Abstract
The serine/threonine kinase Akt has proven to be a significant signaling target, involved in various biological functions. Because of its cardinal role in numerous cellular responses, Akt has been implicated in many human diseases, particularly cancer. It has been established that Akt is a viable and feasible target for anticancer therapeutics. Analysis of all Akt kinases reveals conserved homology for an N-terminal regulatory domain, which contains a pleckstrin-homology (PH) domain for cellular translocation, a kinase domain with serine/threonine specificity, and a C-terminal extension domain. These well defined regions have been targeted, and various approaches, including in silico methods, have been implemented to develop Akt inhibitors. In spite of unique techniques and a prolific body of knowledge surrounding Akt, no targeted Akt therapeutics have reached the market yet. Here we will highlight successes and challenges to date on the development of anticancer agents modulating the Akt pathway in recent patents as well as discuss the methods employed for this task. Special attention will be given to patents with focus on those discoveries using computer-aided drug design approaches.
Keywords: Akt, anticancer therapeutics, computer-aided drug design, drug discovery and development, pleckstrin-homology (PH) domain, serine/threonine kinase
INTRODUCTION
The phosphatidylinositol-3-kinase (PI3K) signaling pathway and its downstream effectors have proven vital to a diverse set of growth and survival functions. Akt (also known as protein kinase B, PKB, or Related to A and C, RAC) is one of the main targets of PI3K. More than 50 proteins have been validated as putative substrates of Akt [1]. Its pivotal role in cell survival, apoptosis, transcription, and proliferation makes it an attractive target for regulatory drugs [2]. Additionally, genetic aberrations such as mutation, amplification, and rearrangement of the Akt pathway are more frequent in human cancer. Genetic screens in model organisms have identified Akt as the primary downstream mediator of the effects of PI3K, and Akt is over-expressed or activated in major cancers [3].
The human Akt family isoforms: Akt1, Akt2, and Akt3 (also known as PKBα, PKBβ, and PKBγ, respectively) are characterized by an amino terminal pleckstrin-homology (PH) domain (~ 110 amino acids), a kinase domain (~260 amino acids) separated by a hinge (LINK or Linker) region of 39 amino acids, and a C-terminal regulatory domain (~ 70 amino acids) Fig. (1). All three Akt kinases have 90–95% homology in the kinase domain, 60% homology in their PH domain, and 25% homology in their LINK region [4]. Structure gives clues as to how it is involved in the signaling cascade. After growth factors, insulin or cytokines recruit PI3K to the plasma membrane, PI3K is allosterically activated, leading to the increase of PtdIns(3,4,5)P3 (PIP3) on the plasma membrane. Akt is translocated from the cytoplasmic space to the plasma membrane by way of PH domain binding to PIP3. Akt is a serine/threonine kinase member of the AGC family of kinases [5]. Akt activation is based on the integrity of the PH domain, which controls membrane translocation, and on phosphorylation of Thr308 in the activation loop and Ser473 in the hydrophobic domain [6]. After these two phosphorylation events, numerous intramolecular interactions are formed within Akt, including an anchoring salt bridge in the C-terminal α-helix and an anchoring hydrogen bond between the C-terminal α-helix and the phosphate of Thr308. The phosphorylation of Thr308 causes four more intramolecular interactions that brings all of the functional loops together – the catalytic, the magnesium binding and the substrate binding loops [7]. The X-ray structure reveals that Phe469 and Phe472 of the C terminus comprise the hydrophobic motif (HM) Phe-X-X-Phe-(Ser/Thr)-Trp. This motif binds in an intramolecular fashion to a hydrophobic groove (HG) on the kinase domain Fig. (1). The HG lies under the kinase domain β4-β5 loop, behind the αB and αC helices, including residues: Lys185, Arg202, Arg208, Lys216, and Gln220.
Fig. 1.

Schematic representation of Akt1. PH: Pleckstrin homology domain; LINK: Linker domain; HG: Hydrophobic groove; P: Phosphate; CTD: C-terminal domain.
While Akt1 (referred to as Akt henceforth) is expressed ubiquitously at high levels with exception to the kidney, liver and spleen [8–10], Akt2 expression is elevated in insulin-responsive tissues such as brown fat, skeletal muscle and liver [11], and Akt3 expression is ubiquitous with low levels in liver and skeletal muscle. Specific functions have been implicated in the different isoforms of Akt; Akt2 amplification and overexpression corresponds with motility/invasion [12], and increased Akt3 activity is suggested to contribute to the aggressiveness of steroid hormone-insensitive cancers [13]. Knockout experiments have shown that the three isozymes are not functionally identical. Akt1−/− mice are healthy, but show impaired fetal and postnatal growth [14] while Akt2−/− knockout mice display normal growth characteristics, but with a mild to severe insulin resistance phenotype [15, 16]. Akt3−/− mice have smaller and fewer brain cells, resulting in a 20% decrease in brain size [17].
Because the three isozymes of Akt are not functionally identical, inhibitors against them have been used to determine the function in different tissues [18]. However, similar to other kinases, the development of Akt specific inhibitors is still problematic due to its high sequence identify to other AGC family proteins (>90% homology among Akt isoforms and >50% with PKA-α and PKC-α) Fig. (2). Moreover, there is a 100% homology between the ATP binding site of Akt1 and Akt2 (96% homology with Akt2/3 and >70% with PKA-α/PKC-α), as indicated in Table 1. This implies that the development of Akt isoform-specific inhibitors is even more challenging and unselective inhibition of these isoforms will lead to various off-target toxicities due to their involvement in different pathways. Nevertheless, the fact that some kinase domain inhibitors display certain selectivity among the isoforms hints towards structural differences in binding. When comparing the kinase domain crystal structures of Akt1 (3CQU) with Akt2 (2UW9), which have similar bound inhibitors, we found that most of the known critical residues interact with the inhibitors in similar manners; but there are several residues binding differently to the inhibitors, suggesting the subtle structural difference of these isoforms. For instance, we found that in Akt1 (3CQU), the Phe161 interacts tightly with the triazole moiety of the ligand, but in Akt2 (2UW9) the residue Phe163 seems not involved in the inhibitor binding. Similar pattern have been observed for Leu156, Thr211, Glu278 and Asn279.
Fig. 2.

Sequence alignment of the kinase domain of all three Akt isoforms and some other AGC proteins.
Table 1.
Homology of the Kinase Binding Site.
| Kinase | % Homology | Variable Residues in Binding Site | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akt1 | 100 | L158 | K160 | T162 | F163 | K165 | E193 | H196 | T197 | T213 | F227 | M229 | E230 | A232 | E236 | E279 | M282 | T292 | L296 |
| Akt2 | 100 | L | K | T | F | K | E | H | T | T | F | M | E | A | E | E | M | T | L |
| Akt3 | 96 | L | K | T | F | K | E | H | T | T | F | M | E | V | E | E | M | T | L |
| PKC-α | 74 | L | K | S | F | K | D | C | T | T | F | M | E | V | D | D | M | A | M |
| PKA-α | 70 | L49 | T51 | S53 | F54 | R56 | Q84 | H87 | T88 | V104 | M118 | M120 | E121 | V123 | E127 | E170 | L173 | T183 | F187 |
Taken collectively, these findings demonstrate that Akt is a promising target, and there have been numerous and intense efforts toward the development of Akt inhibitors as anticancer agents. These attempts can be organized based upon the regions and functions of Akt to which they target. In this review, we will summarize the approaches to develop Akt inhibitors for potential cancer treatments by targeting different regions of Akt, including: the kinase domain, PH domain, LINK domain, and C-terminal extension domain. Then we will discuss peptide based pseudo-substrates/pseudo-regulators to block protein-protein interactions, followed by combined strategies. Some of these representative inhibitors are summarized in Table 2.
Table 2.
Summary of Some Representative Akt Inhibitors.
| Inhibitor Name | Company | Target | Status | References |
|---|---|---|---|---|
| A-443654 | Abbott | ATP binding site | Lead optimization | [23–26] |
| GSK690693 | GlaxoSmithKline | ATP binding site | Withdrawn | [27–31] |
| XL418 | Exelixis | ATP binding site | Phase I clinical trials | [19, 20] |
| Truncated GSK3β pseudosubstrate | Yale/University of South Florida | Substrate binding site on Kinase domain | Lead optimization | [104–106] |
| WAY-178210-A-1 | Wyeth | T-loop of kinase domain | Lead optimization | [32–35] |
| Phosphatidylinositol ether lipid analogues (PIAs) | MD Anderson/NIH | PH domain | Preclinical development | [56–58] |
| Perifosine | Keryx | PH domain | Phase II clinical trials | [60, 63–67] |
| PHT-427 | PHusis Therapeutics | PH domain | Preclinical development | [69] |
| PH-6 | MD Anderson | PH domain | Lead optimization | [71] |
| DPIEL/PX-316 | Prolx | PH domain | Preclinical development | [21] |
| Merck Akt-I-(1,2,3) | Merck | LINK domain | Preclinical development | [74–83] |
TARGETING THE KINASE DOMAIN FOR AKT INHIBITOR DEVELOPMENT
While the central kinase domain of Akt is the most obvious therapeutic target in the Akt pathway, it has proved to be a difficult one. Each kinase domain of Akt, which stretches from amino acid 148 to 411, terminates in a regulatory hydrophobic motif. Because of the high homology of the kinase domain, the majority of Akt inhibitors occupy the ATP binding site as pan-Akt inhibitors [22]. These compounds display promiscuity towards the general AGC kinase family (related to AMP/GMP kinase and protein kinase C). This kinase family is involved in a diverse range of critical cellular functions; the broad targeting of which leads to off-target effects and toxicity. In spite of the daunting challenge presented by targeting the Akt kinase domain, the significance and potential payoff of this has proved too seductive to ignore. Successes from similar kinase domain inhibition of other noteworthy kinases, such as inhibitors against c-Kit and Bcr/Abl, are anticipated to be repeated with Akt.
There have been numerous reports of Akt kinase inhibitors with reasonable selectivities and promising efficacy results. Luo et al. [23], based on structure-based computational approaches, generated indazole-pyridine based pan-Akt inhibitors, the most potent of which is A-443654 from Abbott Laboratories [24] Fig. (3A). A-443654 targets the ATP-binding pocket and has equal potency against Akt1, Akt2, or Akt3 within cells (Akt1 binding Ki=160pM), but is more selective against Akt than other kinases in the AGC family including PKA and PKC. Figure 3B illustrates that there are multiple hydrogen bonds as well as hydrophobic interactions involved in the binding of this inhibitor to the Akt kinase domain, explaining the high binding affinity. A-443654 was also found to induce Akt Ser473 phosphorylation in PTEN- and TSC2-deficient cancer cell lines [25], which possibly lead to its severe hypotension in rats and dogs [26].
Fig. 3.
Fig. (3A). A-443654 from Abbott Laboratories.
Fig. (3B). Analysis of crystal structure demonstrates that Glu230, Ala232 Asn280, Asp293 and several other residues are involved in strong interactions between Akt kinase domain and the inhibitor A-443654.
Another pan-Akt inhibitor, GSK690693 from GlaxoSmithKline, Fig. (4) [27, 28] (Akt1: IC50 = 2nM, Akt2: IC50 = 13nM and Akt3: IC50 = 9nM) is aminofurazan-derived, ATP-competitive inhibitor, and has been reported to be selective against 13 kinases (IC50 < 100nM), including AGC kinase family members: PKA, PrkX and PKC isozymes [29]. GSK690693 showed selective antiproliferative effects towards malignant cells, and downstream Akt phosphorylation events were reduced. In 55% of 112 cell lines tested, acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, and Burkitt lymphoma showed 89%, 73%, and 67% proliferation sensitivity, respectively, to GSK690693. It was withdrawn prior to entering clinical trials [30], possibly because treatment resulted in increased circulating blood glucose and insulin levels [31] due to its interactions with Akt2, which is an integral part of the insulin signaling pathway [11].
Fig. 4.
Structure of GSK690693.
Via high-throughput screening, Wyeth reported lacto-quinomycin (WAY-178210-A-1), Fig. (5A) from the fermentation broth of a microbial strain Streptomyces sp. LL-AF101. It is a potent and selective inhibitor by targeting Akt (Akt1 IC50 = 0.149μM) through a novel allosteric mechanism that involves two critical catalytic activation loop (T-loop) cysteines (Cys296 and Cys310) neighboring the activating residue Thr308 [32]. Lactoquinomycin was shown to be selective against a panel of 45 kinases, but did not discriminate appreciably between Akt1 and Akt2 isozymes. The T-loops of AGC kinases, in contrast to the ATP-binding sites, show a high level of polymorphism [33]. However therapy by lactoquinomycin itself is not feasible because of its redox properties conferring nonspecific cytotoxicity [34]. Development of the proven Akt inactivating pyranonaphthoquinone (PNQ) framework might lead to less toxic compounds. Further PNQ framework exploration by Wyeth has resulted in modified compounds with increased Akt inhibition Fig. (5B) [35].
Fig. 5.


Fig. (5A).Wyeth Akt inhibitor Lactoquinomycin (WAY-178210-A-1).
Fig. (5B). The pyranonaphthoquinone (PNQ) framework of Wyeth compounds derived from structure Fig. (5A).
LIMITATIONS OF TARGETING KINASE DOMAIN FOR AKT INHIBITOR DEVELOPMENT
Despite these preceding results, progress in the development of Akt inhibitors targeting the kinase domain has been slow and disappointing, primarily because of high homology of ATP binding pocket in various kinases as well as the fact that Akt inhibition is often associated with promiscuous inhibition within the AGC kinase family (protein A, G and C kinases) [29]. To date, there have been no reports of specific Akt kinase inhibitors that have been shown to be both active against tumors in vivo and devoid of major toxicities to normal tissues [36]. Extensive conservation of the ATP-binding domain within the AGC kinase family has proved to be a major hurdle in developing small molecule inhibitors against Akt. There have been reports of libraries of small molecule inhibitors of Akt, resulting from screening of larger libraries of compounds. In future, more potent and selective Akt inhibitors may be identified by virtual screening of these libraries using structure-guided approaches based on the 3D structures of Akt as well as the chemical-physical properties of chemical compounds [37–40].
TARGETING THE PH DOMAIN FOR AKT INHIBITOR DEVELOPMENT
Although the majority of small molecule inhibitors are ATP-competitive inhibitors targeting the kinase domain of Akt [41, 42], they provide little specificity because of the high degree of homology in the ATP-binding pocket among different serine/threonine kinases [43] as discussed above. In order to overcome this drawback, alternative therapeutic modes have been pursued by targeting the PH domain of Akt to interfere with its binding to PIP3 and membrane translocation. The PH domain is a region containing 100 to 120 amino acids found in over 250 human proteins. Although the amino acid sequence of PH domains is not universally conserved, the tertiary structure is remarkably conserved [44]. The fact that the sequence identity of different PH domains is usually less than 30%, which offers the possibility to develop selective agents for different PH domain targets [45].
Akt can be activated through binding to 3′-OH phosphorylated phosphatidylinositols via the PH domain. In order to block this interaction, computer-based molecular modeling has been successfully used in drug discovery and development targeting Akt [46–55]. Based on computational modeling of Akt PH domain, a series of structurally modified phosphatidylinositol ether lipid analogues (PIAs) were synthesized to inhibit the interaction [56–58]. Five PIAs with modification at two sites on the inositol ring were found to inhibit Akt with IC50 less than 5μM Fig. (6C). These compounds inhibited Akt activation and phosphorylation of several downstream substrates of Akt in tumor cells without affecting the activities of upstream kinases (such as PI3-K and 3-phosphoinositide-dependent protein kinase 1 (PDK1, also known as PDPK1), or members of other signaling pathways such as ERK2. Moreover, PIAs increased apoptosis 20–30-fold in cancer cell lines with high levels of endogenous Akt activity, but only 4–5-fold in cancer cell lines with low levels of Akt activity. However, whether PIAs are effective in vivo and whether PIAs affect other PH-domain containing proteins is currently unknown.
Fig. 6.
Akt PH domain inhibitors. A. Perifosine. B. Ceramides. C. PIAs. D. The derivates of 4-(1-benzoyl-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-ylazo)-N-pyrimidin-2-yl-benzenesulfonamide. E. 4-Dodecyl-N-(1,3,4-thiadiazol-2-yl)benenesulfonamide.
Perifosine structurally resembles naturally occurring phospholipids, and appears to block the activation and phosphorylation of Akt in cellular settings [59–61]. Perifosine belongs to a class of antitumor alkylphospholipids with a piperidine head group Fig. (6A). It was originally based on the structure of lysophosphatidylcholine. In vivo, perifosine induced myelopoiesis in murine marrow and spleen, whereas it caused apoptosis in myeloma xenografts [62]. In vitro, perifosine inhibits Akt at low micromolar concentrations [60, 63–65], and inhibition has been measured in many xenograft models in vivo [63, 66, 67]. Perifosine is currently being tested in phase 2 clinical trials. So far, the clinical results with perifosine have been modest and have not lived up to the hopes for an orally available Akt inhibitor, because no modulation of Akt has been assessed [59]. In order to measure the binding affinity of perifosine to the Akt PH domain, surface plasmon resonance spectroscopy binding assay was executed, which uses PIP3 biotin-labeled liposomes to measure the displacement [68]. Although perifosine has been reported as an Akt PH domain inhibitor, our recent experimental studies showed that perifosine does not bind to the PH domain of Akt with binding affinity (Ki) > 50μM. [69]. Furthermore, another report demonstrated that the low binding affinity of perifosine to the PH domain was undetectable by titration calorimetry. Ultimately, it remains unclear if the potential effectiveness of perifosine is due to disrupting microdomains crucial to Akt activation or via direct PH domain displacement of PIP2 and PIP3 ligands [59].
Ceramides are molecules also belonging to the lipid family. A ceramide is composed of a sphingosine and a fatty acid Fig. (6B). Ceramides are found in high concentrations within the cell membrane. Experimental findings obtained with separate domains suggest that ceramide blocks the effects of insulin on both the PH domain and the catalytic/regulatory domain of Akt [70]. Major issues with the lipid-based molecules are: limited solubility, moderate potency against Akt kinases, aggregation, and poor pharmacokinetics. Hence, small molecule design was undertaken for inhibitors targeting the PH domain of Akt, but with better ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties.
In order to design small molecule inhibitors of the Akt PH domain, in silico structure-based drug design was performed to guide the chemical synthesis. Using in silico library screening and interactive molecular docking, as described next, a novel class of non-lipid-based compounds were identified Fig. (6D), with binding affinity in the range 0.4 to 3.6μmol/L [71]. Some of them exhibited PH domain-binding selectivity for Akt compared with insulin receptor substrate-1 and PDK1. These compounds inhibited Akt in cells, induced apoptosis, and inhibited cancer cell proliferation. Unfortunately, in vivo, the lead compound failed to achieve the blood concentrations required to inhibit Akt in cells, most likely due to rapid metabolism and elimination, and did not show antitumor activity.
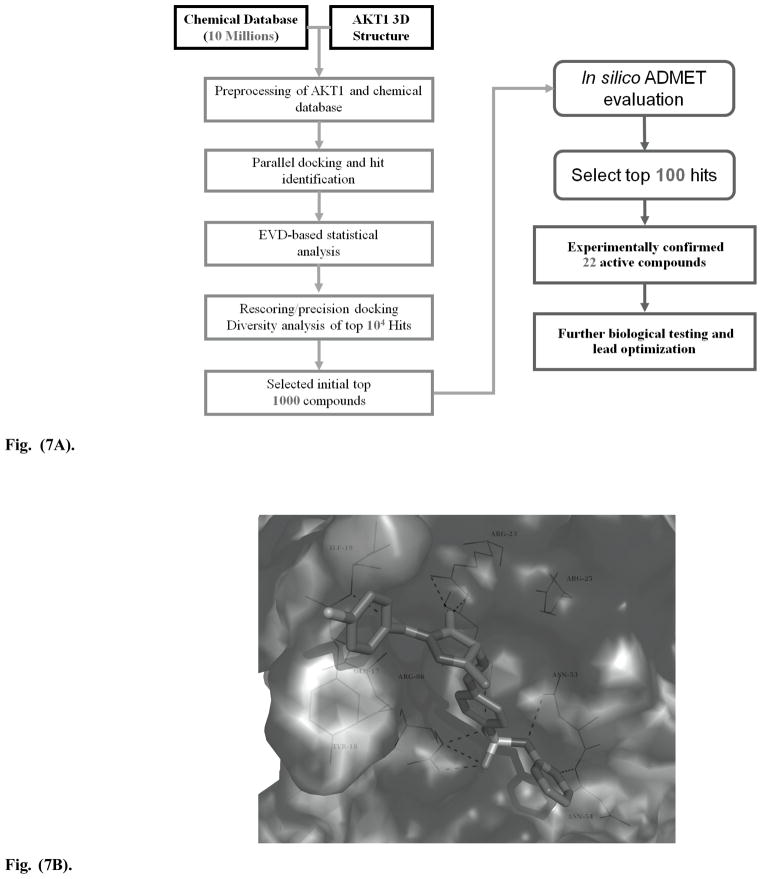
Recently, a small inhibitor with good antitumor activity was discovered by our group [68, 72]. The discovery workflow was summarized in Fig. (7A). Briefly, the Akt1 PH domain crystal structure 1UNQ [73] was retrieved from the protein database (PDB). SYBYL was employed to fix the missing residues and atoms of the protein. Hydrogen atoms were added, and crystal waters and the ligand were removed from the complex structure. For the definition of the binding pocket, no cavity detection was executed and a cavity file was instead generated, which included all residues within 6.5Ǻ around the residues Lys14, Glu17, Arg23 and Arg86 which were reported as essential for the protein-ligand interactions [73]. Via high-throughput virtual screening with our in-house tool HiPCDock [73], top 104 hits were initially selected for further rescoring and diversity analysis. At this stage, GOLD docking was executed using default parameters unless otherwise noted [73]. The internal hydrogen bonds were taken into account to restrict the flexibility of the ligand. No early termination was allowed for the fitness search. For each ligand, the received docking poses were evaluated via virtualization and selected according to the protein pharmacophore. About 1000 compounds were selected at this step for in silico ADMET evaluation, followed with experimental validation of 100 chemical candidates. After this process 22 potential PH domain inhibitors were identified and were experimentally confirmed to be active using surface plasmon resonance spectroscopy. All of these evaluations have been focused on Akt1. Due to the relatively low sequence identity of PH domains (< 20%), compared to kinase domains (> 95%), we expect that optimization of Akt isoform-specificity of our PH domain inhibitors will be not as challenging as ATP competitors. In order to improve the binding affinity and cellular permeability, the structures of these compounds were further optimized using both structure-based docking approaches and ligand-based Caco-2 QSAR models. Figure 7B shows the interaction between one of our hits Fig. (6D) and the Akt PH domain. Another hit, 4-dodecyl-N-(1,3,4-thiadiazol-2-yl) benzenesulfonamide Fig. (6E), was subsequently synthesized and measured to have a binding affinity KD = 40.8 ± 2.5μM and Akt inhibition IC50 = 6.3 ± 0.9μM [68]. This compound inhibited Akt and its downstream targets in cells as well as in pancreatic cancer cell xenografts in immunocompromised mice, exhibiting promising antitumor activity [68].
Fig. 7.
Fig. (7A) The workflow employed for PH domain inhibitor identification with high-throughput virtual screening and other in silico modeling approaches. The gray numbers represent the reduction of numbers of compounds from the initial large chemical database collection to the final experimentally measured active hits for future studies.
Fig. (7B). One of the hits we identified through our in silico library screening. It has been confirmed active in our experimental testing followed with further optimization. Molecular docking demonstrates that this compound mimics the 4 and 5 phosphate groups in PIP3 to interact with the positive Akt PH domain binding pocket involved in Glu17, Arg23, Arg25, Asn53, Asn54, Arg86 and several others.
TARGETING THE LINK DOMAIN FOR AKT INHIBITOR DEVELOPMENT
The LINK domain is the region connecting the PH domain with the catalytic domain of Akt. This domain consists of only about 50 residues, and it is poorly conserved among the Akt isoforms (17–46% identical) and has no significant homology to any other human protein [45]. Because there are no published crystal structures of the LINK region, and the low sequence homology between this domain and other known structures makes the homology modeling difficult, it presents a high challenge for the drug design targeting the LINK domain.
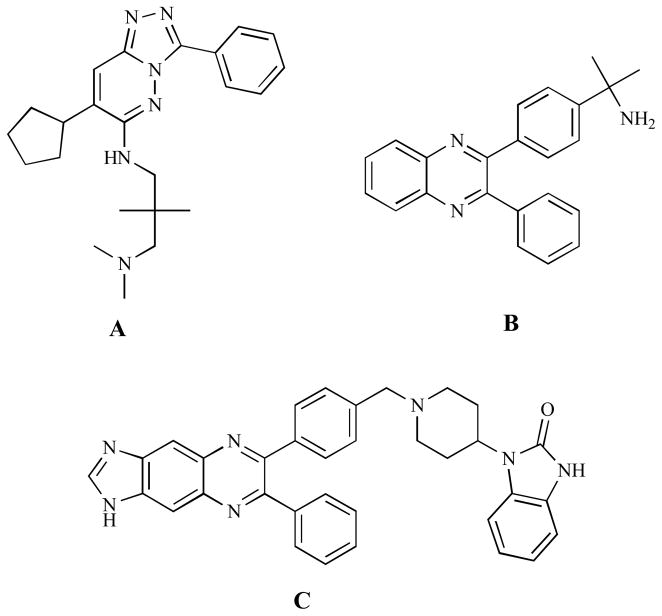
Two LINK domain inhibitors were developed, as claimed by Merck, using high-throughput screening techniques [74–83]. Both inhibitors are capable of inhibiting full-length Akt, and a detailed kinetic analysis indicated that these inhibitors were not competitive with ATP or peptide substrate and could not inhibit Akt lacking the PH domain [84].
Because of the sequence diversity of the LINK region, as mentioned above [45], the selectivity of the LINK domain-targeted inhibitors is presumably high. For instance, Merck Akt-I-1 Fig. (8A) inhibits Akt1 with IC50 = 4.6μM, while IC50 > 250μM for the Akt2 inhibition. Merck-Akt-I-2 Fig. (8B) is also isoform selective against the full length Akt paralogs (Akt1: IC50 = 3.4 μM, Akt2: IC50 = 23μM and Akt3: IC50 > 50μM) [79]. It is inactive against PH domain-truncated Akt [84]. Merck-Akt-I-2 was shown to reversibly inhibit the activation of Akt1 and Akt2, but not Akt3 [84–86]. Furthermore, optimization of the quinoxaline template and extension at the isopropylamine group results in higher potency Fig. (8C) [79, 87]. A series of other analogs were derived from the Merck-Akt-I-2 to improve the potency and selectivity. One of the analogs can selectively inhibit Akt1 at IC50 = 760nM, while another one is Akt2 selective with IC50 = 325nM [88].
Fig. 8.
LINK domain inhibitors. A. Merck-Akt-I-1. B. Merck-Akt-I-2. C. Merck Akt-I-3.
TARGETING THE C-TERMINAL HYDROPHOBIC MOTIF
Akt terminates with a C-terminal hydrophobic domain, in which the HM Phe-X-X-Phe-(Ser/Thr)-Trp is found to be conserved among AGC kinase family [89–91]. These hydrophobic residues play a significant role in triggering allosteric effects and fully activating Akt for substrate phosphorylation. Recent X-ray crystallography studies showed that phosphorylation of Akt2 Ser474 in the HM by a putative PDK2 stabilized the hydrophobic groove (HG) residues located in the N-lobe of the kinase domain, in which αB and αC-helices are highly disordered in unphosphorylated and monophosphorylated Akt2 [33]. This is achieved by the hydrophobic interaction with αB and αC-helices as well as a putative hydrogen bond with residues phospho-Ser474 to Gln219 of the β4 strand [33, 92]. The conformational changes resulting from the interactions between HM and HG augment Akt kinase activity by 7–10 fold [93]. It offers the biological basis of developing novel inhibitors of Akt by hampering intramolecular hydrophobic interactions between the HM and HG structures. Furthermore, Yang et al. have provided insights to this novel target utilizing a mutagenesis study of chimeric protein of Akt and PIFtide [92]. PIFtide, a 23-residue peptide from PRK2 HM, is a potent activator of Akt which mimics the phosphorylated HM of Akt [89]. They found that mutations of conserved phenylalanine or asparate residues in PIFtide might eliminate their potential to activate Akt [92]. These findings indicated an innovative drug design strategy against Akt; that is, to exploit pseudo-unphosphorylated-HM or its small-molecule derivatives that are capable of constitutively binding to HG and making Akt unresponsive to the phosphorylation on Ser473. Although no efficacious inhibitors or drugs have been disclosed so far, the therapeutic potential of C-terminal hydrophobic domain has aroused profound interest from some pharmaceutical companies including Novartis.
TARGETING TRAF6 TO INHIBIT AKT PATHWAY SIGNALING
It has been recently discovered that the E3 ligase TRAF6 forms a complex with Akt, and it is responsible for Akt ubiquitination at a specific residue (Lys8) in the PH domain. This ubiquitination promotes translocation, instead of protein degradation, to the plasma membrane for subsequent Akt activation [94]. This latest study has provided TRAF6 as a new potential target in developing novel Akt pathway inhibitors for human cancer therapy [95]. Although there are no TRAF6 inhibitors currently available, as of this publication, blocking E3 ligase functions indeed has shown great promise in clinical trials. TRAF6 is an especially attractive target due to its roles in the PI3K/Akt/PTEN pathways [96]. Combined with TRAF6 inhibition, rapamycin analogs (rapalogs) that inhibit the mammalian target of rapamycin complex 1 (mTORC1) can shift their stabilization and cytostatic effects to cytotoxic effects, and thus increase their therapeutic potential [95].
INHIBITING AKT WITH PEPTIDE-BASED AKT PSEUDOSUBSTRATES OR PSEUDOREGULATORS
The majority of inhibitors, which target the Akt ATP binding pocket or PH domain, are capable of affecting other ATP or PtdIns-utilizing proteins. Therefore, selective inhibition of Akt activity still remains challenging due to a variety of homologous proteins sharing similarity in structures and functions. In contrast to ATP (or PtdIns) competitive inhibitors, substrate-mimetic inhibitors that target the Akt substrate binding domain hold a great potential in becoming new anticancer therapeutics with high specificity and low toxicity. Akt modulates its downstream substrates, such as BAD, GSK3, FKRHL1 etc, via phosphorylation at specific serine/threonine sites in which the Akt-specific binding motif plays an important role. Recent development of peptide and non-peptidic inhibitors has proven to be selective and potent in inhibiting Akt activity in vitro. A series of hybrid peptides composed of 9aa from human forkhead transcription factors (hFKRHL1) and 11aa Akt-binding consensus motif [97] have been designed to induce apoptosis and sensitize tumor cells [98–100]. With mutations of putative phorsphorylation serine/threonine sites with Alanine, these pseudosubstrates are able to prevent the substrates from binding to Akt1, Akt2 and Akt3. For instance, a peptide with two Alanine mutations inhibits in vitro phosphorylation of biotinylated BAD peptide by Akt1 with Ki 110nM, whereas four out of five peptides exhibit at least 30-fold selectivity against Akt compared with other kinases including p70S6K, PKA, Cdc2, Src, PKCγ, etc. Moreover, these peptide-based inhibitors can also block the phosphorylation of GSK3 and Hela cell growth in vitro.
In another patent, a peptide named Akt-in was explored based on βA strand of TCL1 or its homology MTCL1 [101]. TCL1 is a proto-oncogene and acts as the co-activator of Akt through two functional motifs, which are responsible for Akt association and homodimerization, respectively [102]. Hiromura et al. were the first to hypothesize and studied the inhibitory use of βA peptide from TCL1 (10–24aa), which spans the crucial binding site for Akt association. NMR structure and molecular docking showed that Akt-in was bound to the Akt PH domain and inhibited membrane translocation by inducing conformational change on the PIP3 binding pocket in a dose-dependent manner. Akt-in also exhibited strong specificity against Akt over PDK1, PKC, PKA, and MAPK in both in vitro and cell-based experiments [103]. However, like other peptidomimetic inhibitors, Akt-in still retains poor bioavailability; it needs further optimization for future clinical use.
Although peptide-based Akt inhibitors have shown potent efficacy and selectivity against Akt activity, their large size and high flexibility limit the satisfactory pharmacokinetic properties and cell-permeability. Recently, Kayser-Bricker et al. reported a group of non-peptidomimetic small-molecule inhibitors based on truncated GSK3β binding motif Fig. (9) [104, 105]. Using structure-based modification of the N-terminal, C-terminal, and the core chemical scaffold, followed by ligand-based structure-activity relationship (SAR) optimization, small molecule inhibitors showed improved PK/PD profile compared to its original lead compound [106], with merely slight potency decreases (from 14μM to 17μM).
Fig. 9.
Rational design of Akt pseudosubstrates and lead optimization of non-peptidomimetic small-molecule inhibitors based on human GSK3β binding motif. A series of modifications on chemical moieties (in dashed box), including the deletion of N-terminal Arginine and Proline, substitution of internal scaffold to p-aminobenzoic acid, increase of C-terminal hydrophobicity, etc. It afforded the peptidomimetic inhibitor with IC50 14μM. Lead optimization based on chemical scaffold and molecular docking resulted in several drug-like and synthesizable small-molecular inhibitors which were validated to inhibit Akt kinase activity at low micromolar level.
COMBINED STRATEGIES COMPLEMENTARY TO UNI-TARGET ANTICANCER THERAPEUTICS FOR AKT INHIBITION
Anticancer chemotherapy is more than the inhibition of hyperactivation of Akt. As far as we know, dozens of upstream or downstream regulators or effectors in the Akt signaling pathway are thought to mediate the survival, evasion of apoptosis, proliferation and angiogenesis of cancer cells [107]. Thus, personalized chemotherapy targeting multiple nodes in the Akt pathway may be effective to treat specific cancers which are resistant to a single agent or therapy. Although the combination of other anticancer inhibtors with Akt inhibitors has been extensively studied under preclinical background, only a few patents of combined strategies have been published to date.
It is known that three Akt isoforms are hyperactivated in various types of cancers, and can be phosphorylated and activated by multiple upstream kinases, such as PI3K, HER2, EGFR etc [108, 109]. In addition, irregularities of Akt activity can be achieved by Akt-independent upstream signaling, like PTEN loss and PI3KCA mutation [110]. Also Akt may be constitutively activated due to overexpression of Akt isoforms. Therefore, the rational combination of proximal upstream inhibition with Akt inhibition may solve the single drug resistance in some cases. Barnett et al. first reported a significant increase of caspase-3 activation by treating at least two selective Akt1 inhibitors, an Akt1 and Akt2 inhibitor, a PI3K inhibitor (LY294002), and a HER2 inhibitor (Herceptin) over one test compound alone [78]. Another patent comprising the combination of Bevacizumab, Tarceva and Gleevec (as inhibitors of VEGF mediated angiogenesis, HER1-Akt pathway, and Bcr-abl tyrosine kinase, respectively) has been tested in Phase II clinical trials [111]. This composition was verified as sufficient to treat proliferative diseases with minimized toxicity to the host. Similar results have been observed in gefitinib (tyrosine kinase inhibitor)-resistant cancer cell lines when using a combined approach of LY294002 with EGFR inhibition [112].
Several substrates of Akt, such as mTOR, BAD, and hsp90 mediate Akt cellular functions in protein synthesis, inhibition of apoptosis, and NO signaling. On the other hand, the deregulation of other pathways such as MAPK, and the NO pathway, also contribute to carcinogenesis and metastasis [113, 114]. Hence, the combination of inhibitors of distal targets of Akt with other cancer signaling pathways has been indicated as an alternative methodology to improve and augment their therapeutic effects. For example, the synergistic effect of rapamycin-induced autophagy by a PI3K/Akt inhibitor has been reported previously in malignant glioma cells [115]. A newly-invented drug combination comprising diaryl urea compounds, PI3K inhibitors, Akt kinase inhibitors and mTOR inhibitors was recently published for treating cancer [116]. This composition is targeted to the MAP kinase signaling pathway [117] as well as the PI3K/Akt/mTOR pathway [118] and may enhance the inhibition effect of metastasis and cell proliferation of treated cancer tissue. However, it is still a challenge to effectively inhibit the PI3K pathway by targeting mTORC1 because the blockage of the negative feedback loop can eventually lead to Akt activation [119]. Therefore it is important to inhibit Akt for anticancer therapy. More recently, Akt pathway inhibitors such as LY294002 may coordinate with drugs that target hsp90 or caveolin-1 to treat NO-dependent tumor angiogenesis [120]. Another patent invention concentrates on the synergistic induction of cancer cell apoptosis by inhibiting cyclin-dependent kinases (CDKs) and XIAP [121]. Roscovtine or flavopiridol (CDK9 and XIAP inhibitors) combined with API-2 (Akt inhibitor) or LY294002 (PI3K inhibitor) elicited apoptosis on roscovtine-resistant prostate cancer PC3 cell line, while normal epidermal cells remained viable.
CURRENT & FUTURE DEVELOPMENTS
There have been over five thousand publications on Akt in the last 15 years (based on PubMed search), and as of late a marked increase has occurred in the number of Akt inhibitors. However, there are still unresolved issues in regard to Akt target specificity and isozyme selectivity that must be addressed before these inhibitors can be employed in anti-cancer therapies. The complexity of the Akt pathway provides many signaling events that can be molecular targets for potential therapeutics, both up and downstream of Akt itself. Elucidation of the structure, systemic biology, and complete molecular network of Akt and its isoforms will result in advanced therapies with higher therapeutic index and lower toxicities. In addition, Akt inhibitors can be used in combination with existing chemotherapeutic agents. Moreover, computer-aided in silico and rational design approaches will further help us to accelerate the discovery and development of Akt targeted therapies in a more cost-effective manner.
Acknowledgments
This work was supported in part by the MD Anderson Cancer Center Faculty Startup to SZ and the NIH/NCI grants RO1 CA061015 and P30 CA23074 to GP.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
References
- 1.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/Akt: A major therapeutic target. Biochim Biophys Acta. 2004;1697(1–2):3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsley CW, Barnett SF, Layton ME, Bilodeau MT. The PI3K/Akt pathway: Recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Cancer Drug Targets. 2008;8(1):7–18. doi: 10.2174/156800908783497096. [DOI] [PubMed] [Google Scholar]
- 4.Kumar CC, Madison V. Akt crystal structure and Akt-specific inhibitors. Oncogene. 2005;24(50):7493–501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 5.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995;9(8):576–96. [PubMed] [Google Scholar]
- 6.Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 7.Li Q. Recent progress in the discovery of Akt inhibitors as anticancer agents. Expert Opin Ther Pat. 2007;17(9):1077–130. [Google Scholar]
- 8.Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201(2):475–81. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88(10):4171–5. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, et al. Structure, expression and chromosomal mapping of c-Akt: Relationship to v-Akt and its implications. Oncogene. 1993;8(3):745–54. [PubMed] [Google Scholar]
- 11.Bellacosa A, Testa JR, Moore R, Larue L. A portrait of Akt kinases: Human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3(3):268–75. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- 12.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, et al. Overexpression of Akt2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63(1):196–206. [PubMed] [Google Scholar]
- 13.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, et al. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274(31):21528–32. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276(42):38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 15.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292(5522):1728–31. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 17.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25(5):1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laine J, Kunstle G, Obata T, Noguchi M. Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family. J Biol Chem. 2002;277(5):3743–51. doi: 10.1074/jbc.M107069200. [DOI] [PubMed] [Google Scholar]
- 19.Rice K, Co EW, Kim MH, Bannen LC, Bussenius J, Le D, Tsuhako AL, Nuss J, Wang Y. [1H-pyrazolo[3, 4-D]pyrimidin-4-yl]-piperidine or -piperazine compounds as serine-theoronine kinase modulators (P70S6K, ATK1 and ATK2) for the treatment of immunologcal, inflammatory and proliferative diseases. WO06071819. 2006
- 20.Anand NK, Blazey CM, Bowles OJ, Bussenius J, Canne BL, Chan DS, Chen B, Co EW, Costanzo S, Defina SC, Dubenko L, Franzini M, Huang P, Jammalamadaka V, Khoury RG, Kim MH, Klein RR, Le T, Mac MB, Nuss JM, Parks JJ, Rice KD, Tsang TH, Tsuhako AL, Wang Y, Xu W. WO05117909. Kinase modulators and methods of use. 2005
- 21.Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, et al. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res. 2004;14(10):513–27. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- 22.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of Akt kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4(6):977–86. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 24.Qun L, Woods KW, Zhu GD, Fischer JP, Gong J, Waukegan TL, Gandhi V, Thomas SA, Packard GK, Song X, Abrams JN, Diebold RB, Dinges J, Hutchins CW, Stoll VS, Rosenberg H, Giranda VL. US6831175. Kinase inhibitors. 2004
- 25.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26(38):5655–61. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 26.LoPiccolo J, Granville CA, Gills JJ, Dennis PA. Targeting Akt in cancer therapy. Anticancer Drugs. 2007;18(8):861–74. doi: 10.1097/CAD.0b013e3280cc2c6f. [DOI] [PubMed] [Google Scholar]
- 27.Heerding DA, Clark TJ, Leber JD, Safonov I. WO07058850. Inhibitors of Akt activity. 2007
- 28.Heerding DA, Clark TJ, Leber JD, Safonov I. US20100056523. Inhibitors of Akt activity. 2010
- 29.Maira S-M, Furet P, Stauffer F. Discovery of novel anticancer therapeutics targeting the PPI3K/Akt/mTOR pathway. Future Med Chem [Review] 2009;1(1):19. doi: 10.4155/fmc.09.5. [DOI] [PubMed] [Google Scholar]
- 30.GlaxoSmithKline. Protocol Summary Akt108169. GlaxoSmithKline; [Google Scholar]
- 31.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68(7):2366–74. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 32.Toral-Barza L, Zhang WG, Huang X, McDonald LA, Salaski EJ, Barbieri LR, et al. Discovery of lactoquinomycin and related pyranonaphthoquinones as potent and allosteric inhibitors of Akt/PKB: Mechanistic involvement of Akt catalytic activation loop cysteines. Mol Cancer Ther. 2007;6(11):3028–38. doi: 10.1158/1535-7163.MCT-07-0211. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9(12):940–4. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 34.Nomoto K, Okabe T, Suzuki H, Tanaka N. Mechanism of action of lactoquinomycin A with special reference to the radical formation. J Antibiot (Tokyo) 1988;41(8):1124–9. doi: 10.7164/antibiotics.41.1124. [DOI] [PubMed] [Google Scholar]
- 35.Salaski EJ, Krishnamurthy G, Ding WD, Yu K, Insaf SS, Eid C, et al. Pyranonaphthoquinone lactones: A new class of Akt selective kinase inhibitors alkylate a regulatory loop cysteine. J Med Chem. 2009;52(8):2181–4. doi: 10.1021/jm900075g. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: Molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004;4(3):235–56. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, et al. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem. 2000;43(16):3045–51. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, Meuillet EJ, Berggren M, Powis G, Kozikowski AP. 3-Deoxy-3-substituted-D-myo-inositol imidazolyl ether lipid phosphates and carbonate as inhibitors of the phosphatidylinositol 3-kinase pathway and cancer cell growth. Bioorg Med Chem Lett. 2001;11(2):173–6. doi: 10.1016/s0960-894x(00)00640-5. [DOI] [PubMed] [Google Scholar]
- 39.Rong SB, Hu Y, Enyedy I, Powis G, Meuillet EJ, Wu X, et al. Molecular modeling studies of the Akt PH domain and its interaction with phosphoinositides. J Med Chem. 2001;44(6):898–908. doi: 10.1021/jm000493i. [DOI] [PubMed] [Google Scholar]
- 40.Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125(5):1144–5. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 41.Breitenlechner CB, Wegge T, Berillon L, Graul K, Marzenell K, Friebe W-G, et al. Structure-based optimization of novel azepane derivatives as PKB inhibitors. J Med Chem. 2004;47(6):1375–90. doi: 10.1021/jm0310479. [DOI] [PubMed] [Google Scholar]
- 42.Reuveni H, Livnah N, Geiger T, Klein S, Ohne O, Cohen I, et al. Toward a PKB inhibitor: Modification of a selective PKA inhibitor by rational design. Biochemistry. 2002;41(32):10304–14. doi: 10.1021/bi0202530. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Cron P, Thompson V, Valerie GM, Hess D, Brian HA, et al. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9(6):1227–40. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 44.Rebecchi MJ, Scarlata S. Pleckstrin homology domains: A common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–28. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 45.Kumar CC, Madison V. Akt crystal structure and Akt-specific inhibitors. Oncogene. 2005;24(50):7493–501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 46.Chen LS, Du-Cuny L, Vethantham V, Hawke DH, Manley JL, Zhang S, et al. Chain termination and inhibition of mammalian poly(A) polymerase by modified ATP analogues. Biochem Pharmacol. 2010;79(5):669–77. doi: 10.1016/j.bcp.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen LS, Nowak BJ, Ayres ML, Krett NL, Rosen ST, Zhang S, et al. Inhibition of ATP synthase by chlorinated adenosine analogue. Biochem Pharmacol. 2009;78(6):583–91. doi: 10.1016/j.bcp.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du-Cuny L, Song Z, Moses S, Powis G, Mash EA, Meuillet EJ, et al. Computational modeling of novel inhibitors targeting the Akt pleckstrin homology domain. Bioorg Med Chem. 2009;17(19):6983–92. doi: 10.1016/j.bmc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, et al. Discovery of a novel class of Akt pleckstrin homology domain inhibitors. Mol Cancer Ther. 2008;7(9):2621–32. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moses SA, Ali MA, Zuohe S, Du-Cuny L, Zhou LL, Lemos R, et al. In vitro and in vivo activity of novel small-molecule inhibitors targeting the pleckstrin homology domain of protein kinase B/Akt. Cancer Res. 2009;69(12):5073–81. doi: 10.1158/0008-5472.CAN-08-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oloff S, Zhang S, Sukumar N, Breneman C, Tropsha A. Chemometric analysis of ligand receptor complementarity: Identifying Complementary Ligands Based on Receptor Information (CoLiBRI) J Chem Inf Model. 2006;46(2):844–51. doi: 10.1021/ci050065r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Golbraikh A, Oloff S, Kohn H, Tropsha A. A novel automated lazy learning QSAR (ALL-QSAR) approach: method development, applications, and virtual screening of chemical databases using validated ALL-QSAR models. J Chem Inf Model. 2006;46(5):1984–95. doi: 10.1021/ci060132x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Wei L, Bastow K, Zheng W, Brossi A, Lee KH, et al. Antitumor agents 252. Application of validated QSAR models to database mining: Discovery of novel tylophorine derivatives as potential anticancer agents. J Comput Aided Mol Des. 2007;21(1–3):97–112. doi: 10.1007/s10822-007-9102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Du-Cuny L. Development and evaluation of a new statistical model for structure-based high-throughput virtual screening. Int J Bioinform Res Appl. 2009;5(3):269–79. doi: 10.1504/IJBRA.2009.026419. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Kumar K, Jiang X, Wallqvist A, Reifman J. DOVIS: An implementation for high-throughput virtual screening using AutoDock. BMC Bioinformatics. 2008;9:126. doi: 10.1186/1471-2105-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castillo SS, Brognard J, Petukhov PA, Zhang C, Tsurutani J, Granville CA, et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64(8):2782–92. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 57.Kozikowski AP, Dennis P, Sun H, Brognard J. WO2004022569. Akt Inhibitors, pharmaceutical compositions, and uses thereof. 2004
- 58.Kozikowski A, Qiao L, Powis G. 3-Deoxy-D-myo-inositol ether lipid analogs as inhibitors of phosphatidyl myo-inositol cycle, preparation thereof, and use for inhibition of cancer cell growth. US20036667340. 2003
- 59.Gills JJ, Dennis PA. Perifosine: Update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–10. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2(11):1093–103. [PubMed] [Google Scholar]
- 61.Noessner G, Kutscher B, Engel J, Schumacher W, Stekar J, Hilgard P. US6172050. Preparation of new phospholipid derivatives (phosphates containing heterocycle moieties) as drugs. 1994
- 62.Catley L, Hideshima T, Chauhan D, Neri P, Tassone P, Bronson R, et al. Alkyl phospholipid perifosine induces myeloid hyperplasia in a murine myeloma model. Exp Hematol. 2007;35(7):1038–46. doi: 10.1016/j.exphem.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Hideshima T, Catley L, Raje N, Chauhan D, Podar K, Mitsiades C, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138(6):783–91. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 64.Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I, et al. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia. 2008;22(6):1106–16. doi: 10.1038/leu.2008.79. [DOI] [PubMed] [Google Scholar]
- 65.Elrod HA, Lin Y-D, Yue P, Wang X, Lonial S, Khuri FR, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6(7):2029–38. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- 66.De la Pena L, Burgan WE, Carter DJ, Hollingshead MG, Satyamitra M, Camphausen K, et al. Inhibition of Akt by the alkylphospholipid perifosine does not enhance the radiosensitivity of human glioma cells. Mol Cancer Ther. 2006;5(6):1504–10. doi: 10.1158/1535-7163.MCT-06-0091. [DOI] [PubMed] [Google Scholar]
- 67.Leleu X, Jia X, Runnels J, Ngo HT, Moreau A-S, Farag M, et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110(13):4417–26. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moses S, Ali MA, Zuohe S, Du-Cuny L, Zhou LL, Lemos R, et al. In vitro and in vivo activity of a novel small molecule inhibitor targeting the pleckstrin homology domain of protein kinase B/Akt. Cancer Res. 2009;69:5073–81. doi: 10.1158/0008-5472.CAN-08-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meuillet EJ, Zuohe S, Lemos R, Ihle N, Kingston J, Watkins R, et al. Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 Pleckstrin homology domain inhibitor. Mol Cancer Ther. 2010;9(3):706–17. doi: 10.1158/1535-7163.MCT-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: DUAL mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279(35):36608–15. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 71.Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, et al. Discovery of a novel class of Akt pleckstrin homology domain inhibitors. Mol Cancer Ther. 2008;7(9):2621–32. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du-Cuny L, Song Z, Moses S, Powis G, Mash EA, Meuillet EJ, et al. Computational modeling of novel inhibitors targeting the Akt pleckstrin homology domain. Bioorg Med Chem. 2009;17(19):6983–92. doi: 10.1016/j.bmc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, van Aalten DMF. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem J. 2003;375(3):531–8. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnett SF, Defeo-Jones D, Haskell KM, Huber HE, Nahas DD. WO02083064. A method of treating cancer using a selective inhibitor of serine/threonine protein kinase Akt. 2002
- 75.Barnett SF, Graham SL, Remy DC. WO02083138. Preparation of 2,3-diphenylquinoxalines as inhibitors of Akt/PKB (protein kinase B) activity for the treatment of cancer. 2002
- 76.Carling WR, Castro PJL, Moore KW. WO02083675. Preparation of triazolo[4,3-b]pyridazines as inhibitors of Akt, a serine/threonine protein kinase. 2002
- 77.Barnett SF, Owens AP. WO02083140. Preparation of triazolo[3,4-a]phthalazines as inhibitors of Akt/PKB (protein kinase B) activity for the treatment of cancer. 2002
- 78.Barnett SF, Defeo-Jones DD, Hartman GD, Huber HE, Stirdivant SM, Heimbrook DC. US20040102360. Preparation of quinazolines and analogs as Akt inhibitors and indoles as protein kinase inhibitors for use in synergistic combination therapy for the treatment of cancer. 2004
- 79.Wu Z, Robinson RG, Fu S, Barnett SF, Defeo-Jones D, Jones RE, et al. Rapid assembly of diverse and potent allosteric Akt inhibitors. Bioorg Med Chem Lett. 2008;18(6):2211–4. doi: 10.1016/j.bmcl.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 80.Graham SL, Remy DC, Barnett SF, David C. WO02083138. Inhibitors of Akt activity. 2002
- 81.Chen C, Eastman BW, Hu EH. US7705014 B2. Inhibitors of Akt activity. 2010
- 82.Bilodeau MT, Chua PC, Cosford NDP, Hoffman JM, Nagasawa JY. US20100075970 A1. Inhibitors of Akt activity. 2010
- 83.Bilodeau MT, Chua PC, Cosford NDP, Hoffman JM, Nagasawa JY. US7655649. Inhibitors of Akt activity. 2010
- 84.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385(2):399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, et al. Discovery of 2,3,5-trisubstituted pyridine derivatives as potent Akt1 and Akt2 dual inhibitors. Bioorg Med Chem Lett. 2005;15(4):905–9. doi: 10.1016/j.bmcl.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 86.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4(2):271–9. [PubMed] [Google Scholar]
- 87.Bilodeau MT, Chen C, Cosford NDP, Eastman BW, Hartnett JC, Hu EH, Manley PJ, Neilson LA, Tehrani LR, Wu Z. US7750151. Inhibitors of Akt activity. 2010
- 88.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, et al. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15(3):761–4. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 89.Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 2000;19(5):979–88. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Q. Recent progress in the discovery of Akt inhibitors as anticancer agents. Expert Opin Ther Pat. 2007;17(9):1077–130. [Google Scholar]
- 91.Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;2001(108):re17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- 92.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, et al. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9(6):1227–40. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 93.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541–51. [PMC free article] [PubMed] [Google Scholar]
- 94.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325(5944):1134–8. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lakshmanan M, Bughani U, Duraisamy S, Diwan M, Dastidar S, Ray A. Molecular targeting of E3 ligases-a therapeutic approach for cancer. Expert Opin Ther Targets. 2008;12(7):855–70. doi: 10.1517/14728222.12.7.855. [DOI] [PubMed] [Google Scholar]
- 96.Restuccia DF, Hemmings BA. Cell signaling. Blocking Akt-ivity Science. 2009;325(5944):1083–4. doi: 10.1126/science.1179972. [DOI] [PubMed] [Google Scholar]
- 97.Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, et al. Peptide and protein library screening defines optimal substrate motifs for Akt/PKB. J Biol Chem. 2000;275(46):36108–15. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- 98.Luo Y, Smith RA, Guan R, Liu XS, Klinghofer V, Shen JW, et al. Pseudosubstrate peptides inhibit Akt and induce cell growth inhibition. Biochemistry. 2004;43(5):1254–63. doi: 10.1021/bi034515p. [DOI] [PubMed] [Google Scholar]
- 99.Luo Y, Giranda VL, Richardson PL, Smith RA. US20030181366. Peptide inhibitors of Akt and uses thereof. 2003
- 100.Luo Y, Giranda VL, Richardson PL, Smith RA. US20040038883. Peptide inhibitors of Akt and uses thereof. 2004
- 101.Noguchi M, Okada F, Hiromura S. WO2005056594. Akt activity specifically inhibiting polypeptide. 2005
- 102.Kunstle G, Laine J, Pierron G, Kagami S, Nakajima H, Hoh F, et al. Identification of Akt association and oligomerization domains of the Akt kinase coactivator TCL1. Mol Cell Biol. 2002;22(5):1513–25. doi: 10.1128/mcb.22.5.1513-1525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiromura M, Okada F, Obata T, Auguin D, Shibata T, Roumestand C, et al. Inhibition of Akt kinase activity by a peptide spanning the beta A strand of the proto-oncogene TCL1. J Biol Chem. 2004;279(51):53407–18. doi: 10.1074/jbc.M403775200. [DOI] [PubMed] [Google Scholar]
- 104.Kayser-Bricker KJ, Glenn MP, Lee SH, Sebti SM, Cheng JQ, Hamilton AD. Non-peptidic substrate-mimetic inhibitors of Akt as potential anti-cancer agents. Bioorg Med Chem. 2009;17(4):1764–71. doi: 10.1016/j.bmc.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sebti SM, Cheng JQ, Hamilton AD, Kayser K. WO2008070823. Substrate-mimetic Akt inhibitor. 2008
- 106.Kayser KJ, Glenn MP, Sebti SM, Cheng JQ, Hamilton AD. Modifications of the GSK3 beta substrate sequence to produce substrate-mimetic inhibitors of Akt as potential anti-cancer therapeutics. Bioorg Med Chem Lett. 2007;17(7):2068–73. doi: 10.1016/j.bmcl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 107.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24(50):7482–92. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 108.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22(21):3205–12. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 109.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31(Pt 6):1203–8. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 110.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. Akt-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Linbeck L, Lindenberg M, Nemunaitis JJ, Senzer N, Shanahan D. WO2007106892 A2. Combined targeted therapy for the treatment of proliferative disease. 2007
- 112.She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9(12):4340–6. [PubMed] [Google Scholar]
- 113.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 114.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–35. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 115.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65(8):3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 116.Scheuring U, Bernard I. US20090192127 A1. Combination therapy comprising a diaryl urea compound and a PI3, Akt kinase or MTOR inhibitors (Rapamycins) for cancer treatment. 2009
- 117.Boyer S, Dumas J, Riedl B, Wilhelm S. US20050038080. Fluoro substituted omega-caboxyaryl diphenyl urea for the treatment and prevention of diseases and conditions. 2005
- 118.Garcia-Echeverria C, Maria SM. WO2010049481. Combination of a phosphatidylinositol-3-kinase (PI3K) inhibitor and a mTOR inhibitor. 2010
- 119.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27(41):5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 120.Balligand JC, Feron O. US20090226427. Novel pharmaceutical compositions for modulating angiogenesis. 2009
- 121.Mohapatra S, Pledger WJ. WO2007117653. CDK9-PI3K-Akt Inhibitors for cancer treatment. 2007