 40mg for the remaining 4 weeks of the study and escitalopram dose increased to 20mg. Subjects unable to tolerate the increased dose of medication had their dosage reduced to the maximum previously tolerated dose. Subjects brought pill bottles to weekly visits so that a pill count could be performed. In the case of severe insomnia, subjects were permitted zolpidem 10mg per day.
Statistical Analyses
Descriptive statistics are expressed as means and standard deviations or percentages. Chi-square analyses and independent samples t-tests were used to compare subjects in the placebo-controlled and comparator tracks on demographic and clinical features.
A number of analytic approaches can be used to analyze two-time-point data such as those in the current study, including the analysis of simple change scores and the analysis of regressed change (Cohen et al. 2003). Analyzing two-time-point data using simple change scores can be problematic because subtracting the pre-score from post-score does not take unreliability into account. Reliability of change scores tends to be quite low and decreases as the correlation between the pre-test and post-test increases. This problem can be averted by adopting a partial or regressed change procedure using multiple regression, which simply treats the pre-randomization expectancy as a covariate effectively removing all correlation from the post-randomization expectancy score (Cohen et al. 2003).
For these reasons, the regressed change approach was adopted for analyzing the pre-post randomization expectancy data in this study (Cohen et al. 2003). According to this approach, the post-randomization expected likelihood of improvement and expected magnitude of improvement were treated as the outcome variables and baseline expectancy scores as covariates. To test the hypothesis that randomization to the placebo-controlled vs. comparator groups significantly changed subjects' expectancy of the likelihood and magnitude of improvement, a group term dummy-coded as 0 (placebo-controlled) or 1 (comparator) was included in this regressed change model. Therefore, this model tests for differences in change among the two experimental conditions while removing the correlation between pre- and post-randomization expectancy scores. In addition to covarying for baseline expectancy, we also adjusted for subject age, gender, and baseline level of depression in all models. All covariates were centered at their respective means and all significance tests were evaluated at the 5% level.
Results
Descriptive statistics
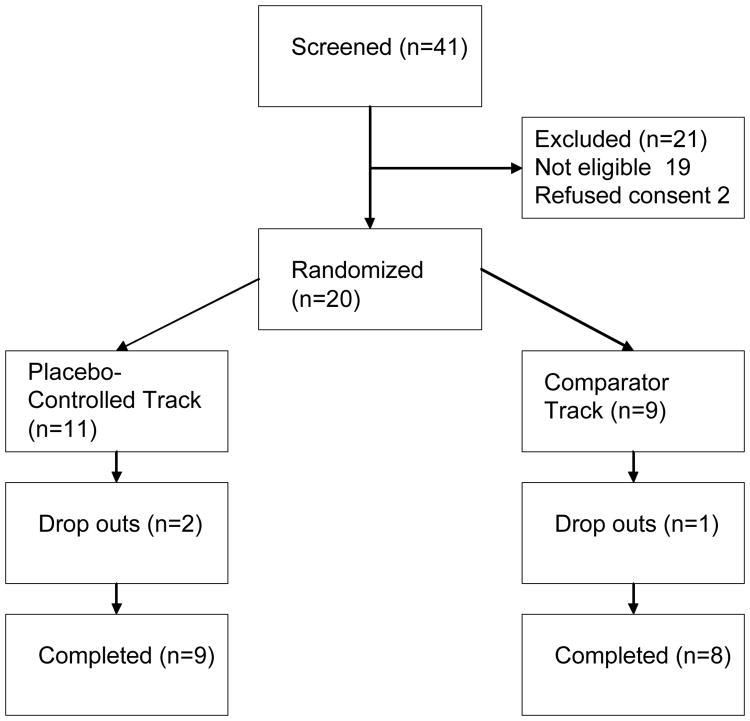
41 subjects were screened for the study, 20 enrolled, and 11 were randomized to the placebo group and 9 to the comparator group (see Figure 2). These groups have not yet been broken down by specific treatment assignment, since these pilot data are part of a larger study that is still ongoing. Subjects in the placebo-controlled group were 55% male and had mean age 57.4 ± 12.2, baseline HRSD 25.7 ± 6.1, baseline BDI 26.6 ± 7.1, baseline CGI—Severity 4.1 ± 0.3. Subjects in the comparator group were 44% male and had mean age 55.3 ± 12.0, baseline HRSD 22.3 ± 3.7, baseline BDI 22.5 ± 4.8, baseline CGI—Severity 3.9 ± 0.3. Both groups demonstrated improvement in their depression over the course of the study (see Table 1). No significant differences in baseline characteristics or final symptom measures were found between groups.
Figure 2. participant flow through the RCT.
Table 1.
Clinical and demographic characteristics of subjects.
| Characteristic | Placebo-Controlled Track (mean ± SD) | Comparator Track (mean ± SD) |
|---|---|---|
| N | 11 | 9 |
| Age (years) | 57.4 ± 12.2 | 55.3 ± 12.0 |
| % male | 55 | 44 |
| Baseline 24-item HRSD | 25.7 ± 6.1 | 22.3 ± 3.7 |
| Baseline BDI | 26.6 ± 7.1 | 22.5 ± 4.8 |
| Baseline CGI—Severity | 4.1 ± 0.3 | 3.9 ± 0.3 |
| % drop out | 18.2 | 11.1 |
| % Final HRSD ≤ 7 | 36 | 50 |
| Final CGI—Improvement | 2.3 ± 1.4 | 1.4 ± 0.8 |
Expectancy manipulation
Subjects' pre- and post-randomization expectancy scores are presented in Table 2. To test the hypothesis that randomization to placebo-controlled and comparator groups significantly affected subjects' expected likelihood of improvement, a regressed change model with post-randomization expected likelihood as the outcome variable was fit to the data. In this model, the effect of group on expected likelihood of improvement was not significant (B=0.040, t=0.096, p=0.925). Covarying for subject age, gender, and baseline HRSD scores did not make a substantive difference in the model.
Table 2.
Subject expectancy of improvement before and after randomization in clinical trial.
| Expectancy score | Placebo-Controlled Track (mean ± SD) | Comparator Track (mean ± SD) |
|---|---|---|
| Pre-randomization expected likelihood of improvement | 5.7 ± 1.4 | 6.1 ± 2.2 |
| Post-randomization expected likelihood of improvement | 5.9 ± 1.2 | 6.0 ± 1.9 |
| Pre-randomization expected magnitude of improvement | 6.7 ± 1.7 | 6.4 ± 3.3 |
| Post-randomization expected magnitude of improvement | 6.0 ± 1.0 | 6.5 ± 2.7 |
To test the hypothesis that randomization to placebo-controlled and comparator groups significantly affected subjects' expected magnitude of improvement, a regressed change model with post-randomization expected magnitude as the outcome variable was fit to the data. The effect of group on expected magnitude of improvement was significant (B=1.247, t=2.340, p=0.041). Subjects in the comparator group had 1.2 units higher expected magnitude of improvement following randomization compared to subjects in the placebo-controlled group, adjusting for their pre-randomization scores of expected magnitude. Covarying for subject age, gender, and baseline HRSD scores did not make a substantive difference in the model. The effect size for the observed difference was [1.2−(-0.1)]/0.859=1.5, which corresponds to a large effect size for the experimental manipulation of expectancy in this study.
Discussion
The initial results from this randomized controlled trial indicate that subjects randomized to a comparator vs. a placebo-controlled group reported higher expected magnitude of improvement. These data support our hypothesis that subjects who know they are receiving effective medication expect to improve more than subjects who are aware they may be receiving placebo. They also raise the possibility that patient expectancies may explain the repeated finding that antidepressant response rates are higher in open and comparator trials vs. placebo-controlled trials.
It is interesting that subjects' reported expectancies about the likelihood they would improve during antidepressant treatment were not significantly different between the placebo-controlled and comparator groups. The reasons for this finding are unclear but may have to do with the question about likelihood being less intuitively understandable to subjects than considering how much they will improve (i.e., magnitude). All studies of expectancy in subjects with depression performed to date have measured the expected magnitude rather than the likelihood of improvement. Therefore, expectancies about how much change will occur in depressive symptoms rather than the likelihood of change may be the more important facet of expectancy to measure.
While the finding that subjects who know they are receiving active medication as opposed to placebo have higher expectancies of improvement may appear intuitive, it has been surprisingly difficult to demonstrate expectancy differences in clinical trials. To our knowledge, this is the first study in depressed subjects to manipulate subject expectancy, measure expectancy before and after the experimental manipulation, and confirm the effectiveness of the manipulation. The most common prior method of manipulating expectancy in patient samples has been varying the clinician's behavior in a standardized way (e.g., optimistic, neutral, pessimistic). For example, a recent study by Kemeny et al (2007) randomized patients with asthma to a 2 × 2 study design examining the effect of salmeterol vs. placebo and “enhanced” vs. “efficient” physician style. “Enhanced” encounters were intended to transmit a positive expectancy for improvement to the patient, while physicians providing the “efficient” care were trained to convey equivocal expectancies by being less authoritative and supportive. Robust placebo responses were achieved in the study (as measured by objective physiologic change in forced expiratory volumes), but the investigators were unsuccessful in inducing differential expectancies using different physician styles.
It is critical to measure expectancy because research subjects may forget what they are told, be apathetic about what they were told, talk to another subject given different instructions, or else improve despite a negative expectancy (Lick and Bootzin 1975). As pointed out by Wilkins (1973), experimenters who fail to measure expectancy after attempting to manipulate them may engage in circular reasoning. An investigator attempting to instill positive outcome expectancies in a group of subjects who then demonstrate greater improvement than subjects given lower expectancies may conclude that the subject indeed had different expectancy states and that these caused the disparate outcomes. This is circular, since the presence of high or low expectancy states in the research subjects is identified indirectly by the treatment outcome which expectancy is said to produce—subjects were known to have high expectancies since they improved, and they improved since they had high expectancies.
Finally, the results of this study should be interpreted with certain limitations in mind. First, the number of subjects enrolled to date in this study is relatively small, and these results must be replicated by larger studies. Moreover, even if it were established that study design does significantly affect expectancy, the critical next step is to establish that expectancy is associated with differences in treatment outcome. This study is designed to answer this question when subject recruitment is complete. Second, the main objective of this study is to focus on patient expectancy effects and their correlation with depression outcome, but expectancies of health care providers and outcome raters may also affect treatment outcome. To minimize clinician and rater bias affecting the study results, a self-report measure of expectancy was used and outcome raters in this study were blinded to subjects' group assignments.
Acknowledgments
This research was supported by a National Institutes of Health grant T32-MH20004 and a Clinical and Translational Science Award from the Irving Center of Columbia University.
Appendix
Patient name:______________________ Date: ___________________________
Visit: ___________________
Credibility and Expectancy Scale
1. The rationale for this treatment seems logical.
| Completely Disagree | Somewhat Agree | Completely Agree | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
2. This treatment will be successful in reducing my symptoms of depression.
| Completely Disagree | Somewhat Agree | Completely Agree | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
3. I would confidently recommend this treatment to a friend who was experiencing depression.
| Completely Disagree | Somewhat Agree | Completely Agree | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
4. How much improvement in your symptoms of depression do you think will occur?
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
Footnotes
The authors report no conflicts of interest relevant to this manuscript.
Contributor Information
Bret Rutherford, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY.
Joel Sneed, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY.
Dev Devanand, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY.
Rachel Eisenstadt, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY.
Steven Roose, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY.
References
- Beck AT, Ward CH, Mendelson M. An inventory of measuring depression. Archives of General Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Maggi G, Lopiano L, Lanotte M, Rainero I, Vighetti S, Pollo A. Open versus Hidden Medical Treatments: The Patient's Knowledge about a Therapy Affects the Therapy Outcome. Prevention and Treatment. 2003;6 Available: http://journals.apa.org/journals/pre/6/1/1a.html. [Google Scholar]
- Borkovec TD, Nau SD. Credibility of Analogue Therapy Rationales. Journal of Behavioral Therapy and Experimental Psychiatry. 1972;3:257–260. [Google Scholar]
- Borkovec TD, Costello E. Efficacy of Applied Relaxation and Cognitive-Behavioral Therapy in the Treatment of Generalized Anxiety Disorder. Journal of Consulting and Clinical Psychology. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd. New York: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavioral Therapy and Experimental Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Guy W. New Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. Vol. 1976. Rockville, MD: National Institute of Mental Health; 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- Hamilton MW. A rating scale for depression. Journal of Neurology and Neurosurgical Psychiatry. 1960;23:56–60. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haour F. Mechanisms of the Placebo Effect and of Conditioning. Neuroimmunomodulation. 2005;12:195–200. doi: 10.1159/000085651. [DOI] [PubMed] [Google Scholar]
- Jiang W, Krishnan RR, O'Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111–127. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose R, Berg-Smith S, Kline J. Placebo Response in Asthma: A Robust and Objective Phenomenon. Journal of Allergy and Clinical Immunology. 2007;119:1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. Journal of Affective Disorders. 2004;80:135–144. doi: 10.1016/S0165-0327(03)00054-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Specifying Nonspecifics: Psychological Mechanisms of Placebo Effects. In: Harrington A, editor. The placebo effect: An interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- Kirsch I, Sapirstein G. Listening to prozac but hearing placebo: A meta-analysis of antidepressant medication. Prevention and Treatment. 1998 Available at: http://journals.apa.org/prevention/volumeI/pre0010002a.html.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scorboria A, Moore TJ, Johnson BT. Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration. Public Library of Science Medicine. 2008;5:260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject Expectations of Treatment Effectiveness and Outcome of Treatment with an Experimental Antidepressant. Journal of Clinical Psychiatry. 2004;65:1174–1179. doi: 10.4088/jcp.v65n0904. [DOI] [PubMed] [Google Scholar]
- Lick J, Bootzin R. Expectancy Factors in the Treatment of Fear: Methodological and Theoretical Issues. Psychological Bulletin. 1975;82:917–931. [PubMed] [Google Scholar]
- Marmar C, Gaston L. California Pharmacotherapy Alliance Scale. Unpublished manual 1998 [Google Scholar]
- Noble LM, Douglas BC, Newman SP. What do patients expect of psychiatric services? A systematic and critical review of empirical studies. Social Science & Medicine. 2001;52:985–998. doi: 10.1016/s0277-9536(00)00210-0. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychological Medicine. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Pollo A, Torre E, Lopiano L, Rizzone M, Lanotte M, Cavanna A, Bergamasco B, Benedetti F. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. NeuroReport. 2002;13:1383–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Sneed JR, Roose SP. Does Study Design Affect Outcome? The Effects of Placebo Control and Treatment Duration in Antidepressant Trials. Psychotherapy and Psychosomatics. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design Makes a Difference: Antidepressant Response Rates in Placebo-controlled versus Comparator Trials in Late Life Depression. American Journal of Geriatric Psychiatry. 2008;16:65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- Sotsky AM, Glass DR, Shea MT, Pilkonis PA, Collins F, Elkin I, Watkins JT, Imber SD, Leber WR, Moyer J, Oliveri ME. Patient Predictors of Response to Psychotherapy and Pharmacotherapy: Findings in the NIMH Treatment of Depression Collaborative Research Program. American Journal of Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo Response in Studies of Major Depression: Variable, Substantial, and Growing. Journal of the American Medical Association. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wilkins W. Expectancy of Therapeutic Gain: An Empirical and Conceptual Critique. Journal of Consulting and Clinical Psychology. 1973;40:69–77. doi: 10.1037/h0034056. [DOI] [PubMed] [Google Scholar]
- The World Health Organization. The World Health Report 2004: Changing History, Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. Geneva: WHO; 2004. [Google Scholar]