Abstract
Background
Several studies have shown that use of medications to treat chronic conditions is highly sensitive to out-of-pocket price and influenced by changes in insurance coverage. Because antibiotics target infections and are used for a short period, one may expect antibiotic use to be less responsive to price. However, no studies have evaluated how antibiotic use changes with drug coverage. We evaluate changes in ambulatory oral antibiotic use following implementation of the Medicare drug benefit (Part D).
Methods
We conducted a pre-post-intervention-with-a-comparison-group analysis using insurance claims data from a large Medicare Advantage plan two years before and after Part D (2004–2007). Outcomes included likelihood of using any oral antibiotics and major subclasses among 35,102 older adults, and rates of antibiotics use among those with pneumonia and other acute respiratory infections (ARI).
Results
Overall antibiotic use increased most among those who did not previously have drug coverage (relative odds ratio 1.58; 95% CI 1.36–1.85). Use of broad-spectrum antibiotics—quinolones (1.70; 95% CI 1.35–2.15) and macrolides (1.59; 95% CI 1.26–2.01)—increased more than other subclasses, especially for those with prior drug coverage. Rates of ambulatory antibiotic use associated with pneumonia increased (3.60; 95% CI 2.35–5.53), more than those associated with other ARI visits (2.29; 95% CI 1.85–2.83)
Conclusions
Antibiotic use increased among older adults whose drug coverage improved post Part D with the largest increases for broad-spectrum, newer and more expensive antibiotics. Our study suggests reimbursement may play a role in addressing inappropriate antibiotic use.
Introduction
Overuse of antibiotics is a common and important problem, potentially leading to unnecessary prescription drug spending, increased risks of side effects with no associated benefit and the development of antimicrobial resistance.1, 2 Multiple programs have aimed to reduce inappropriate antibiotic use in inpatient and ambulatory care settings.3, 4 Although many of these interventions have helped curb antibiotic prescribing for acute respiratory infections and other conditions,5 there may still be substantial room for additional reductions. While quality improvement programs have traditionally focused on altering prescriber behavior through education and/or audit and feedback, interventions are increasingly including a patient education component, because researchers have found that patient expectation of and demand for antibiotic prescriptions affect physicians’ prescribing behavior.6, 7
An important moderator of patient demand for prescription drugs is out-of-pocket cost. Numerous studies have shown that pharmacy copayment increases are followed by reductions in the likelihood of use, and refill adherence.8 Likewise, when drug coverage becomes more generous patients fill more prescriptions9–11 with resultant increases in both appropriate and inappropriate use. However, nearly all studies of the impact of prescription drug coverage on utilization have focused on either medication use overall or those used to treat chronic conditions (e.g., antidepressants, cholesterol lowering therapies, and anti-hypertensives).
If antibiotic prescription were appropriate, one might expect antibiotic utilization to be somewhat less sensitive to out-of-pocket price changes because antibiotics are for short term use and to treat specific infections which could worsen fairly rapidly without adequate antimicrobial treatment. In other words, price should not affect the incidence of infections, and the consequences of failing to treat will manifest within a short time frame.
We use the 2006 implementation of the new Medicare drug benefit (Part D) as a natural experiment to study how changes in drug coverage affect utilization of antibiotics. Studies indicate that, on average, Part D increased drug use 6–74 percent depending on level of prior coverage and reduced out-of-pocket spending 13–23 percent.9, 11 To our knowledge, no studies have evaluated how use of antibiotics changed with Part D’ implementation and very few have examined the impact of patient financial incentives on use of antibiotics.12, 13
In this study, we evaluate the impact of Part D on the likelihood of any oral antibiotic use as well as major subclasses to determine, for example, whether use of newer, more expensive, and broader-spectrum macrolides might be more responsive to changes in coverage than older, less expensive, and narrower-spectrum penicillins. In addition, we examine whether changes in oral antibiotic use differ for pneumonia, a condition that could be life threatening for which antibiotics are often though not always indicated, versus other acute respiratory infections, conditions for which overuse is more common.14, 15
Methods
Study design
We used the implementation of Medicare Part D as a natural experiment to compare changes in antibiotic use for four groups of elderly beneficiaries who were continuously enrolled in Medicare-Advantage plans offered by a large Pennsylvania insurance company. Our study period was January 01, 2004 to December 31, 2007, two years before and after the January 2006 implementation of Part D. We had three intervention groups who had no or limited drug coverage before the implementation of Part D whose coverage improved in January 2006 because they enrolled in the Part D products of the same Medicare-Advantage plan. One intervention group had no coverage prior to Part D (no-coverage group), the other two groups had an $8 or $20 copayment before their total pharmacy spending reached quarterly caps on plan payment for drugs of $150 or $350 ($150-cap or $350-cap group). The level of drug coverage pre-Part D in the latter two groups depended on members’ county of residence (i.e., the insurer only offered either $150-cap or $350-cap in one county). This mitigates the selection bias.
In January 2006, all members in the intervention groups switched to Part D products. The standard Part D benefit includes a $250 deductible, a 25% coinsurance before drug spending reaches $2,250, a coverage gap for drug spending between $2,250 and $5,100, and a 5% coinsurance in the catastrophic coverage period for drug spending over $5,100 or out-of-pocket drug spending over $3,600 (2006 figures). However, Part D plans are permitted to offer a benefit actuarially equivalent to the standard benefit or better; and most plans including the study plan modified the benefit by eliminating the deductible and substituting copayments for co-insurance. Thus, beneficiaries in our three intervention groups did not have a deductible; faced tiered copayments ($8/$20 generic/brand) rather than 25% coinsurance; and could, for an additional premium, add coverage of generic drugs in the coverage gap (70% in our sample chose the plan that covered generics vs. 63% at the national level).16 The Part D plans that members chose were comparable across the three intervention groups.
Our comparison group consisted of enrollees who had stable drug coverage, without quarterly caps, through their former employers throughout four years (no-cap group). Because the comparison group’s coverage depended on decisions by their former employers to offer supplementary drug coverage, and few people decline this coverage because it is normally more generous, we believe selection bias is minimal.
All enrollees in the four groups obtained their non-drug medical coverage from the same Medicare Advantage plans and this coverage did not change over the four-year study period.
Study cohorts
The first study cohort consisted of a random sample of 36,858 members who were continuously enrolled in the Medicare Advantage plans from January 2004 through December 2007. We excluded 1,756 members who were under the age of 65 years in 2004 and enrolled in Medicare because of a disability. This is our continuously-enrolled overall study cohort.
We then constructed two condition-specific sub-cohorts. For each 2-year study period, pre- and post-Part D, we identified individuals who had outpatient visits or inpatient hospital admissions for pneumonia or other acute respiratory tract infections. Individuals were identified as having pneumonia if they had outpatient visits or inpatient admissions with ICD-9 codes 481, 482, 483, 485, 486. For other acute respiratory tract infections (ARI) we identified visits or admissions for which the primary diagnosis carried International Classification of Diseases Version 9 (ICD-9) codes for sinusitis (ICD-9 461, 473), pharyngitis (ICD-9 034, 462, 463), bronchitis (ICD-9 466, 490), or nonspecific upper respiratory tract infection (ICD-9 460, 465). Any return office visits occurring within 28 days after the first diagnosis of pneumonia or ARI were defined as the same incident. This approach to identifying these diagnoses in administrative claims data has been shown to have a sensitivity of 68–79% and a specificity of 84–91% depending on the condition.17, 18
Outcomes
We calculated the proportion of members in the overall cohort who filled at least one oral antibiotic prescription at both quarterly and yearly levels between January 2004 and December 2007. We also calculated the proportion of using any antibiotics by major antibiotic drug subclass, including cephalosporins, penicillins, tetracyclines, quinolones, macrolides, sulfonamides, and others.
For the pneumonia and ARI sub-cohorts, we constructed a measure of antibiotic use based on whether the individual filled an antibiotic prescription within 2 days (before or after) of the incident visit or admission. This method for linking outpatient pharmacy claims to incident diagnoses of pneumonia and ARIs in medical claims has high level of sensitivity and specificity.17, 18 We then calculated the rate of outpatient antibiotic prescriptions filled conditional on having a diagnosis of pneumonia or other ARIs, for pre- and post-Part D periods.
We also conducted a sensitivity analysis relaxing the number of days between diagnosis and prescription fill from 2 to 5 days, as well as excluding those incident visits associated with an institutional stay (including acute care hospital or nursing home stay).
Statistical analysis
Although selection bias was small, we used propensity score weighting to enhance comparability between each intervention group and the comparison group.9, 19, 20 Propensity score weights were calculated in two steps. First, we calculated the probability of being in each intervention group versus the comparison group using three logistic regressions (one for each intervention group) and adjusting for zip-code level income, race, poverty rate, urban/suburb, and individual-level variables such as age, sex, and health status measured by annual prospective risk scores during the baseline years (2004 and 2005). The risk scores were calculated using risk-grouper software involving a series of proprietary algorithms (D×CG) based on ICD-9 diagnoses or Healthcare Common Procedure Coding System codes reported on claims.21 Higher risk scores indicate worse health status and greater expected future medical care spending.
Second, the estimated propensity scores were assigned to each individual that was proportional to the estimated probability of the enrollee’s assignment to the other group in the pairwise comparisons. That is, enrollees in each intervention group who had characteristics similar to those of enrollees in the comparison group were given higher weights.
We then calculated weighted averages of the proportion of members in each study group who used any antibiotics and plotted changes over time for each intervention group and the comparison group.
We used random effects logistic regression models to estimate the changes in outcomes between 2 years before and 2 years after the implementation of Medicare Part D. Random effects models estimate subject-specific changes and pre and post Part D changes. We applied propensity scores weights in the logistic models to balance study groups. In particular, logistic regression estimated the effects on the likelihood of using any antibiotics as well as each major subclass, including cephalosporins, penicillins, tetracyclines, quinolones, macrolides, sulfonamides, and others. Random effects logistic regressions were also used to estimate changes in the likelihood of ambulatory antibiotic prescriptions among those with diagnoses of pneumonia or ARI before and after Part D between each intervention and the comparison group. All reported P values are two-sided. We used SAS 9.2 and Stata 10 for estimation.
Results
Study population
Characteristics of the study population are reported in Table 1. The comparison group was slightly more likely to be male and younger than the intervention groups. The $150-cap group was more likely to live in suburban areas and zip codes with a higher proportion of whites. This is consistent with the fact that the level of the quarterly caps ($350 and $150) depended on the county of residence. The group with no prior drug coverage was slightly less likely to have diagnoses of hypertension or diabetes than the comparison group whereas the comparison group was more likely to have a diagnosis of hyperlipidemia than the intervention groups. Importantly, there were no statistically significant differences among the four groups in prospective risk scores, our measure of overall status and predictor of health service use, even before propensity-score weighting.
Table 1.
Characteristics of the Study Population in 2005†
| Intervention Groups | Comparison Group |
|||
|---|---|---|---|---|
| No-Coverage | $150-Cap | $350-Cap | No Cap | |
| N(%) | 3,939(11) | 2,662(8) | 19,014(54) | 9,487(27) |
| Female (%) | 55.4 | 62.3 | 62.1 | 52.5* |
| Age (%) | ||||
| 65<=age<=74 | 47.4 | 49.7 | 52.5 | 60.7* |
| 75<=age<=84 | 44.3 | 40.6 | 39.3 | 34.1* |
| age>=85 | 8.3 | 9.7 | 8.2 | 5.3* |
| Whites (%)‡ | 93.0 | 96.0* | 91.9 | 92.1 |
| African Americans (%) ‡ | 5.2 | 2.4* | 6.0 | 5.7 |
| Below poverty line (%) ‡ | ||||
| <100% | 10.6 | 11.1 | 10.1 | 9.9 |
| between 100% and 200% | 18.3 | 20.9* | 17.2 | 16.9 |
| >200% | 71.2 | 68.0* | 72.7 | 73.2 |
| Urban (%) ‡ | 73.7* | 57.7* | 79.1 | 79.7 |
| Diagnosed chronic conditions (%) | ||||
| Hypertension | 54.5* | 62.8 | 62.6 | 61.2 |
| Hyperlipidemia | 48.2 | 55.7 | 57 | 60.4* |
| Diabetes | 19.6* | 22.5 | 22.3 | 23.3 |
| Prospective Risk Scores§ | ||||
| 2004 | 0.83±0.011 | 0.85±0.014 | 0.86±0.005 | 0.84±0.008 |
| 2005 | 0.92±0.012 | 0.95±0.016 | 0.94±0.006 | 0.92±0.009 |
| 2006 | 1.03±0.015 | 1.04±0.017 | 1.04±0.007 | 1.03±0.010 |
| 2007 | 1.15±0.017 | 1.19±0.020 | 1.18±0.008 | 1.14±0.011 |
| Use of medical services in 2005 | ||||
| Emergency room visits (%) | 26.8* | 24.1 | 25.9 | 24.4 |
| Hospitalizations (%) | 18.5 | 16.8 | 18.3 | 17.1 |
| Mean outpatient visits | 23±0* | 25±1 | 25±0 | 26±0 |
| Mean outpatient costs | 3498±93 | 3533±124 | 3741±47 | 3869±66 |
| Mean medical costs | 6000±187 | 5838±227* | 6209±88 | 6267±130 |
Note:
These numbers are unweighted raw data. Plus-minus values are means±standard errors.
p<0.05. If * is indicated for the comparison group, it means the variable is statistically significant difference between each intervention group and the comparison group. If * is indicated for an intervention group, it means for that particular intervention group compared with the comparison group. We used chi-square tests for categorical variables and one-way analysis of variance (ANOVA) test for continuous variables. Some percentages do not sum up to one because of rounding effects.
These numbers are at zip-code level.
Prospective risk scores were calculated with the use of an algorithm that is described in the text, with higher scores indicating greater expected future medical spending.
Effects on likelihood of use of any antibiotics and each subclass of antibiotics
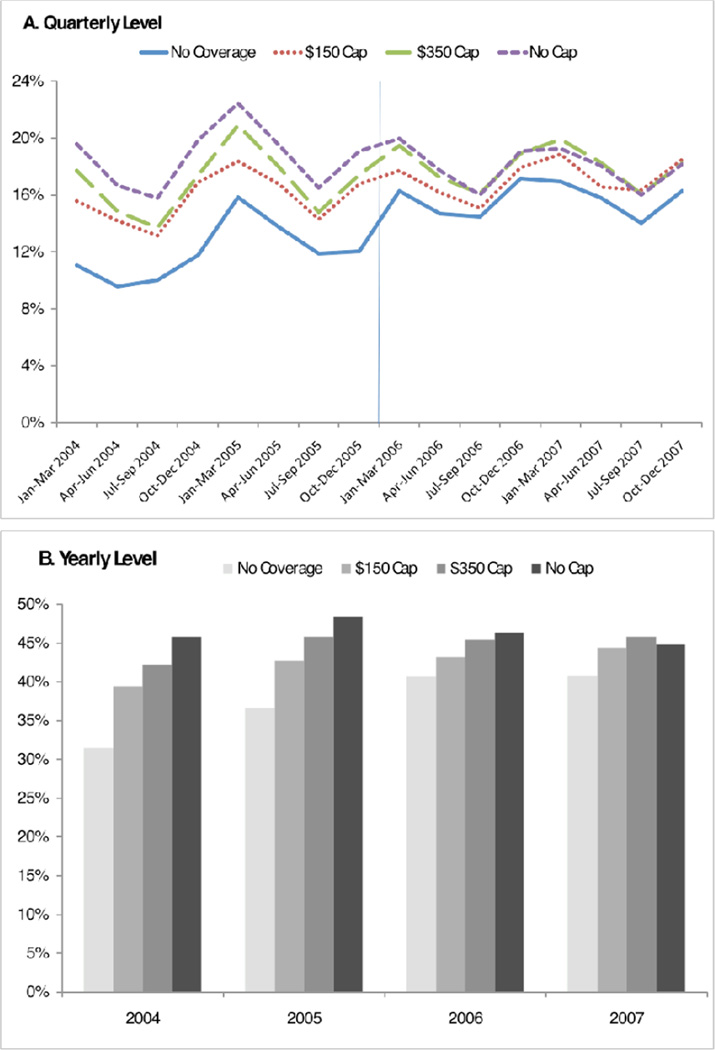
Figure 1 shows the proportion of the overall study population that ever filled any prescription for any antibiotics, by quarter (Figure 1 Panel A) and year (Figure 1 Panel B), between January 2004 and December 2007. There were clear seasonal trends in antibiotic use with the highest use occurring in each year's first quarter and lowest use in each year's third quarter (p<0.05). One can also observe a slight declining trend in use of antibiotics over four-year study period.
Figure 1.
Proportion of Study Population Filled at least One Antibiotic Prescription
Table 2 presents numerical results. Between December 2005 and December 2007, relative to the comparison group, the likelihood of use of any antibiotics increased by 1.58 (relative odds ratios, 95% confidence interval [CI], 1.36 to 1.85) among enrollees who transitioned from no drug coverage to Part D. Relative odds of use of any antibiotics in a year increased slightly in the $150-cap group and $350-cap group, by 1.27 (95% CI 1.06–1.53) and 1.19 (95% CI 1.10–1.30), respectively.
Table 2.
Effects of Part D on Likelihood of Use of Antibiotics
| Study Groups |
Pre 2 yr (%)* |
Post 2 yr (%)* |
Relative Odds Ratios† |
95% CI | ||
|---|---|---|---|---|---|---|
| Any antibiotics | Intervention groups | No-coverage | 34.1 | 40.7 | 1.58 | (1.36, 1.85) |
| $150-cap | 41.1 | 43.8 | 1.27 | (1.06, 1.53) | ||
| $350-cap | 44.0 | 45.6 | 1.19 | (1.10, 1.30) | ||
| Comparison | No-cap | 47.2 | 45.5 | 1.00 | ref | |
| Cephalosporins | Intervention groups | No-coverage | 7.0 | 9.2 | 1.43 | (1.11, 1.84) |
| $150-cap | 9.5 | 10.5 | 1.16 | (0.88, 1.53) | ||
| $350-cap | 9.0 | 9.5 | 1.08 | (0.95, 1.24) | ||
| Comparison | No-cap | 10.2 | 10.0 | 1.00 | ref | |
| Penicillins | Intervention groups | No-coverage | 13.1 | 14.9 | 1.38 | (1.12, 1.71) |
| $150-cap | 16.2 | 16.1 | 1.12 | (0.88, 1.43) | ||
| $350-cap | 17.6 | 17.2 | 1.10 | (0.36, 3.32) | ||
| Comparison | No-cap | 19.5 | 18.1 | 1.00 | ref | |
| Tetracyclines | Intervention groups | No-coverage | 2.5 | 3.4 | 1.60 | (1.05, 2.42) |
| $150-cap | 3.8 | 4.1 | 1.20 | (0.77, 1.88) | ||
| $350-cap | 3.4 | 3.5 | 1.16 | (0.94, 1.44) | ||
| Comparison | No-cap | 4.2 | 3.8 | 1.00 | ref | |
| Quinolones | Intervention groups | No-coverage | 8.2 | 13.4 | 1.70 | (1.35, 2.15) |
| $150-cap | 10.0 | 14.6 | 1.48 | (1.14, 1.93) | ||
| $350-cap | 13.1 | 16.0 | 1.16 | (1.04, 1.31) | ||
| Comparison | No-cap | 13.6 | 15.0 | 1.00 | ref | |
| Macrolides | Intervention groups | No-coverage | 9.1 | 11.7 | 1.59 | (1.26, 2.01) |
| $150-cap | 12.5 | 13.0 | 1.20 | (1.00, 1.56) | ||
| $350-cap | 13.2 | 13.4 | 1.17 | (1.03, 1.31) | ||
| Comparison | No-cap | 14.4 | 13.3 | 1.00 | ref | |
| Sulfonamides | Intervention groups | No-coverage | 0.0 | 0.1 | 1.65 | (0.11, 23.77) |
| $150-cap | 0.1 | 0.1 | 0.67 | (0.03, 14.22) | ||
| $350-cap | 0.1 | 0.1 | 1.21 | (0.31, 4.74) | ||
| Comparison | No-cap | 0.2 | 0.2 | 1.00 | ref | |
| Others | Intervention groups | No-coverage | 4.7 | 6.6 | 1.37 | (1.00, 1.89) |
| $150-cap | 6.4 | 7.4 | 1.05 | (0.74, 1.50) | ||
| $350-cap | 5.9 | 7.1 | 1.12 | (0.95, 1.32) | ||
| Comparison | No-cap | 6.1 | 6.7 | 1.00 | ref | |
Note:
Pre and Post comparison are unadjusted raw numbers. These numbers are proportions of members ever filled an antibiotic prescription in the study period.
“Relative Odds Ratios” are adjusted difference-in-difference estimates from logistic regression random-effect regression models with propensity score weighting. The adjusted variables used in calculating propensity score include zip-code level of income, race, poverty rate, urban, and individual-level variables such as age categories, sex, and 2004 and 2005 risk scores.“Relative Odds Ratios” measure changes in outcomes pre-two-year and post-two-year Part D in each intervention group, relative to the changes in outcomes in the comparison group.
Relative to the comparison group, the no-coverage group was more likely to fill prescriptions for each of the pharmacologic subclasses we studied after Part D than before, with the exception of sulfonamides (Table 2). The $150 cap and $350 cap groups were more likely to fill prescriptions for broad spectrum antibiotics after Part D. For example, the relative odds ratios of filling prescriptions for quinolones were 1.48 (95% CI 1.14–1.93) and 1.16 (95% CI 1.04–1.31) for the $150- and $350- cap groups respectively. Relative odds ratios of filling prescriptions for macrolides were 1.20 (95%CI 1.00–1.56) for the $150 cap group and 1.17 (95% CI 1.03–1.31) for the $350 cap group.
Effects on rates of outpatient antibiotic prescriptions for pneumonia or other ARIs
Table 3 shows the rates of ambulatory antibiotic use for those diagnosed with pneumonia or other ARIs, pre- and post- Part D, as well as changes associated with Part D. There were 5,079 and 6,534 visits related to pneumonia in the pre- and post- two years, respectively; of which 670 and 715 were institutional visits. There were 10,246 and 10,390 visits related to other ARIs in the pre- and post- two years, respectively; of which 155 and 182 were institutional visits. The rates of outpatient antibiotic prescriptions for those visits for which a pneumonia or ARI diagnosis was recorded declined in the no-cap comparison group over time. However, rates increased among members switching to more generous Part D drug coverage from either no or limited (only $150-cap) drug coverage. The increase in rates of antibiotic use was larger for pneumonia-related visits than the increase for other ARI-related visits. After controlling for the declining trend in antibiotic use in general in the comparison group, the proportion of members in the no-coverage group with visits for pneumonia who filled outpatient antibiotics prescriptions more than doubled with relative odds ratio of 3.60 (95% CI 2.35–5.53). The relative odds ratio in the $150-cap group was 1.64 (95% CI 1.01–2.67).
Table 3.
Rates of Outpatient Antibiotic Prescriptions Filled Among Those with Visits for Pneumonia or Acute Respiratory Tract Infections
| Study Groups |
Pre 2 yr (%)* |
Post 2 yr (%)* |
Relative Odds Ratios† |
95% CI | ||
|---|---|---|---|---|---|---|
| Pneumonia | Intervention groups | No-coverage | 28.4 | 46.8 | 3.60 | (2.35, 5.53) |
| $150-cap | 44.4 | 48.7 | 1.64 | (1.01, 2.67) | ||
| $350-cap | 42.2 | 40.0 | 1.27 | (0.98, 1.65) | ||
| Comparison | No-cap | 47.6 | 40.2 | 1.00 | ref | |
| Pneumonia (institutional stays excluded) | Intervention groups | No-coverage | 28.2 | 44.0 | 3.07 | (1.94, 4.85) |
| $150-cap | 42.4 | 49.8 | 1.97 | (1.17, 3.32) | ||
| $350-cap | 40.9 | 38.9 | 1.29 | (0.97, 1.70) | ||
| Comparison | No-cap | 46.6 | 38.8 | 1.00 | ref | |
| Acute Respiratory Tract Infections (ARI) | Intervention groups | No-coverage | 45.1 | 59.5 | 2.29 | (1.85, 2.83) |
| $150-cap | 57.8 | 62.6 | 1.40 | (1.10, 1.79) | ||
| $350-cap | 59.5 | 62.5 | 1.36 | (1.18, 1.57) | ||
| Comparison | No-cap | 64.9 | 62.0 | 1.00 | ref | |
| ARI (institutional stays excluded) | Intervention groups | No-coverage | 45.2 | 59.7 | 2.32 | (1.87, 2.87) |
| $150-cap | 57.7 | 62.7 | 1.42 | (1.11, 1.82) | ||
| $350-cap | 59.7 | 62.6 | 1.38 | (1.19, 1.59) | ||
| Comparison | No-cap | 65.3 | 62.2 | 1.00 | ref | |
Note:
Pre and Post comparison are unadjusted raw numbers. These numbers are proportions of members ever filled an antibiotic prescription in the study period.
“Relative Odds Ratios” are adjusted difference-in-difference estimates from logistic regression random-effect regression models with propensity score weighting. The adjusted variables used in calculating propensity score include zip-code level of income, race, poverty rate, urban, and individual-level variables such as age categories, sex, and 2004 and 2005 risk scores. “Relative Odds Ratios” measure changes in outcomes pre-two-year and post-two-year Part D in each intervention group, relative to the changes in outcomes in the comparison group.
All three intervention groups with other ARI visits increased rates of antibiotics filled at a smaller magnitude of change compared with those with pneumonia visits. The no-coverage group increased the highest at relative odds ratio of 2.29 (95% 1.85–2.83), and relative odds ratios were 1.40 (95% 1.10–1.79) for the $150-cap group and 1.36 (95% CI 1.18–1.57) for the $350-cap group.
The sensitivity analysis relaxing the number of days between diagnosis and prescription fill to 5 days did not change the rates of antibiotics use associated with two conditions (results not shown). Results after excluding institutional stays were quantitatively similar (Table 3).
Discussion
We found that utilization of antibiotics increased in response to reductions in out-of-pocket price post Part D. However, it is difficult to discern whether these increases represent appropriate use, inappropriate use or some combination of both, because we cannot accurately assess the quality of antibiotic prescribing using insurance claims data. For pneumonia, we found Part D was associated with a triple increase in rates of antibiotic treatment among those previously lacking drug coverage, with a relative odds ratio of 3.60 (95% CI 2.35–5.53), after adjusting for secular trends in the comparison group. Given the high mortality associated with community-acquired pneumonia among the elderly,22 the finding that changes in drug coverage improve the likelihood of treatment is encouraging.
However, we also found increases in antibiotic use for other acute respiratory infections (sinusitis, pharyngitis, bronchitis, and nonspecific upper respiratory tract infection) for which antibiotics are generally not indicated.18 We found rates of antibiotic use for ARIs declined between 2004–2005 and 2006–2007 for the group whose drug coverage did not change. In contrast, the three groups who moved from limited or no drug coverage to Part D increased their use of antibiotics for ARIs. The magnitude of these increases was smaller than that for pneumonia. Inappropriate use of antibiotics has contributed to the development of antibiotic-resistant bacteria and has as a result been the target of numerous interventions to reduce use.3 Our findings suggest that changes in drug coverage among the elderly may exacerbate problems with antibiotic overuse.
Increased antibiotic prescription fill rates may be explained by a change in patient behavior, physician behavior or by some combination. Patients with generous drug coverage may be more likely to request an antibiotic prescription and more likely to fill it. Likewise, surveys suggest physicians’ decisions about whether and what to prescribe may be influenced by their perceptions about their patient’s ability to pay for drugs although communication between older adults and physicians about drug cost burden is known to be inadequate.23, 24 Systematic reviews suggest that more complex, multifaceted interventions are more effective at reducing inappropriate antibiotic prescribing.3, 4 Our findings suggest that health systems may consider changes to patient cost-sharing as another potential lever to alter patient and provider behavior.
We also found different responses to changes in drug coverage across antibiotic subclasses. While the group transitioning from no or limited coverage to Part D increased use of nearly all antibiotics (with the exception of sulfonamides), they increased use of broad spectrum antibiotics (e.g., macrolides and quinolones), more so than other older, cheaper subclasses. We expected a larger effect of insurance coverage changes for these subclasses which can cost up to $30 per day compared to less than $1 per day of treatment for older classes such as penicillin (authors’ calculation based on average costs in our study sample). This might be a concern because broad-spectrum antibiotics generally are more likely to lead to bacterial resistance.25
Our study is subject to certain limitations. First, because the individuals we studied were all enrolled in MA-PD plans offered from one insurance company, the results might not generalize to all older adults. Second, our results are based on drugs purchased at network pharmacies, but any bias from missing claims is likely negligible for several reasons. For the time period in which beneficiaries paid entirely out-of-pocket those using a network pharmacy received a 15% discount from the plan’s negotiated prices which were already well below a retail price. In addition, network pharmacies were numerous and covered almost all local pharmacies. Third, we could not distinguish between bacterial and viral pneumonia using claims data. Antibiotics are not indicated for viral pneumonia. However, our pre-post-with-comparison-group approach should control for temporal trends in viral and/or bacterial pneumonia that affect study groups and we do not expect differences across study groups in the underlying causes of pneumonia. Similarly, we are not able to determine from claims data whether or not the use of broad spectrum antibiotics is appropriate.
In sum, use of antibiotics increased as individuals gained better drug coverage, especially for broad-spectrum, newer and more expensive, antibiotics. We found increases in the likelihood of antibiotic treatment for both pneumonia and other ARIs. These increases took place against a backdrop of declines in antibiotics overall nationally.5 Our study suggests that reimbursement may play a role in addressing the substantial role of inappropriate antibiotic prescribing and use.
Acknowledgments
This publication was supported by the National Center for Research Resources, a component of the National Institutes of Health (NIH), NIH Roadmap for Medical Research (Grant no.KL2-RR024154-04 to Donohue), the University of Pittsburgh’s Graduate School of Public Health Computational and Systems Models in Public Health Pilot Program (Zhang), the NIH Models of Infectious Agent Study (MIDAS) grant 1U54GM088491-0109 (Lee), and the Pennsylvania Department of Health Center of Excellence in Prevention and Control of Antibiotic-resistant Bacterial Infections (Lee).
The University of Pittsburgh Institutional Review Board approved this study. The study design and analysis were done by the authors.
Footnotes
This manuscript was presented at the 7th World Congress meeting of the International Health Economics Association (iHEA) on July 13 2009 in Beijing China.
Disclosure: Dr. Zhang is the Co-Principal Investigator for a project in part funded by Highmark Inc (a Medicare-Advantage plan) to evaluate the economic impact of high-deductible health plan on medical care spending.
References
- 1.Arason VA, Sigurdsson JA, Erlendsdottir H, Gudmundsson S, Kristinsson KG. The role of antimicrobial use in the epidemiology of resistant pneumococci: A 10-year follow up. Microb Drug Resist. 2006;12(3):169–176. doi: 10.1089/mdr.2006.12.169. [DOI] [PubMed] [Google Scholar]
- 2.Bergman M, Huikko S, Huovinen P, Paakkari P, Seppala H. Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50(11):3646–3650. doi: 10.1128/AAC.00234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranji SR, Steinman MA, Shojania KG, Gonzales R. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care. 2008;46(8):847–862. doi: 10.1097/MLR.0b013e318178eabd. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005;4:CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients' expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315(7117):1211–1214. doi: 10.1136/bmj.315.7117.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kravitz RL, Bell RA, Azari R, Kelly-Reif S, Krupat E, Thom DH. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163(14):1673–1681. doi: 10.1001/archinte.163.14.1673. [DOI] [PubMed] [Google Scholar]
- 8.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003;349(23):2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Donohue JM, Lave JR, O’Donnell G, Newhouse JP. The impact of the Medicare Part D drug benefits on pharmacy and medical care spending. N Engl J Med. 2009;361(1):52–61. doi: 10.1056/NEJMsa0807998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Lave JR, Newhouse JP, Donohue JM. How the Medicare Part D Drug Benefit Changed the Distribution of Out-of-Pocket Pharmacy Spending among Older Beneficiaries. J Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbp111. doi: 10.1093/geronb/gbp111(Advance Access published online on December 14, 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin W, Basu A, Zhang JX, Rabbani A, Meltzer DO, Alexander GC. The effect of the Medicare Part D prescription benefit on drug utilization and expenditures. Ann Intern Med. 2008;148(3):169–177. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]
- 12.Flottorp S, Oxman AD, Havelsrud K, Treweek S, Herrin J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002;325(7360):367–374. doi: 10.1136/bmj.325.7360.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxman B, Valdez RB, Lohr KN, Goldberg GA, Newhouse JP, Brook RH. The effect of cost sharing on the use of antibiotics in ambulatory care: results from a population-based randomized controlled trial. J Chronic Dis. 1987;40(5):429–437. doi: 10.1016/0021-9681(87)90176-7. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278(11):901–904. [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Medicare prescription drug benefit symposium: Part D data symposium fact sheet. Baltimore, MD, USA: 2008. [Google Scholar]
- 17.Maselli JH, Gonzales R. Measuring antibiotic prescribing practices among ambulatory physicians: accuracy of administrative claims data. J Clin Epidemiol. 2001;54(2):196–201. doi: 10.1016/s0895-4356(00)00269-9. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales R, Sauaia A, Corbett KK, et al. Antibiotic treatment of acute respiratory tract infections in the elderly: effect of a multidimensional educational intervention. J Am Geriatr Soc. 2004;52(1):39–45. doi: 10.1111/j.1532-5415.2004.52008.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: an application to data on right heart catheterization. Health Serv Outcome Res Meth. 2001;2(3):259–278. [Google Scholar]
- 20.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 21.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 22.National Heart Lung and Blood Institute. [accessed on November 8, 2009];Pneumonia. 2008
- 23.Alexander GC, Casalino LP, Meltzer DO. Physician strategies to reduce patients' out-ofpocket prescription costs. Arch Intern Med. 2005;165(6):633–636. doi: 10.1001/archinte.165.6.633. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IB, Schoen C, Neuman P, et al. Physician-patient communication about prescription medication nonadherence: a 50-state study of America's seniors. J Gen Intern Med. 2007;22(1):6–12. doi: 10.1007/s11606-006-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson LR. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect. 2005;11(Suppl 5):4–16. doi: 10.1111/j.1469-0691.2005.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]