Abstract
Introduction
Perampanel is a first-in-class antiepileptic drug approved for adjunctive treatment of partial-onset seizure in patients aged 12 years or older. Published randomised controlled trials (RCTs) had small sample sizes, and meta-analyses have included too few studies to draw conclusive results for the assessment of tolerability, efficacy and safety of perampanel. There is a need to conduct a meta-analysis with a larger dataset and an appropriate study design.
Objective
The aim of this study was to systematically review the efficacy and safety of perampanel in the treatment of partial-onset epilepsy.
Methods
Electronic and clinical trials databases were searched for RCTs of perampanel published up to March 2013. Outcomes of interest were 50 % responder rates, seizure freedom, treatment-emergent adverse events (TEAEs) and incidence of withdrawal. Meta-analysis was performed to investigate the outcomes of interest.
Results
Five RCTs with a total of 1,678 subjects were included. The 50 % responder rates were significantly greater in patients receiving 4, 8 and 12 mg perampanel versus placebo, with risk ratios of 1.54 (95 % CI 1.11–2.13), 1.80 (95 % CI 1.38–2.35) and 1.72 (95 % CI 1.17–2.52), respectively. There was no statistical evidence of a difference in seizure freedom between 8 or 12 mg perampanel and placebo. Of the five commonly reported TEAEs included, both dizziness and somnolence were statistically associated with 8 mg perampanel, whilst dizziness was statistically associated with 12 mg perampanel. Incidences of withdrawal due to adverse events were significantly higher in the 8 mg and 12 mg perampanel groups versus placebo.
Conclusion
The use of perampanel resulted in a statistically significant reduction of seizure frequency with respect to the 50 % responder rate in patients with partial-onset epilepsy. Perampanel is well tolerated at 4 mg and reasonably tolerated at 8 and 12 mg. Further clinical and pharmacovigilance studies are required to investigate the long-term efficacy and safety of perampanel in the management of other types of epilepsy.
Electronic supplementary material
The online version of this article (doi:10.1007/s40263-013-0091-9) contains supplementary material, which is available to authorized users.
Introduction
Epilepsy is a set of chronic neurological disorders involving a predisposition to generate seizures [1]. According to the WHO, approximately 50 million people in the world have epilepsy, and about 80 % of cases are found in developing countries [2]. Pharmacological treatment is the first-line intervention for partial-onset epilepsy. Antiepileptic drugs (AEDs) that aim to suppress seizure occurrence have been widely used in epilepsy treatment. Clinical guidelines have been issued for the management of epilepsy [3, 4]. These guidelines suggest that the use of AEDs should be personalised, and the choice of AEDs should be based on factors including the patient’s epilepsy syndrome, seizure type and lifestyle. A variety of AEDs have been designed to target different mechanisms involved in seizure development. Common mechanisms of AEDs include the blocking of sodium or calcium channels, activation of potassium channels, enhancement of gamma aminobutyric acid (GABA) activity and inhibition of excitatory amino acids [5].
Perampanel is a highly selective and non-competitive antagonist of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor [6]. AMPA receptors are found on the excitatory synapses in the central nervous system [7]. These receptors mediate fast synaptic signalling by the binding of glutamate, which is an excitatory neurotransmitter. Overexpression of AMPA receptors plays a crucial role in the forming and spreading of seizures. Therefore, as an AMPA receptor antagonist, perampanel produces an antiepileptic effect. The European Medicines Agency (EMA) and the US FDA approved perampanel under the trade name Fycompa® in July 2012 and October 2012, respectively [8]. It is a first-in-class AED approved for adjunctive treatment of partial-onset seizure in patients aged 12 years or older.
A meta-analysis study conducted by Gao et al. [9] investigated the efficacy and safety of six AEDs, including eslicarbazepine, retigabine (or ezogabine), carisbamate, lacosamide, brivaracetam and perampanel. Gao et al. assessed efficacy using the 50 % responder rate, and the odds ratio (OR) of perampanel compared with placebo was reported to be 1.79 (95 % CI 0.88–3.63; p = 0.11). Randomised controlled trials (RCTs) of perampanel included in the study by Gao et al. (labelled as study 206 and 208) were designed to investigate tolerability, and therefore only provided preliminary efficacy results [10]. The small sample sizes in the two studies may not be sufficient to obtain a conclusive result.
There is a need to conduct a meta-analysis with a larger dataset and an appropriate study design. We conducted a meta-analysis to combine evidence from currently available RCTs and further investigated the efficacy and safety of perampanel. Two dose-escalation phase II studies (labelled as studies 206 and 208) and three placebo-controlled phase III studies (labelled as studies 304, 305 and 306) were included in our analysis [10–13].
Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. The Cochrane Library [Cochrane Central Register of Controlled Trials (CENTRAL); Cochrane Database of Systematic Reviews; and Cochrane Epilepsy Group Register], EMBASE and PubMed electronic databases were used to perform the literature search. The following search terms were used: (perampanel) OR (Fycompa) OR (E2007). In order to identify relevant potential studies, the following trial registers were used: the WHO International Clinical Trials Registry Platform [ICTRP] (http://www.who.int/ictrp/en/), the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov) and the metaRegister of Controlled Trials [mRCT] (http://www.controlled-trials.com). The search was performed on 11 March 2013. Titles, abstracts and the content of the articles were screened to determine whether the articles met the inclusion criteria. The reference lists of the articles that met the inclusion criteria were also screened to identify potentially relevant studies. The searching workflow is shown in Fig. 1.
Fig. 1.
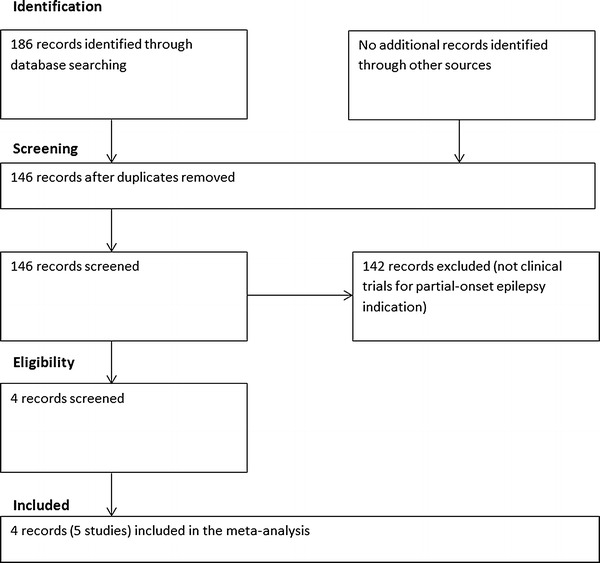
Review flowchart (PRISMA flowchart)
Inclusion Criteria
This meta-analysis includes published RCTs investigating the efficacy and safety of perampanel in patients (aged 12 years or older) diagnosed with partial-onset seizure with or without secondary generalisation. Partial-onset seizure was defined according to the 1981 International League Against Epilepsy Classification of Epileptic Seizures [15]. Since our meta-analysis aimed to investigate efficacy and safety of perampanel as adjunctive therapy, all the included studies targeted patients who were receiving one to three AEDs prior to study commencement. Conference proceedings were excluded. There were no restrictions on language. Full texts were evaluated for accessing the inclusion criteria.
Outcome Measures
The primary outcome for measuring efficacy was the 50 % responder rate, which is defined as the proportion of patients who had a ≥50 % reduction of seizure frequency in the maintenance period when comparing with baseline [16]. Another outcome was seizure freedom. The ‘pragmatic intent-to-treat (ITT)’ was used to define seizure freedom in this meta-analysis. Seizure freedom is defined as the proportion of seizure-free patients who completed treatment through the maintenance period in the ITT population [17].
Secondary outcomes were the number of patients who experienced treatment-emergent adverse events (TEAEs). A TEAE is an adverse event that occurred or became worse during the treatment period. An adverse event was defined as a TEAE if it arose within 30 days after the patient’s last treatment date [11]. TEAEs assessed in this meta-analysis included dizziness, fatigue, headache, somnolence and nasopharyngitis. These TEAEs were assessed as they were the most common TEAEs in the included studies. Worsening seizures were also investigated and are defined as a >50 % increase in seizure frequency during the maintenance period when compared with baseline. Another secondary outcome was the incidence of patient withdrawal from treatment.
Data Extraction
YH performed the initial searches and screened abstracts for eligibility. WQH and YH retrieved and screened full texts of potential articles. The relevant articles were assessed independently by both reviewers for inclusion in the meta-analysis.
Primary and secondary outcome data were extracted from all included studies by two independent reviewers, CWS and WQH. The extracted data were cross-checked by the two reviewers for data accuracy. Data on 50 % responder rates, seizure freedom, TEAEs and withdrawal were extracted from the eligible studies for the meta-analysis. Non-statistical data extracted included author, study location, study duration, perampanel dose, sample size, number/type of concomitant AEDs and seizure type at baseline.
Evaluation of Bias
The Cochrane Collaboration’s tool was used to assess the risk of bias of the identified RCT articles [18] (Online Resource Table 1). Assessment was conducted and cross-checked by two independent reviewers (CWS and WQH). Discrepancies were resolved by consensus.
Statistical Analysis
All the outcomes of interest (i.e. 50 % responder rates, seizure freedom, withdrawal from trials and TEAEs) were dichotomous. Risk ratios (RRs) were calculated for all the outcomes. DerSimonian and Laird random-effects model was used to account for heterogeneity between studies [19]. Studies 206 and 208 were designed to investigate tolerability and safety of perampanel, while the other included studies were designed to investigate efficacy and safety. It may not be appropriate to use the fixed-effect model which makes the assumption that the treatment effect in all the included studies are identical [20]. Therefore, the random-effects model was chosen. I 2 statistic was calculated to describe the proportion of the variability that was due to heterogeneity rather than sampling error. ITT data were used in the efficacy analysis, and the safety population data were used for the safety analysis. Publication bias was not assessed using funnel plot as there were few included studies. However, we were able to conduct a meta-analysis as the included studies had a sufficiently large number of patients. Review Manager 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013) was used to carry out all statistical analyses.
Results
Search Results and Study Selection
Figure 1 summarises the review flowchart in accordance with the PRISMA statement [14]. The electronic search in the Cochrane Library, EMBASE and PubMed yielded a total of 186 studies. No additional clinical trials on perampanel use in patients with partial-onset epilepsy were identified in the trial registers. After removing the duplicate studies, the titles and abstracts of 146 records were screened. Of these, 142 records were further removed since they were not RCTs related to perampanel use in patients with partial-onset epilepsy. Full texts of the four remaining records were retrieved for detailed evaluation, and all were included in this meta-analysis. As a result, four records (with five studies since one record contained two studies) were included in the meta-analysis, giving a total sample size of 1678 (1176 for the perampanel group and 502 for the placebo group). Tables 1 and 2 summarise the characteristics of the included studies.
Table 1.
Patient characteristics of randomised controlled trials included in this meta-analysis
| Article | Region | No. of patients (ITT) | Sex (n = female) | Age (year; mean ± SD) | No. of concomitant AEDs at baseline | Duration of epilepsy (month ± SD) | Seizure type (n) | ||
|---|---|---|---|---|---|---|---|---|---|
| Simple partial | Complex partial | Complex partial with secondary generalization | |||||||
| French et al. 2012 [11] (study 304) | North/South America | 133 | 68 | 35.8 ± 14.2 | 1–3 | 282.8 ± 162.2 | 271 | 345 | 279 |
| 133 | 65 | 36.7 ± 14.6 | 279.5 ± 172.4 | ||||||
| 121 | 67 | 35.6 ± 14.7 | 289.6 ± 154.4 | ||||||
| Total 387 | Total 200 | ||||||||
| French et al. 2013 [12] (study 305) | North/South | 129 | 64 | 36.7 ± 14.4 | 1–3 | 270.3 ± 163.4 | 240 | 328 | 262 |
| America | 121 | 71 | 35.5 ± 14.1 | 255.9 ± 158.6 | |||||
| Europe | 136 | 65 | 34.4 ± 13.6 | 264.2 ± 155.3 | |||||
| Asia | Total 386 | Total 200 | |||||||
| Australia | |||||||||
| Africa | |||||||||
| Krauss et al. 2012 [13] (study 306) | Europe | 180 | 95 | 33.8 ± 13.6 | 1–3 | 232.4 ± 145.2 | 423 | 593 | 487 |
| Asia | 172 | 84 | 33.6 ± 12.2 | 236.9 ± 145.3 | |||||
| Australia | 169 | 92 | 34.6 ± 12.8 | 239.4 ± 142.9 | |||||
| 184 | 90 | 33.4 ± 12.6 | 209.9 ± 128.1 | ||||||
| Total 705 | Total 361 | ||||||||
| Krauss et al. 2012 [10] (study 206) | Australia | 50 | 29 | 40.0 ± 11.38 | 1 or 2 | 301.2 ± 161.4 | 76 | 148 | 92 |
| Europe | 51 | 29 | 42.5 ± 12.06 | 276.0 ± 155.9 | |||||
| North America | 51 | 28 | 38.1 ± 11.62 | 274.8 ± 164.3 | |||||
| Total 152 | Total 86 | ||||||||
| Krauss et al. 2012 [10] (study 208) | Australia | 38 | 20 | 40.7 ± 11.99 | 1–3 | 267.6 ± 180.84 | 15 | 41 | 41 |
| Europe | 10 | 5 | 45.5 ± 12.05 | 216.0 ± 111.24 | |||||
| Total 48 | Total 25 | ||||||||
AEDs antiepileptic drugs, ITT intention-to-treat, SD standard deviation
Table 2.
Study design of randomised controlled trials included in this meta-analysis
| Article | Study design | Dosage of perampanel |
|---|---|---|
| French et al. 2012 [11] (study 304) | 6 weeks baseline | 8 mg QD |
| 6 weeks titration | 12 mg QD | |
| 13 weeks maintenance | Placebo | |
| 4 weeks follow-up | ||
| French et al. 2013 [12] (study 305) | 6 weeks baseline | 8 mg QD |
| 6 weeks titration | 12 mg QD | |
| 13 weeks maintenance | Placebo | |
| 4 weeks follow-up | ||
| Krauss et al. 2012 [13] (study 306) | 6 weeks baseline | 2 mg QD |
| 6 weeks titration | 4 mg QD | |
| 13 weeks maintenance | 8 mg QD | |
| 4 weeks follow-up | Placebo | |
| Krauss et al. 2012 [10] (study 206) | 4 weeks baseline | Maximum dose of 2 mg BID |
| 8 weeks titration | Maximum dose of 4 mg QD | |
| 4 weeks maintenance | Placebo | |
| 2 weeks follow-up | ||
| Krauss et al. 2012 [10] (study 208) | 4 weeks baseline | Maximum dose of 12 mg QD |
| 12 weeks titration | Placebo | |
| 4 weeks maintenance |
BID twice per day, QD once per day
Methodological Quality
All the included studies were reported to be double-blind. Methods of sequence generation and allocation concealment were reported in three studies. There were risks of bias in all studies since some of the outcomes stated in the trial protocol were not reported. In the included studies, efficacy and safety data were reported with different doses of perampanel. In this meta-analysis, studies were grouped by dose groups and 50 % responder rates/seizure freedom at each dose, with data from at least two studies reported in the forest plot (Figs. 2 and 3). The responder rate at 2 mg was only reported in one study (Online Resource Fig. 1). The seizure freedom at 2 mg and 4 mg were also reported in one study only (Online Resource Fig. 2).
Fig. 2.
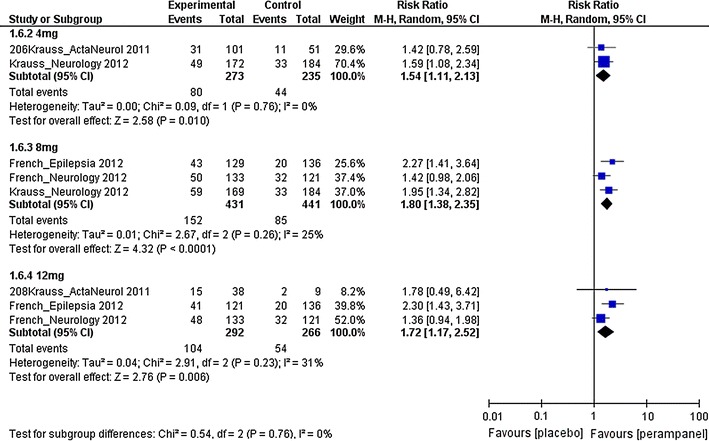
Risk ratios of 50 % responder rates for different doses of perampanel. df degree of freedom, M–H Mantel–Haenszel
Fig. 3.
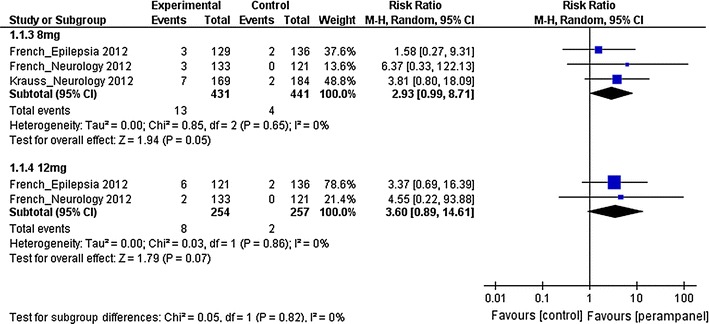
Risk ratios of seizure freedom for 8 mg and 12 mg perampanel. df degree of freedom, M–H Mantel–Haenszel
The primary objective of studies 206 and 208 [10] was to assess tolerability of different doses of perampanel, while the other included studies [11–13] primarily assessed efficacy. Studies 206 and 208 only reported overall efficacy results, and did not report the individual dose subgroup results. In the subgroup analysis, the two studies were assigned to a dose group according to the maximum dose tested in that study. Study 206 was assigned to the 4 mg group since 82.4 % of the subjects were able to reach 4 mg. Study 208 was assigned to the 12 mg group. As only 37.5 % of subjects were able to reach 12 mg, sensitivity analysis was conducted to investigate the effect of removing and assigning study 208 to different dose groups.
Efficacy
The 50 % responder rates of patients with partial-onset seizures receiving 4 mg, 8 mg and 12 mg perampanel were investigated. The estimated RRs were 1.54 (95 % CI 1.11–2.13), 1.80 (95 % CI 1.38–2.35) and 1.72 (95 % CI 1.17–2.52) for the 4 mg, 8 mg and 12 mg groups, respectively. This suggests that there was statistical evidence of higher 50 % responder rates in patients receiving perampanel treatment in all three dose groups when compared with the placebo group. For the seizure freedom, the estimated RRs were 2.93 (95 % CI 0.99–8.71) and 3.60 (95 % CI 0.89–14.61) for the 8 mg and 12 mg groups, respectively. The results suggest that there was no statistical evidence of a difference in seizure freedom in the 8 mg or 12 mg perampanel groups when compared with the placebo group.
A 50 % responder rate with 2 mg perampanel was only reported in one study. The RR was 1.15 with a 95 % CI of 0.75–1.75, which did not show evidence of a difference in 50 % responder rate when comparing 2 mg perampanel with placebo. Seizure freedom at 2 mg and 4 mg perampanel were also not included in this meta-analysis due to too few studies being available. The RRs were 1.53 (95 % CI 0.26–9.07) and 3.74 (95 % CI 0.79–17.78) for the 2 mg and 4 mg groups, respectively. There was no evidence of a difference in seizure freedom when comparing 2 mg or 4 mg perampanel with placebo.
Safety
Table 3 shows the most commonly reported TEAEs. Dizziness, somnolence, headache, fatigue and nasopharyngitis were included in this meta-analysis. The association between perampanel and TEAEs was assessed using 95 % CIs of the RRs. There was no evidence of a statistically significant association between the use of 4 mg perampanel and the five TEAEs. In contrast, there was statistical evidence of differences between use of 8 mg perampanel and placebo in the incidences of dizziness/somnolence. The use of 12 mg perampanel was also shown to be associated with dizziness. In all the cases where evidence of association existed, the risks of the TEAEs were higher in the perampanel group when comparing with the placebo group. In the 4 mg and 8 mg subgroups, the incidence of worsening seizures was significantly lower with perampanel when compared with placebo. In the 12 mg group, there was no statistical difference between perampanel and placebo with respect to the incidence of worsening seizures.
Table 3.
TEAEs with perampanel and withdrawal from trials
| TEAEs | Dose of perampanel | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 mg | 4 mg | 8 mg | |||||||
| No. of studies | Sample size (perampanel/placebo) | Risk ratio (95 % CI) | No. of studies | Sample size (perampanel/placebo) | Risk ratio (95 % CI) | No. of studies | Sample size (perampanel/placebo) | Risk ratio (95 % CI) | |
| Dizziness | 1 | 180/185 | 1.03 (0.55–1.91) | 2 | 274/236 | 1.29 (0.69–2.41) | 3 | 431/442 | 3.44 (2.48–4.77) |
| Somnolence | 1 | 180/185 | 1.88 (0.96–3.69) | 2 | 274/236 | 1.19 (0.66–2.17) | 3 | 431/442 | 2.17 (1.19–3.93) |
| Headache | 1 | 180/185 | 1.03 (0.53–1.99) | 2 | 274/236 | 1.05 (0.61–1.80) | 3 | 431/442 | 1.00 (0.68–1.46) |
| Fatigue | 1 | 180/185 | 1.64 (0.55–4.93) | 2 | 274/236 | 1.85 (0.69–4.97) | 2 | 298/321 | 1.73 (0.95–3.14) |
| Nasopharyngitis | 1 | 180/185 | 2.40 (0.63–9.13) | 2 | 274/236 | 0.76 (0.04–13.84) | 1 | 169/185 | 1.09 (0.22–5.35) |
| Worsening seizures | 1 | 180/185 | 0.73 (0.43–1.25) | 1 | 172/185 | 0.54 (0.29–0.99) | 3 | 431/442 | 0.63 (0.42–0.93) |
| Fall | – | – | – | – | – | – | 1 | 133/121 | 1.48 (0.63–3.44) |
| Irritability | – | – | – | – | – | – | 1 | 133/121 | 1.52 (0.57–4.05) |
| Ataxia | – | – | – | – | – | – | 1 | 133/121 | 15.48 (0.90–265.34) |
| Upper respiratory tract infection | 1 | 180/185 | 2.26 (0.80–6.38) | 1 | 172/185 | 1.29 (0.40–4.15) | 1 | 169/185 | 0.66 (0.16–2.71) |
| Gait disturbance | 1 | 180/185 | 0.51 (0.05–5.62) | 1 | 172/185 | 1.08 (0.15–7.55) | 1 | 169/185 | 4.93 (1.08–22.48) |
| Sleep disorder | – | – | – | – | – | – | 1 | 129/136 | 3.16 (0.33–30.02) |
| Anxiety | – | – | – | – | – | – | 1 | 129/136 | 5.27 (0.26–108.72) |
| Aggression | – | – | – | – | – | – | 1 | 129/136 | 2.11 (0.19–22.97) |
| Confusional state | – | – | – | – | – | – | 1 | 129/136 | Not estimablea |
| Anger | – | – | – | – | – | – | 1 | 129/136 | Not estimablea |
| Withdrawal | |||||||||
| Any cause | 1 | 180/185 | 1.41 (0.81–2.45) | 2 | 274/236 | 0.85 (0.49–1.48) | 3 | 431/442 | 1.31 (0.93–1.85) |
| Adverse events | 1 | 180/185 | 1.71 (0.64–4.62) | 2 | 274/236 | 0.94 (0.39–2.27) | 3 | 431/442 | 1.82 (1.01–3.25) |
| Lack of therapeutic effect | 1 | 180/185 | 7.19 (0.37–138.28) | 1 | 172/185 | Not estimablea | 3 | 431/442 | 0.57 (0.09–3.48) |
| TEAEs | Dose of perampanel | ||||
|---|---|---|---|---|---|
| 12 mg | Overall | ||||
| No. of studies | Sample size (perampanel/placebo) | Risk ratio (95 % CI) | Sample size (perampanel/placebo) | Risk ratio (95 % CI) | |
| Dizziness | 3 | 293/267 | 4.94 (3.27–7.48) | 1178/503 | 2.86 (2.16, 3.79) |
| Somnolence | 3 | 293/267 | 3.11 (0.81–11.97) | 1178/503 | 1.96 (1.40, 2.76) |
| Headache | 3 | 293/267 | 1.04 (0.67–1.60) | 1178/503 | 0.99 (0.74, 1.33) |
| Fatigue | 2 | 159/146 | 1.25 (0.34–4.49) | 911/382 | 1.54 (0.96, 2.45) |
| Nasopharyngitis | – | – | – | 623/236 | 0.88 (0.41, 1.90) |
| Worsening seizures | 2 | 255/257 | 0.74 (0.44–1.24) | 1038/442 | 0.67 (0.49, 0.91) |
| Fall | 1 | 134/121 | 1.92 (0.86–4.29) | 267/121 | 1.70 (0.80, 3.60) |
| Irritability | 1 | 134/121 | 2.86 (1.18–6.92) | 267/121 | 2.19 (0.93, 5.14) |
| Ataxia | 1 | 134/121 | 29.82 (1.81–491.80) | 267/121 | 22.31 (1.37, 363.80) |
| Upper respiratory tract infection | – | – | – | 521/185 | 1.42 (0.54, 3.73) |
| Gait disturbance | – | – | – | 521/185 | 2.13 (0.48, 9.43) |
| Sleep disorder | 1 | 121/136 | 2.25 (0.21–24.48) | 250/136 | 2.72 (0.32, 23.05) |
| Anxiety | 1 | 121/136 | 5.61 (0.27–115.81) | 250/136 | 4.91 (0.27, 90.56) |
| Aggression | 1 | 121/136 | 1.12 (0.07–17.78) | 250/136 | 1.63 (0.17, 15.54) |
| Confusional state | 1 | 121/136 | 7.86 (0.41–150.66) | 250/136 | 3.82 (0.20, 73.43) |
| Anger | 1 | 121/136 | 5.61 (0.27–115.81) | 250/136 | 2.73 (0.13, 56.44) |
| Withdrawal | |||||
| Any cause | 3 | 293/267 | 1.78 (1.11–2.87) | 1178/503 | 1.35 (1.02, 1.78) |
| Adverse events | 3 | 293/267 | 3.18 (1.18–8.58) | 1178/503 | 2.05 (1.30, 3.25) |
| Lack of therapeutic effect | 2 | 255/257 | 0.97 (0.20–4.76) | 1038/442 | 0.99 (0.26, 3.82) |
TEAEs treatment-emergent adverse events
aZero count for both the perampanel and placebo groups
Patient withdrawal from the included trials was investigated and categorized into three groups (Table 3): withdrawal due to any cause, adverse events or lack of therapeutic effect. There was statistically significant evidence of a higher incidence of withdrawal due to any cause with the use of 12 mg perampanel when compared with placebo. There was also evidence of a higher incidence of withdrawal due to adverse events in patients receiving 8 mg or 12 mg perampanel when compared with those patients receiving placebo. For withdrawal due to lack of therapeutic effect, the RRs for 8 mg and 12 mg perampanel compared with placebo were 0.57 (95 % CI 0.09–3.48) and 0.97 (95 % CI 0.20–4.76), respectively. No statistically significant differences were observed between perampanel and placebo in withdrawal due to lack of therapeutic effect.
Sensitivity Analysis
The effect of including study 208 [10] was tested by moving it to the 4/8 mg subgroup and removing it from the meta-analysis (Online Resource Table 2). Moving or removing this study did not significantly alter the RRs of 50 % responder rate. For example, excluding study 208 from the meta-analysis only led to a change in RR in the 12 mg perampanel group from 1.72 (95 % CI 1.17–2.52) to 1.74 (95 % CI 1.04–2.90). Although excluding or moving study 208 might lead to changes in heterogeneity (e.g. excluding study 208 led to an increase of I 2 statistic from 31 to 66 % in the 12 mg perampanel group), it did not materially alter the estimations.
Discussion
The use of perampanel at doses of 4, 8 and 12 mg resulted in statistically significant reductions in seizure frequency with respect to the 50 % responder rate in patients with partial-onset epilepsy compared with those treated with placebo. Our meta-analysis showed that the withdrawal rate due to any cause in the 12 mg perampanel group was significantly higher than that of the placebo group with OR of 2.03 (95 % CI 1.15–3.58). However, no statistically significant differences were observed between 4 mg or 8 mg perampanel and placebo in withdrawal due to any cause.
The previous meta-analysis of Gao et al. [9] did not show statistical evidence of a difference between perampanel and placebo in reducing seizure frequency in terms of 50 % responder rate. It is likely due to the limited number of studies available at the time of their meta-analysis. Our meta-analysis included more patients and has sufficient power to confirm the efficacy. Gao et al. also reported the 50 % responder rate ORs for eslicarbazepine, retigabine, carisbamate, lacosamide and brivaracetam to be 2.43 (95 % CI 1.77–3.35), 2.81 (95 % CI 2.09–3.78), 1.49 (95 % CI 1.19–1.88), 2.11 (1.58–2.82) and 3.78 (95 % CI 1.73–8.26), respectively. Our results on 4, 8 and 12 mg perampanel were all comparable with that results of Gao et al. on the five individual AEDs, suggesting significantly higher 50 % responder rates in the AEDs when compared with placebo.
The individual ORs (treatment groups/placebo groups) for withdrawal rates of eslicarbazepine, retigabine, carisbamate, lacosamide and brivaracetam were 1.12 (95 % CI 0.71–1.79), 2.33 (95 % CI 1.56–3.45), 1.52 (95 % CI 0.78–2.94), 2.78 (95 % CI 1.39–5.56) and 0.29 (0.09–0.99) in the study by Gao et al. Our results on 4 mg and 8 mg perampanel were comparable with the results for eslicarbazepine and carisbamate in the study by Gao et al., suggesting non-significant differences in withdrawal rate in the AEDs when compared with placebo. On the other hand, our result on the withdrawal rate due to any cause of 12 mg perampanel was consistent with the ORs of Gao et al. for retigabine and lacosamide, which showed significantly higher withdrawal rates in the AED groups when compared with placebo. In order to confirm the results and compare perampanel with other AEDs, further head-to-head direct comparison studies are needed.
Perampanel treatments at 4, 8 and 12 mg had all resulted in higher 50 % responder rates compared with the placebo group. A non-statistically significant dose-dependent effect was observed. Comparing the three dose groups on the forest plot (Fig. 2), the use of 8 mg perampanel appeared to result in a slightly higher responder rate than when using 4 mg. This dose-dependent effect seems to be saturated when the dose reached 8 mg, as a difference could not be observed when comparing 8 mg and 12 mg. The manufacturer of perampanel recommends a starting dose of 2 mg daily and a maximum daily dose of 12 mg as adjunctive treatment of partial-onset seizure in patients aged 12 years or older [21]. They also state in their prescribing information that the use of a daily dose of 12 mg may lead to a moderately improved reduction of seizure frequency compared with the use of a daily 8 mg dose [21]. However, our study did not show any clear difference in 50 % responder rate when comparing the use of 8 mg and 12 mg perampanel.
Freedom from seizures is one of the main goals of AED treatment. However, our meta-analysis did not demonstrate a statistically significant improvement in seizure freedom with 8 or 12 mg perampanel when compared with placebo. This finding is consistent with that of the meta-analysis conducted by Martyn-St James et al. [22] who also reported a non-statistically significant improvement in seizure freedom in patients treated with eslicarbazepine acetate, lacosamide and tiagabine compared with patients treated with placebo. These results probably reflected the fact that the populations selected for the study by Martyn-St James et al. and our included add-on clinical trials were patients with difficult-to-treat epilepsy. All the patients recruited in our included RCTs were patients with partial-onset epilepsy despite being treated with at least two AEDs prior to the baseline period. This suggests that the recruited patients were likely to be drug resistant; therefore, the poor outcome in seizure freedom was not unexpected.
Dizziness and somnolence are common adverse events found in many AEDs [22, 23]. In the study by James et al., the AEDs eslicarbazepine acetate, lacosamide, pregabalin, retigabine, tiagabine and zonisamide were statistically associated with the increased incidence of dizziness compared with placebo. Notably, there was a statistically significant increase in the incidence of somnolence with pregabalin, retigabine and zonisamide versus placebo. Our meta-analysis demonstrated a statistically significant increase in the incidence of dizziness for 8 mg or 12 mg perampanel versus placebo. Our study also showed a statistically significant association between the use of 8 mg perampanel and an increased incidence of somnolence. We were not able to investigate rare adverse events since the included studies only reported the common TEAEs. Furthermore, rare adverse events may not be apparent in the relatively small number of patients treated with perampanel over such short follow-up periods.
Data for the use of 2 mg perampanel could only be found in study 306 [13], and it was not included in the meta-analysis. Based on data from study 306 only, the RR for 50 % responder rate was calculated to be 1.15 (95 % CI 0.75–1.75). There was no evidence of a statistically significant association between the use of 2 mg perampanel and a change in the 50 % responder rate. RRs were also calculated for the TEAEs and withdrawal rates, and there was no statistical evidence of an association between the use of 2 mg perampanel and the incidence of withdrawal for the five commonly reported TEAEs (dizziness, somnolence, headache, fatigue and nasopharyngitis).
Our study included all relevant published RCTs investigating perampanel to date. No unpublished or ongoing RCTs on perampanel treatment for patients with partial-onset epilepsy were identified in the three trial registers; therefore, it is reasonable to assume that the results of our meta-analysis are unlikely to change in the foreseeable future. Data extraction and statistical analysis were carried out by independent reviewers and carefully cross-checked.Several potential study limitations are worthy of mention. Firstly, the number of studies and RCTs exploring perampanel was relatively small. Only five studies were included in this meta-analysis and all were pharmaceutical company funded.
The second limitation was the inclusion of studies 206 and 208, which were relatively different from those others included in terms of design and data presentation. The reported responder rates in studies 206 and 208 were calculated using pooled data across different dose groups, while the other included studies reported responder rates independently for each dose group. Despite this, the sensitivity analysis showed that the overall conclusions were not materially altered by removing or moving study 208. Traditionally, newly-marketed AEDs are evaluated in patients with severe epilepsy as on-add treatments for short-term RCTs. Perampanel is no exception and we were only able to identify add-on trials. Consequently, we were not able to identify RCTs that evaluated perampanel as a monotherapy or long-term treatment. We identified one extension study (307) which involved patients from three of the included studies (namely 304, 305 and 306). Study 307 investigated the long-term effect of perampanel [24]; however, it was not included in our analysis as it was an open-label study targeting patients who had already participated in those included studies. Nevertheless, study 307 reported that tolerability and efficacy of perampanel were maintained in the long term. Further long-term RCTs with newly diagnosed patients will be useful in studying the long-term effect of perampanel.
Lastly, it is worthy to note that the common outcome measures in short-term clinical trials investigating epilepsy include 50 % seizure reduction, mean seizure reduction and short-term tolerability. Such parameters are traditionally designed for regulatory purposes. These trials do not provide the impact of the new drugs on mortality and morbidity rates, and often do not report the proportion of patients becoming seizure free [25]. Other important outcomes including ‘time to treatment failure’, ‘time to achieve a 12-month remission of seizures’, quality of life outcomes and health economic outcomes are also used in clinical trials [26]. Future clinical and pharmacoepidemiological studies should be conducted to evaluate the real-life clinical use and define the roles of each new AED in the pragmatic setting [27, 28].
Conclusion
This meta-analysis showed that the use of perampanel resulted in a statistically significant reduction of seizure frequency with respect to the 50 % responder rate when compared with placebo. The safety analysis showed that perampanel was well tolerated at 4 mg and reasonably tolerated at 8 and 12 mg. Further clinical and pharmacovigilance studies will be needed to investigate the long-term efficacy and safety of perampanel and, specifically, the efficacy of perampanel in the management of other types of epilepsy.
Electronic supplementary material
Below is the link to the online resource material.
Acknowledgment
We thank Mr. H.H. Pan for his contribution to the study analysis. No external funding was received for this study.
Conflict of interest
There are no potential conflicts of interest to declare.
Footnotes
W. W. Q. Hsu and C. W. Sing contributed equally to this work.
Contributor Information
Warrington W. Q. Hsu, Email: warhsu@hku.hk
Esther W. Chan, Phone: +86-852-28315116, Email: ewchan@hku.hk
References
- 1.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349(13):1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact sheets-epilepsy. 2012. http://www.who.int/mediacentre/factsheets/fs999/en/index.html. Accessed 27 Feb 2013.
- 3.National Institute for Health and Clinical Excellence. CG137 epilepsy: NICE guidance. 2012. http://guidance.nice.org.uk/CG137/NICEGuidance/pdf/English. Accessed 27 Feb 2013.
- 4.French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62(8):1261–1273. doi: 10.1212/01.WNL.0000123695.22623.32. [DOI] [PubMed] [Google Scholar]
- 5.Lason W, Dudra-Jastrzebska M, Rejdak K, Czuczwar SJ. Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamic interactions: an update. Pharmacological reports PR. 2011;63(2):271–292. doi: 10.1016/s1734-1140(11)70497-2. [DOI] [PubMed] [Google Scholar]
- 6.Ceolin L, Bortolotto ZA, Bannister N, Collingridge GL, Lodge D, Volianskis A. A novel anti-epileptic agent, perampanel, selectively inhibits AMPA receptor-mediated synaptic transmission in the hippocampus. Neurochem Int. 2012;61(4):517–522. doi: 10.1016/j.neuint.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr Am Epilepsy Soc. 2011;11(2):56–63. doi: 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shvarts V, Chung S. Perampanel: newly approved, novel antiepileptic medication for partial-onset seizures. Exp Rev Neurother. 2013;13(2):131–134. doi: 10.1586/ern.12.154. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Xia L, Zhao FL, Li SC. Clinical efficacy and safety of the newer antiepileptic drugs as adjunctive treatment in adults with refractory partial-onset epilepsy: a meta-analysis of randomized placebo-controlled trials. Epilepsy Res. 2013;103(1):31–44. doi: 10.1016/j.eplepsyres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Krauss GL, Bar M, Biton V, Klapper JA, Rektor I, Vaiciene-Magistris N, et al. Tolerability and safety of perampanel: two randomized dose-escalation studies. Acta Neurologica Scandinavica. 2012;125(1):8–15. doi: 10.1111/j.1600-0404.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- 11.French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 13.Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ILAE. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22(4):489–501. [DOI] [PubMed]
- 16.European Medicines Agency. Clinical investigation of medicinal products in the treatment of epileptic disorders. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070043.pdf. Accessed 27 Feb 2013.
- 17.Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48(7):1303–1307. doi: 10.1111/j.1528-1167.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Sterne JA. Chapter 8: assessing risk of bias in included studies. 2011. http://www.cochrane.org/sites/default/files/uploads/handbook/Handbook510pdf_Ch08_RiskOfBias.pdf. Accessed 12 March 2013.
- 19.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Mak Int J Soc Med Decis Mak. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 21.Eisai Inc. Prescribing information of Fycompa. 2012. http://us.eisai.com/package_inserts/FycompaPI.pdf. Accessed 27 Feb 2013.
- 22.Martyn-St James M, Glanville J, McCool R, Duffy S, Cooper J, Hugel P, et al. The efficacy and safety of retigabine and other adjunctive treatments for refractory partial epilepsy: a systematic review and indirect comparison. Seizure J Brit Epilepsy Assoc. 2012;21(9):665–678. doi: 10.1016/j.seizure.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Zaccara G, Gangemi PF, Cincotta M. Central nervous system adverse effects of new antiepileptic drugs. A meta-analysis of placebo-controlled studies. Seizure J Brit Epilepsy Assoc. 2008;17(5):405–421. doi: 10.1016/j.seizure.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Krauss GL, Perucca E, Ben-Menachem E, Kwan P, Shih JJ, Squillacote D, et al. Perampanel, a selective, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307. Epilepsia. 2013;54(1):126–134. doi: 10.1111/j.1528-1167.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong ICK, Chadwick DW, Fenwick PBC, Mawer GE, Sander JWAS. The long-term use of gabapentin, lamotrigine, and vigabatrin in patients with chronic epilepsy. Epilepsia. 1999;40(10):1439–1445. doi: 10.1111/j.1528-1157.1999.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 26.Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong IC, Mawer GE, Sander JW, Lhatoo SD. A pharmacoepidemiologic study of factors influencing the outcome of treatment with lamotrigine in chronic epilepsy. Epilepsia. 2001;42(10):1354–1358. doi: 10.1046/j.1528-1157.2001.02101.x. [DOI] [PubMed] [Google Scholar]
- 28.Ackers R, Murray ML, Besag FM, Wong IC. Prioritizing children’s medicines for research: a pharmaco-epidemiological study of antiepileptic drugs. Brit J Clin Pharmacol. 2007;63(6):689–697. doi: 10.1111/j.1365-2125.2006.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


