Abstract
Objective
The aim of this retrospective study is to evaluate the efficacy of adjuvant chemotherapy following radical hysterectomy for intermediate risk stage IB cervical cancer.
Methods
From January 1993 to December 2007, a total of 100 patients of stage IB were enrolled in this study who had at least two of the following three intermediate risk factors (deep stromal invasion, lymphovascular space involvement, and large tumor size) after radical hysterectomy and all patients had no high risk factors and no radiotherapy. Of these patients, 22 patients had surgery only and 78 patients had cisplatin-based combination chemotherapy as adjuvant therapy postoperatively to improve survival. Kaplan-Meier survival curves and Cox's proportional-hazards regression model and log-rank test were used for survival analysis and to estimate the impact of prognostic factors on survival.
Results
The mean age was 52 years (range, 28 to 76 years). The overall survival rate of all intermediate tumors are 92% (92/100). Surgery only group is 81.8% (18/22) and adjuvant chemotherapy group is 94.9% (74/78). Comparison of survival between two groups revealed significant statistical difference in both univariant and multivariant survival analysis (P<0.05). The main toxicities of adjuvant chemotherapy were bone marrow suppression (18%), nausea and vomiting (5.2%) and alopecia in etoposide-cisplatin chemotherapy group (100%) but most side effects of postoperative adjuvant chemotherapy were transient, reversible and within acceptable limits to all patients.
Conclusion
Cisplatin based combined adjuvant chemotherapy for intermediate risk tumors after radical hysterectomy is promising with significant improvement of overall survival and with acceptable toxicity profile.
Keywords: Uterine cervical neoplasm; Chemotherapy, Adjuvant; Cisplatin; Survival analysis
Introduction
Stage IB cervical cancer has a relatively favorable prognosis but the 20% treatment failure has yet remains. The survival of patients with early stage cervical cancer after radical hysterectomy and pelvic lymphadenectomy depends on the presence or absence of several poor prognostic clinicopathologic risk factors. High-risk factors for recurrent disease are as follow: positive lymph nodes, positive or close surgical margins, parametrial involvement. Intermediate risk factors for recurrent disease are as follow: large tumor size, deep cervical stromal invasion, lymphovascular space invasion. Adjuvant therapy is recommended for both these intermediate and high risk patients to improve survival [1-5]. For high risk tumors, concurrent chemoradiotherapy is now regarded as the standard of treatment [6-8].
But for intermediate risk cervical cancer patients, method of adjuvant therapy has been debating. Radiotherapy has been used as postoperative adjuvant therapy to reduce recurrence in patients with intermediate risk tumors and the effectiveness of radiotherapy has been widely accepted, based on the results of a randomized studies reported by Sedlis et al. [9]. But radiotherapy have local effects only, so are not effective in distant metastatic diseases, and have high incidence of morbidity and mortality in patients receiving full course radiotherapy following radical surgery [10,11]. Several studies have suggested that chemotherapy alone is promising as postoperative adjuvant therapy for intermediate risk cervical cancer patients. Postoperative adjuvant chemotherapy without radiotherapy is expected to have several advantages including efficacy for control of both microscopic local and distant metastatic diseases, preservation of ovarian function in young female and avoidance of long term serious radiation sequelae. Also the toxicities of chemotherapy were mostly reversible and within acceptable limits [12-14].
Cisplatin-based chemotherapy usually has been accepted as effective adjuvant therapy against cervical carcinomas. So we have applied postoperative adjuvant cisplatin based combination chemotherapy to the patients who had at least two of the intermediate risk factors and who had no high risk factors and who refused radiotherapy to avoid serious postoperative radiation complication.
Materials and Methods
1. Patients
In this retrospective study we reviewed the medical records of stage IB cervical cancer patients who had radical hysterectomy and pelvic lymphadenectomy at the Gospel Hospital, Busan, Korea from January 1993 to December 2007. Among them, 100 intermediate risk tumors were eligible. Of these patients, 22 patients had surgery only and 78 patients had cisplatin based combination chemotherapy postoperatively as adjuvant therapy to improve survival. Patient characteristics are listed in Table 1. Criteria for intermediate risk group are still debating and there are two commonly used criterias for intermediate risk tumors now; Classic vs. gynecological oncology group (GOG) 92 criteria (Table 2).
Table 1.
Clinicopathologic characteristics of 100 patients with postoperation intermediate risk factors
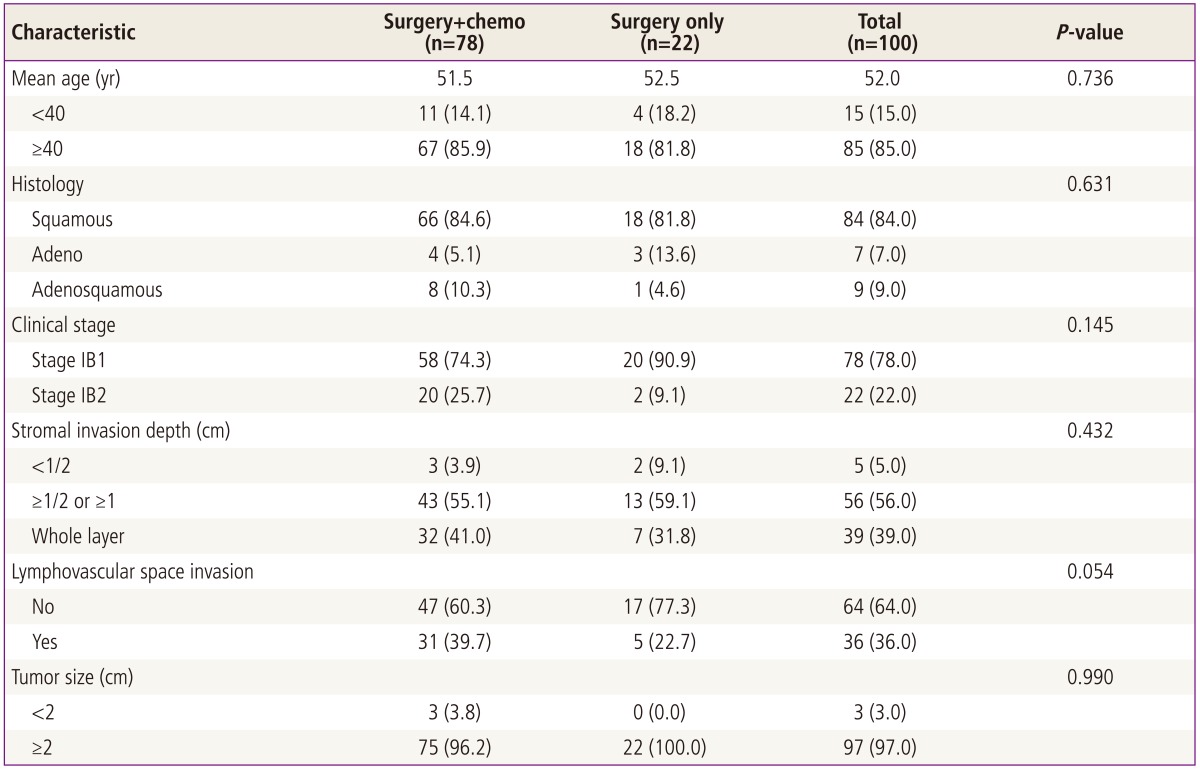
Table 2.
Criterias of intermediate risk tumors: Classic vs. GOG criteria
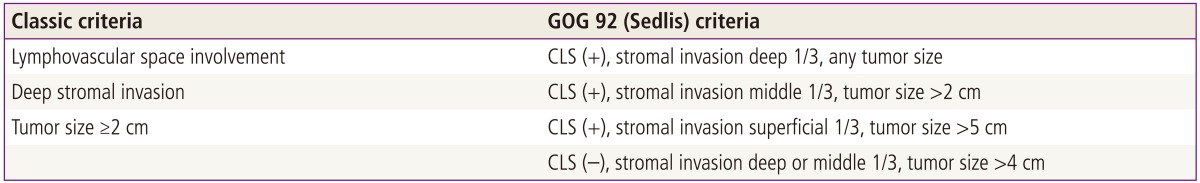
GOG, gynecological oncology group; CLS, capillary lymphatic space invasion.
We applied classic criteria for our intermediate risk tumors because of its simplicity and usefulness. Our eligible criteria for intermediate risk tumors are stage IB cervical cancer patients who had type III radical hysterectomy and pelvic lymphadenectomy and who had at least two of the following three intermediate risk factors (deep stromal invasion ≥1/2 or ≥1 cm, lymphovascular space involvement, large tumor size ≥2 cm) and all patients had no high risk factors and no obious any residual tumors.
2. Surgery and chemotherapy
All eligible 100 patients had type III radical hysterectomy with pelvic/paraaortic lymph node dissection. Among them, total 78 (78%) patients received postoperative cisplatin based combination adjuvant chemotherapy (1 to 6 cycles/patient, total 300 cycles/78 patients; average 3.8 cycles/patient). The schedules of the cisplatin based combination chemotherapy are as follows. Five-fluorouracilcisplatin combination chemotherapy regimen consisted of cisplatin 50 mg/m2 on day 1 and followed by 5-fluorouracil 1,000 mg/m2/day given intravenously from day 2 to day 5 daily with 3 weekly intervals (total 127 cycles/34 patients) and etoposide-cisplatin combination chemotherapy regimen consisted of cisplatin 30 mg/m2 and etoposide 60 mg/m2 given intravenously daily for 3 days with 3 weekly intervals (total 173 cycles/44 patients). Toxicities were recorded and categorized using the National Cancer Institute Common Toxicity Criteria (ver. 3.0, 2003) and chemotherapy was withheld if any of the following criteria were observed: leukocytes <3,000/mm3, granulocytes <1,500 mm3, platelets <100,000/mm3, serum creatinine level >2.0 mg/dL. In case of severe toxicity, chemotherapy was delayed until the symptoms of toxicities had disappeared.
3. Follow-up and statistics
Median follow-up period is 109 months. Overall survival was offered by the Korean National Stastistical Office. Patient selections between two groups (surgery only and postoperative adjuvant chemotherapy group) were done randomly and Kaplan-Meier survival curves and Cox's proportional-hazards regression model and log-rank test were used for survival analysis and to estimate the impact of prognostic factors on survival and SPSS PASW ver. 18.0 software (IBM, Armonk, NY, USA) was used for the statistical analysis. P-value less than 0.05 was considered significant.
Results
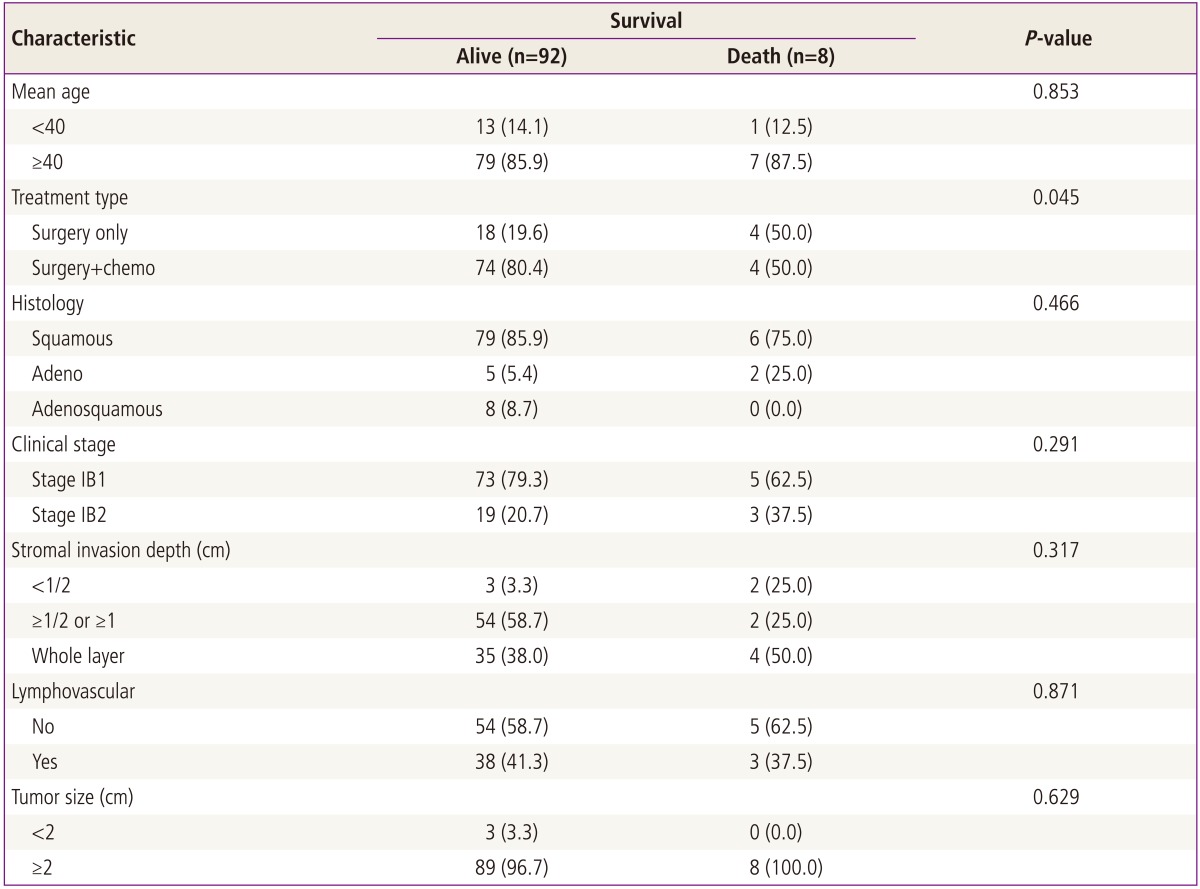
The mean age of patients at diagnosis was 52 years (range, 28 to 76 years), and 84.0% (84/100) had squamous cell type, and 78.0% (78/100) had stage IB1, and 56.0% (56/100) had stromal invasion ≥1/2 or ≥1 cm but not whole layer, and 36.0% (36/100) had lymphovascular space invasion, and 97.0% (97/100) had tumor size ≥2 cm (Table 1).
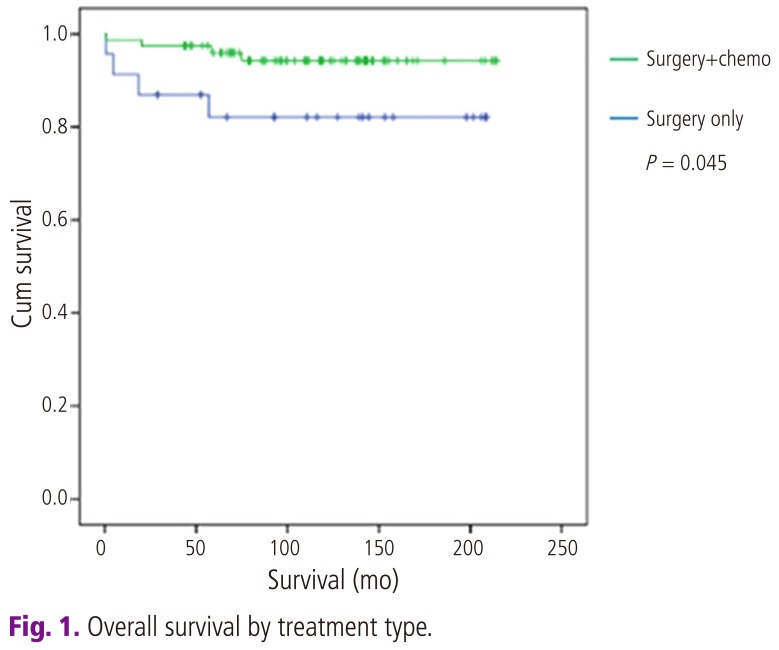
Comparison of patient characteristics between two groups revealed no significant differences. In overall survival, there are 8 deaths (4 deaths in surgery only group, and 4 deaths in adjuvant chemotherapy group), the overall survival rate of all intermediate tumors are 92% (92/100); surgery only group is 81.8% (18/22) and postoperative adjuvant chemotherapy group is 94.9% (74/78), respectively. Mean survival time from disease diagnosis to death are 26 months in surgery only group and 38 months in postradical adjuvant chemotherapy group. Comparison of survival curves between two groups revealed significant statistical difference in both univariant and multivariant survival analysis (P<0.05) (Tables 3-5; Fig. 1).
Table 3.
Deaths analysis of 100 patients with intermediate risk factors
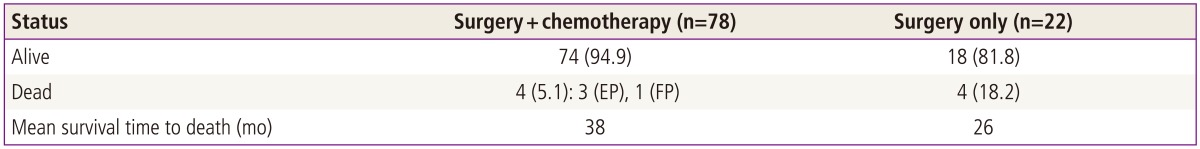
Values are presented as number (%).
EP, etoposide-cisplatin; FP, 5-fluorouracil-cisplatin.
Table 5.
Cox proportional hazard regression model for survival
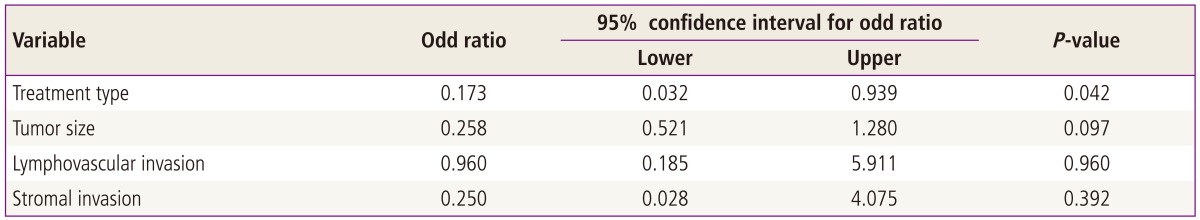
Fig. 1.
Overall survival by treatment type.
Toxicities of adjuvant chemotherapy were mostly transient, reversible and within acceptable limits. Grade 3 or 4 myelosuppression was observed in 18%, grade 3 or 4 gastrointestinal toxicity was observed in 5.2%, grade 3 or 4 hepatotoxicity was observed in two cases, and cardiac toxicity was observed in one case in etoposide-cisplatin group. Grade 3 or 4 alopecia was observed in all patients in etoposide-cisplatin group but was not observed in 5-fluorouracil-cisplatin group.
Discussion
The survival of patients with stage IB cervical cancer after radical hysterectomy and pelvic lymphadenectomy depends on the presence or absence of several poor prognostic clinicopathologic risk factors. The poor risk factors are now classified into two groups: classic high risk and newly developed intermediate risk factors [1-5]. In 1989 and 1990, the GOG published the results of prospective clinicopathologic studies of patients with stage IB cervical carcinoma treated by radical hysterectomy and bilateral pelvic lymphadenectomy. These studies revealed that certain patients with poor prognostic factors related to the primary tumor but without classic high risk factors had a risk of recurrence after radical hysterectomy and pelvic lymphadenectomy. The poor prognostic factors related to the primary tumor are as follow: clinical tumor size, presence or absence of capillary lymphatic space invasion, depth of tumor stromal invasion; the so called newly developed intermediate risk factors [4,5]. Postoperative adjuvant therapy is recommended for these poor risk tumors to reduce recurrence and to prolong survival. Concurrent chemoradiotherapy is now regarded as the standard of treatment for high risk tumors [6-8].
But adjuvant treatment modalities for intermediate risk tumors have been debating. Use of radiotherapy postoperatively for stage IB, intermediate risk tumors was shown to be beneficial in reducing the risk of recurrence by various studies [9,10]. In 1999, Sedlis et al. [9] showed that adjuvant pelvic radiotherapy reduced recurrence from 28% to 15% at 2 years after treatment and had better 2-year disease free survival than no treatment group in their prospective study for intermediate risk cervical cancer. This result suggested that radiotherapy has a role in adjuvant therapy, and after this report radiotherapy has been used as postoperative adjuvant therapy for stages Ib intermediate risk cervical cancer patients [9]. In 2006, Rotman et al. [11] reported the final results of this study, in which adjuvant pelvic radiotherapy significantly prolonged disease free survival but the improvement in overall survival did not reach statistical significance (Hazard ratio, 0.70; 90% confidence interval [CI], 0.45 to 1.05). Possible reasons of Rotman's results are that radiotherapy have local effects only, so are not effective in distant metastatic disease, and have high incidence of morbidity and mortality in patients receiving full course radiotherapy following radical surgery [11].
The use of cisplatin based combination chemotherapy was found to improve overall survival in women with advanced cervical cancers (large IB2 tumors, high risk early stage diseases and recurrent or metastatic cancer of the cervix) [12-21]. Experiences with adjuvant chemotherapy for poor risk cervical carcinomas suggested that cisplatin-based combination chemotherapy was effective not only in local tumor and may also reduce the incidence of lymph node metastasis and even eradicate microscopic metastasis in distant organs [12-14].
In 1998, Iwasaka et al. [12] performed postoperative adjuvant chemotherapy only for high risk cervical cancer and compared their patients with those from another hospital where postoperative adjuvant radiotherapy was performed during the same period. All eligible patients had no obvious residual lesions after radical surgery. The results of chemotherapy were similar to those of radiotherapy (83% vs. 81.7% 5-year survival rate). But in the adjuvant chemotherapy group, 5 of 11 (45%) patients with pelvic recurrences survived more than 5 years after retreatment with irradiation to sites of recurrence, which suggests that adjuvant chemotherapy might be preferable because unnecessary irradiation can be avoided and radiotherapy can be reserved for late locoregional recurrence [12]. In 2006, Takeshima et al. [13] have reported a favorable outcome for intermediate and high risk patients treated with adjuvant chemotherapy following radical hysterectomy. They consistently treated their patients with adjuvant chemotherapy alone for poor risk cervical carcinomas postoperatively for several reasons. First, distant metastasis is a major problem when radiotherapy alone is used, and chemotherapy is considered the most powerful means of eradicating subclinical distant metastastic lesions. Second, their chemotherapy regimen yielded good response rate in patients with recurrent cervical cancer with an acceptable incidence of side effects. Third, when chemotherapy alone is used for adjuvant therapy, radiotherapy can be reserved for late locoregional recurrence. Finally, this treatment strategy may provide a better postoperative quality of life by ovarian preservation and precluding radiation related morbidity. Three courses of bleomycin, vincristine, mitomycin, and cisplatin were given for intermediate risk cases (n=30) and 5 courses for high risk cases (n=35). Estimated 5-year disease free survival was 93.3% with intermediate risk tumors, and 85.7% with high risk tumors. The incidence of locoregional recurrence was 3.3% in the intermediate risk group and 8.6% in the high risk group [13]. These reports suggest that postoperative adjuvant chemotherapy for intermediate risk tumors after radical hysterectomy is promising and intrapelvic recurrence is not a major obstacle when radical operation was performed successfully and all tumor was resected completely [12,13]. In our study, overall survival in adujvant chemotherapy group was 94.9% (74/78), this result was similar to Takeshima's result in intermediate risk group. And the improvement of overall survival in adjuvant chemotherapy group reached statistical significance in both univariant and multivariant survival analysis (odds ratio [OR], 0.173; 95% CI for OR, 0.032 to 0.939; P<0.05) (Tables 4, 5; Fig. 1).
Table 4.
Survival analysis of 100 patients with intermediate risk factors (Kaplan Meier)
Another issue that must be discussed is complications of these adjuvant therapies following radical surgery. Radiotherapy after radical hysterectomy has been reported to be associated with high incidence of complications and many of these conditions are very tragic and irreversible and result in poor quality of life [22,23]. Barter et al. [23] reported that 30% of patients treated with radiation after radical hysterectomy had serious complications; gastrointestinal and genitourinary complications and leg lymphedema were the major problems of radiation combination therapy. The major toxicities of chemotherapy (etoposide-cisplatin and 5-fluorouracil-cisplatin combination) were bone marrow depepression, mild to moderate gastrointestinal troubles, mild to moderate nephrotoxicities, total alopecia (in etoposide-cisplatin group), relatively low incidence of neurotoxicities and hepatotoxicities, rare incidence of pulmonary and cardiac toxicities and some reported secondary malignancies (acute leukemia and some solid tumors) but rare. And the levels of all toxicities were acceptable and most are reversible and transient. But unexpected severe side effects and idiosyncratic drug reactions can be occurred especially in severe systemic debility, advanced age, poor nutritional status during chemotherapy. Therefore careful monitoring of patients is recommended [17-21,24].
In summary, several studies suggest that, when radical operation was performed successfully and all tumor was resected completely, chemotherapy is worth considering as postoperative adjuvant therapy for stage IB intermediate risk cervical cancer from the view of treatment efficacy, morbidity and quality of life.
Our study suggests that postoperative cisplatin based combination adjuvant chemotherapy for intermediate risk stage IB cervical cancer after radical hysterectomy is promising with improvement of overall survival (OR, 0.173; 95% CI for OR, 0.032 to 0.939; P<0.05) and with reversible and acceptable toxicity profile. But experiences in postoperative adjuvant chemotherapy for cervical cancer are limited. We think that well balanced prospective studies are needed to confirm the efficacy and safety of the postoperative adjuvant chemotherapy.
Acknowledgments
This study was funded by the Oncology Center of Kosin University Gospel Hospital.
References
- 1.Hacker NF, Friedlander ML. Cervical cancer. In: Berek JS, Hacker NF, editors. Berek & Hacker's gynecologic oncology. 5th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2010. pp. 341–395. [Google Scholar]
- 2.Bidus MA, Elcas JC. Cervical and vaginal cancer. In: Berek JS, Novak E, editors. Berek & Novak's gynecology. 14th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2007. pp. 1403–1456. [Google Scholar]
- 3.Boyce J, Fruchter RG, Nicastri AD, Ambiavagar PC, Reinis MS, Nelson JH., Jr Prognostic factors in stage I carcinoma of the cervix. Gynecol Oncol. 1981;12:154–165. doi: 10.1016/0090-8258(81)90145-1. [DOI] [PubMed] [Google Scholar]
- 4.Delgado G, Bundy BN, Fowler WC, Jr, Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35:314–320. doi: 10.1016/0090-8258(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 5.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 8.Ryu HS. Concurrent chemoradiotherapy in cervical cancer (a new paradigm in cervical cancer treatment) Yonsei Med J. 2002;43:749–753. doi: 10.3349/ymj.2002.43.6.749. [DOI] [PubMed] [Google Scholar]
- 9.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 10.Soisson AP, Soper JT, Clarke-Pearson DL, Berchuck A, Montana G, Creasman WT. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990;37:390–395. doi: 10.1016/0090-8258(90)90374-t. [DOI] [PubMed] [Google Scholar]
- 11.Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65:169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaka T, Kamura T, Yokoyama M, Matsuo N, Nakano H, Sugimori H. Adjuvant chemotherapy after radical hysterectomy for cervical carcinoma: a comparison with effects of adjuvant radiotherapy. Obstet Gynecol. 1998;91:977–981. doi: 10.1016/s0029-7844(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 13.Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 2006;103:618–622. doi: 10.1016/j.ygyno.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Tattersall MH, Ramirez C, Coppleson M. A randomized trial of adjuvant chemotherapy after radical hysterectomy in stage Ib-IIa cervical cancer patients with pelvic lymph node metastases. Gynecol Oncol. 1992;46:176–181. doi: 10.1016/0090-8258(92)90251-d. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Garcia D, Mason-Liddil N. Cisplatin in advanced cancer of the cervix: an update. Semin Oncol. 1991;18:11–24. [PubMed] [Google Scholar]
- 16.Runowicz CD, Wadler S, Rodriguez-Rodriguez L, Litwin P, Shaves M, O'Hanlan KA, et al. Concomitant cisplatin and radiotherapy in locally advanced cervical carcinoma. Gynecol Oncol. 1989;34:395–401. doi: 10.1016/0090-8258(89)90180-7. [DOI] [PubMed] [Google Scholar]
- 17.Al-Saleh E, Hoskins PJ, Pike JA, Swenerton KD. Cisplatin/etoposide chemotherapy for recurrent or primarily advanced cervical carcinoma. Gynecol Oncol. 1997;64:468–472. doi: 10.1006/gyno.1996.4567. [DOI] [PubMed] [Google Scholar]
- 18.Olive ST, Kiser WR. Diagnosis of appendicitis. J Am Board Fam Pract. 1996;9:306–307. [PubMed] [Google Scholar]
- 19.Bonomi P, Blessing J, Ball H, Hanjani P, DiSaia PJ. A phase II evaluation of cisplatin and 5-fluorouracil in patients with advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;34:357–359. doi: 10.1016/0090-8258(89)90173-x. [DOI] [PubMed] [Google Scholar]
- 20.Namkoong SE, Park JS, Kim JW, Bae SN, Han GT, Lee JM, et al. Comparative study of the patients with locally advanced stages I and II cervical cancer treated by radical surgery with and without preoperative adjuvant chemotherapy. Gynecol Oncol. 1995;59:136–142. doi: 10.1006/gyno.1995.1280. [DOI] [PubMed] [Google Scholar]
- 21.Alberts DS, Kronmal R, Baker LH, Stock-Novack DL, Surwit EA, Boutselis JG, et al. Phase II randomized trial of cisplatin chemotherapy regimens in the treatment of recurrent or metastatic squamous cell cancer of the cervix: a Southwest Oncology Group Study. J Clin Oncol. 1987;5:1791–1795. doi: 10.1200/JCO.1987.5.11.1791. [DOI] [PubMed] [Google Scholar]
- 22.Dische S. Radiotherapy of cervical cancer. Clin Obstet Gynaecol. 1985;12:203–227. [PubMed] [Google Scholar]
- 23.Barter JF, Soong SJ, Shingleton HM, Hatch KD, Orr JW., Jr Complications of combined radical hysterectomy-postoperative radiation therapy in women with early stage cervical cancer. Gynecol Oncol. 1989;32:292–296. doi: 10.1016/0090-8258(89)90627-6. [DOI] [PubMed] [Google Scholar]
- 24.Markmam M. Chemotherapy. In: Berek JS, Hacker NF, editors. Berek & Hacker's gynecologic oncology. 5th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2010. pp. 57–82. [Google Scholar]