Abstract
Objective
To determine the frequency and clinical significance of intra-amniotic inflammation (IAI) defined as an elevated amniotic fluid (AF) matrix metalloproteinase-8 (MMP-8) concentration in patients with preterm labor and intact membranes (PTL) and low AF white blood cell (WBC) counts.
Methods
Adverse pregnancy outcomes were compared according to the presence or absence of IAI in 220 singleton gestations who underwent amniocentesis due to PTL (gestational age<35.7 weeks) and had low AF WBC counts (<19 cells/mm3). Adverse pregnancy outcomes included preterm birth within 5 days of amniocentesis, acute histologic chorioamnionitis (acute-HCA) and positive AF culture. IAI was defined as an elevated AF MMP-8 concentration (≥23 ng/mL).
Results
IAI was present in 19% of study population. Adverse pregnancy outcomes were significantly more frequent in patients with IAI than in those without IAI (preterm birth within 5 days of amniocentesis, 88% vs. 41%; acute-HCA, 47% vs. 11%; positive AF culture, 10% vs. 2%; each for P<0.05). Patients with IAI had a significantly shorter median amniocentesis-to-delivery interval than those without IAI (7.8 hours [0.01-3,307.3 hours] vs. 310.3 hours [0.01-2,973.8 hours]; P<0.001 from survival analysis). Multiple logistic regression analysis demonstrated that only an IAI (odds ratio, 3.3; 95% confidence interval, 1.5-7.3; P<0.005) retained a statistical significance in the prediction of acute-HCA after other confounding variables were adjusted.
Conclusion
Approximately one-fifth of patients with PTL and low AF WBC counts have an evidence of IAI and are at risk for impending preterm delivery and acute-HCA when AF MMP-8 concentration is used.
Keywords: Amniotic fluid, Chorioamnionitis, Inflammation, Matrix metalloproteinase-8, Preterm labor, White blood cell
Introduction
Matrix metalloproteinase-8 (MMP-8), human neutrophil collagenase, is released from the secondary or specific granules of polymorphonuclear leukocytes stimulated by chemotactic cytokines [1], and has the potent capabilities for degrading extracellular matrix, in particular collagen type I [2,3]. The degradation of extracellular matrix of fetal membranes is an important biochemical process in chorio-decidua activation, which is a part of the common terminal pathway of human parturition [4]. Indeed, MMP-8 has been documented in the amniotic cavity, fetal membranes and lower uterine segment in the context of preterm or term parturition [5-7].
Intra-amniotic inflammation (IAI) is associated with impending preterm delivery, acute histologic chorioamnionitis (acute-HCA), positive amniotic fluid (AF) culture and significant neonatal morbidity [8-19]. AF white blood cell (WBC) count has been generally used as a method for the rapid analysis of IAI, and a low AF WBC count has been thought to reflect the amniotic cavity without inflammation. However, AF MMP-8 has recently emerged as a reliable indicator of IAI [20-28]. We have previously observed that some patients with preterm labor and intact membranes (PTL) had low AF WBC counts but high AF MMP-8 concentrations. However, there is a paucity of data about the frequency and clinical significance of IAI defined as an elevated AF MMP-8 concentration in patients with PTL and low AF WBC counts. We hypothesized that only a low AF WBC count could not guarantee the continuation of pregnancy after 5 days of amniocentesis, placenta without any inflammation and a negative AF culture when AF MMP-8 concentration was elevated in patients with PTL. The objective of the study is to examine this hypothesis.
Materials and methods
1. Study design
Study population consisted of 220 singleton gestations who underwent amniocentesis due to PTL (gestational age [GA] <35.7 weeks) and had low AF WBC counts (defined as AF WBC count<19 cells/mm3) at Seoul National University Hospital between January 1993 and December 2007. Adverse pregnancy outcomes were compared according to the presence or absence of IAI. Adverse pregnancy outcomes included preterm birth within 5 days of amniocentesis, acute-HCA and a positive AF culture. Of adverse pregnancy outcomes, the relationship between IAI and acute-HCA was examined in 110 cases delivered within 7 days of amniocentesis. This criterion was used to preserve a meaningful temporal relationship between the results of AF studies and the placental pathologic findings at birth. At our institution, transabdominal amniocentesis for retrieval of AF and placental pathologic examination after delivery were routinely offered to all patients who were admitted with the diagnosis of PTL. AF was analyzed for microbiologic and inflammatory status and fetal lung maturity. Written informed consent was obtained from all these patients. The Institutional Review Board of Seoul National University Hospital approved the collection and use of these samples and information for research purposes. The Seoul National University has a Federal Wide Assurance with the Office for Human Research Protections of the Department of Health and Human Services of the United States. Many of patients in this study were included in our previous studies.
2. Clinical characteristics and pregnancy outcomes
The demographic and clinical characteristics of the mothers and their neonates were examined through a review of the medical records. We investigated parity (≥1), maternal age, antenatal use of corticosteroid, GA at amniocentesis, gender of newborn, route of delivery, GA at delivery, birth weight, 1 minute Apgar score and 5 minutes Apgar score.
3. Clinical chorioamnionitis
Clinical chorioamnionitis was diagnosed when maternal body temperature was elevated to 37.8℃ and ≥2 of the following criteria were present according to the definitions previously described in detail [29]: uterine tenderness, malodorous vaginal discharge, maternal leukocytosis (>15,000 cells/mm3), maternal tachycardia (>100 beats/min) and fetal tachycardia (>160 beats/min).
4. Placental pathology
Placental tissue samples obtained for pathologic evaluation included the chorio-decidua, amnion, chorionic plate and umbilical cord. These samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections of prepared tissue blocks were stained with hematoxylin and eosin. Pathologists were masked to the clinical information. Acute-HCA was defined in the presence of acute inflammatory changes on examination of a membrane roll and chorionic plate of the placenta; inflammation of the chorio-decidua or amnion was diagnosed in the presence of at least 1 focus of >5 neutrophils in the chorio-decidua or amnion; inflammation of the chorionic plate was diagnosed in the presence of more than 1 focus of at least 10 neutrophilic collections or diffuse inflammation in subchorionic fibrin, or diffuse and dense inflammation, neutrophilic infiltration into connective tissue of the placental plate, or placental vasculitis; funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton's jelly with the use of criteria previously published [30]. The presence of acute inflammation was classified as grade 1 or 2 in each anatomical region of placenta (amnion, chorio-decidua, chorionic plate and umbilical cord), and total grade was used to determine the severity of acute-HCA from 1 to 8 with the use of criteria previously published [30].
5. Amniotic fluid
AF was cultured for aerobic and anaerobic bacteria and for genital mycoplasmas (Ureaplasma urelyticum and Mycoplasma hominis) and analyzed for WBC count according to the methods previously described [31,32]. The remaining fluid was centrifuged and stored in polypropylene tubes at -70℃. MMP-8 concentrations in stored AF were measured with a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech Inc., Little Chalfont, Bucks, UK). The sensitivity of the test was <0.3 ng/mL. Both intra- and inter-assay coefficients of variation were <10%. Details about this assay and its performance have been previously described [24]. IAI was defined as an elevated AF MMP-8 concentration (>23 ng/mL) as previously reported [25].
6. Statistical analysis
Mann-Whitney U test was used for comparison of continuous variables. Comparisons of proportions were performed with the Fisher's exact test. Logistic regression analysis was used to examine the relationship between IAI and acute-HCA, controlling for the effect of any other confounding variables. The generalized Wilcoxon test for survival analysis was used to compare the amniocentesis-to-delivery interval according to the presence or absence of IAI in study population. Patients who were delivered for maternal or fetal indications had their procedure-to-delivery interval treated as censored observations, with a censoring time equal to the amniocentesis-to-delivery interval. Cox proportional hazards modeling was used to compare the interval to delivery between groups after adjustment for GA. Data were analyzed using SPSS Statistics ver. 20.0 (IBM Corporation, Somers, NY, USA). Statistical significance was defined as a P<0.05.
Results
1. Clinical characteristics according to the presence or absence of IAI
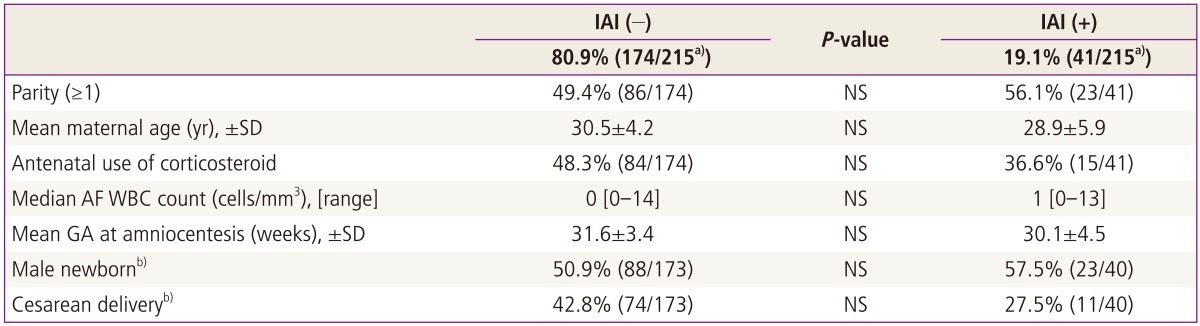
IAI and positive AF cultures were present in 19% and 3% of study population. Table 1 demonstrated that there was no significant difference in any clinical characteristics including GA at amniocentesis according to the presence or absence of IAI (defined as AF MMP-8≥23 ng/mL) (each for P > 0.05).
Table 1.
Clinical characteristics according to the presence or absence of IAI (defined as an elevated AF MMP-8 concentration ≥23 ng/mL) in 220 patients with preterm labor and low AF WBC counts (<19 cells/mm3)
IAI, intra-amniotic inflammation; AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; WBC, white blood cell; NS, not significant; GA, gestational age.
a)Of 220 cases, 215 patients were included in this analysis, because the test of AF MMP-8 concentration was not performed in 5 cases due to the limited amount of the remaining AF; b)Two neonates were excluded from the analysis among 215 patients with PTL and low AF WBC counts, because they were transferred to other hospitals due to various causes. Therefore, they could not be evaluated with respect to the presence or absence of male newborn or Cesarean delivery.
2. Pregnancy outcomes according to the presence or absence of IAI
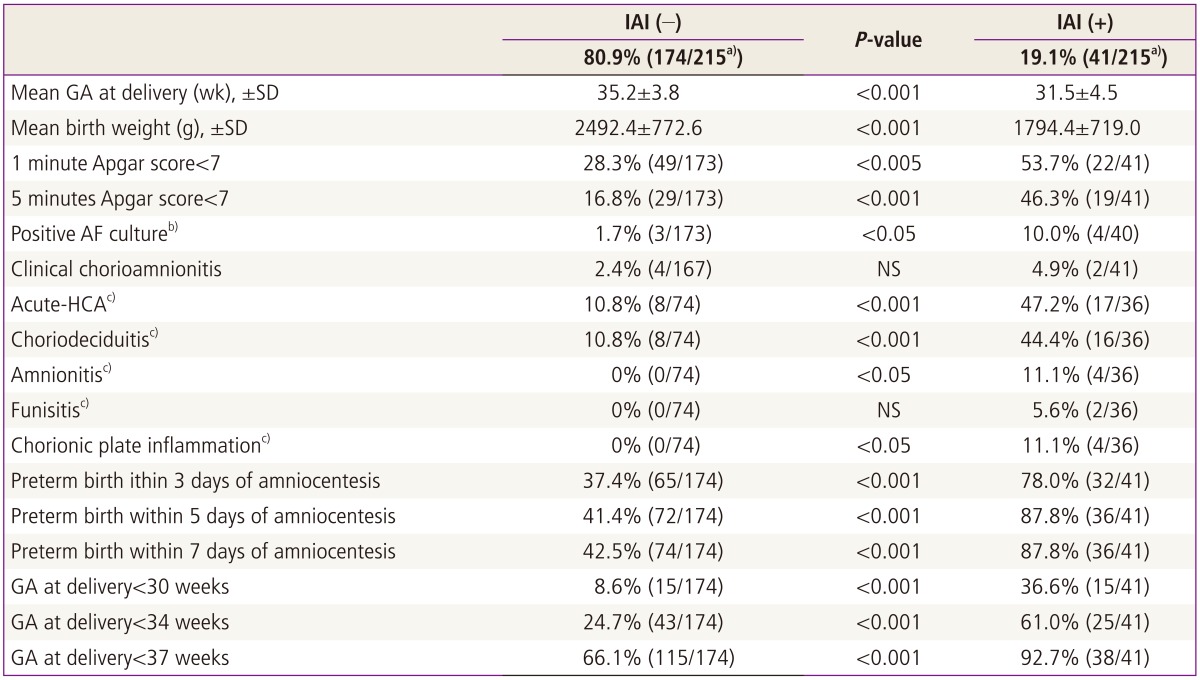
Table 2 shows that patients with IAI had a significantly lower mean GA at delivery and higher rate of preterm birth within 5 days of amniocentesis than those without IAI (GA at delivery, 31.5±4.5 weeks vs. 35.2±3.8 weeks; preterm birth within 5 days of amniocentesis, 88% vs. 41%; each for P<0.001). Acute-HCA was significantly more frequent in patients with IAI than in those without IAI (47% vs. 11%, P<0.001) (Table 2). Moreover, AF with inflammation was associated with a significantly higher rate of positive AF culture than AF without inflammation (10% vs. 2%, P<0.05) (Table 2).
Table 2.
Pregnancy outcomes according to the presence or absence of IAI (defined as an elevated AF MMP-8 concentration≥23 ng/mL) in 220 patients with preterm labor and low AF WBC counts (<19 cells/mm3)
IAI, intra-amniotic inflammation; AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; WBC, white blood cell; GA, gestational age; NS, not significant; Acute-HCA, acute histologic chorioamnionitis.
a)Of 220 cases, 215 patients were included in this analysis, because the test of AF MMP-8 concentration was not performed in 5 cases due to the limited amount of the remaining AF; b)Of 215 cases with available AF MMP-8 results, 213 patients were included in this analysis, because AF culture result was not available in two cases; c)One hundred ten patients who underwent amniocentesis within 7 days of birth were included in this analysis to preserve a meaningful temporal relationship between the results of AF studies and those of placental pathologic examination.
3. Amniocentesis-to-delivery interval according to the presence or absence of IAI
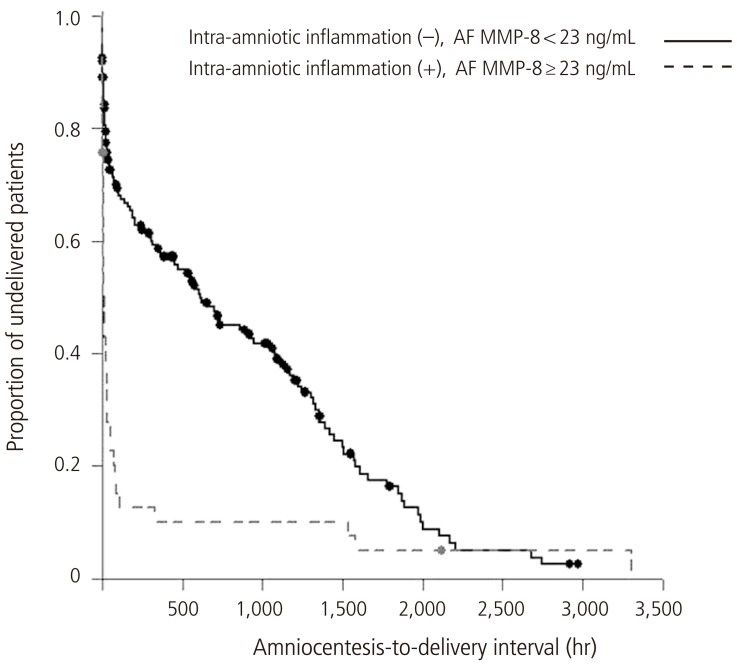
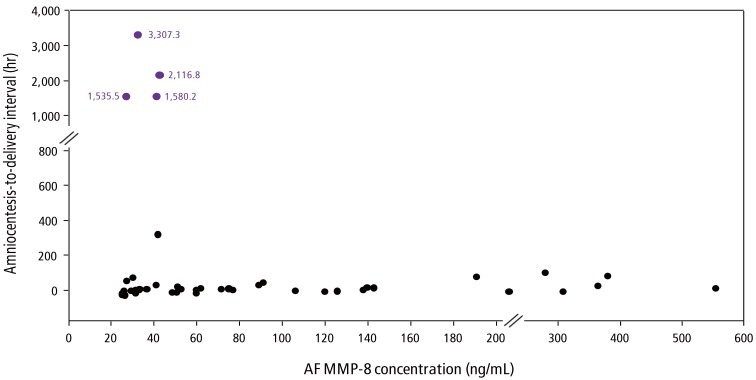
Fig. 1 illustrated that patients with IAI had a significantly shorter median amniocentesis-to-delivery interval than did those without IAI (P<0.001 from survival analysis) and this difference remained significant after adjustment for GA at amniocentesis. Moreover, among 25 cases with AF MMP-8 >43 ng/mL, no patients continued the pregnancy for more than 5 days after amniocentesis even in the context of low AF WBC counts (<19 cells/mm3) (Fig. 2).
Fig. 1.
Survival analysis of amniocentesis-to-delivery interval according to the presence or absence of IAI in patients with preterm labor and low AF WBC counts (IAI (+), median 7.8 hours [range, 0.01-3,307.3 hours] vs. IAI (-), median 310.3 hours [range, 0.01-2,973.8 hours]; P<0.001). This difference remained significant after adjustment for gestational age at amniocentesis. IAI, intra-amniotic inflammation; AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; WBC, white blood cell.
Fig. 2.
Amniocentesis-to-delivery interval according to AF MMP-8 concentrations in cases with intra-amniotic inflammation (defined as AF MMP-8 concentration≥23 ng/mL) in patients with preterm labor and low AF WBC counts. AF, amniotic fluid; MMP-8, matrix metalloproteinase-8; WBC, white blood cell.
4. Relationship between clinical or laboratory parameters and acute-HCA
To determine the relative value of clinical and laboratory parameters in the prediction of acute-HCA, we conducted multiple logistic regression analysis with potential risk factors for acute-HCA. Of all these independent variables, only an IAI retained a statistical significance in the prediction of acute-HCA after other confounding variables were adjusted (odds ratio, 3.3; 95% confidence interval, 1.5-7.3; P<0.005) (Table 3).
Table 3.
Relationship of various independent variables with acute histologic chorioamnionitis among patients with preterm labor and low AF WBC counts (<19 cells/mm3) by overall logistic regression analysis
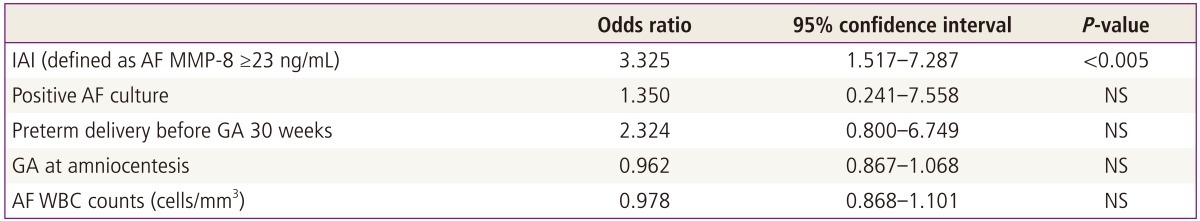
AF, amniotic fluid; WBC, white blood cell; IAI, intra-amniotic inflammation; MMP-8, matrix metalloproteinase-8; GA, gestational age; NS, not significant.
5. Clinical characteristics of patients with IAI in the context of AF WBC count zero
Table 4 displays the clinical information and laboratory results for the patients with IAI in the context of AF WBC count zero. In this group, there was only one patient (5.9%) with positive AF culture. However, it should be noted that 52.9% (9/17) of this group had acute-HCA although total grade of acute-HCA was less than 4 in all cases of this group (Table 4). Moreover, all patients except two cases (case no. 16 and 17) in this group delivered preterm neonates within 4 days of amniocentesis even in the context of AF WBC count zero (Table 4).
Table 4.
Clinical characteristics of 17 cases with IAI among patients with preterm labor and AF WBC count zero
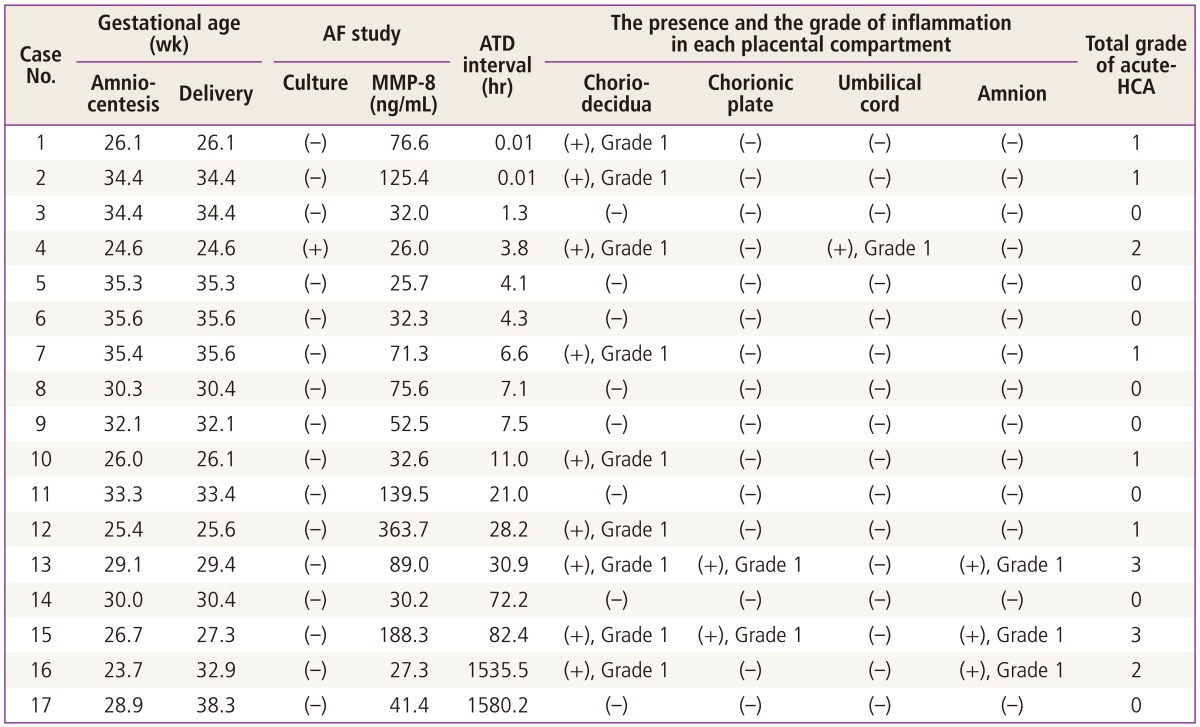
IAI, intra-amniotic inflammation; AF, amniotic fluid; WBC, white blood cell; ATD interval, amniocentesis-to-delivery interval; acute-HCA, acute histologic chorioamnionitis; MMP-8, matrix metalloproteinase-8.
Discussion
Principal findings of this study are as following. Firstly, IAI (defined as AF MMP-8 ≥23 ng/mL) was present in approximately one-fifth of patients with PTL and low AF WBC counts (<19 cells/mm3). Secondly, patients with IAI were at risk for impending preterm delivery and acute-HCA even in the context of low AF WBC counts among cases with PTL.
Previous studies demonstrated that AF MMP-8 had a better performance than AF interleukin-6 and AF WBC count in the prediction of pregnancy duration, positive AF culture and significant neonatal morbidity among cases with PTL [27] and among case with preterm premature rupture of membranes [28]. Indeed, our data shows that patients with IAI had a significantly shorter median amniocentesis-to-delivery interval than did those without IAI (Fig. 1), and most cases (88%) of patients with IAI were delivered within 5 days of amniocentesis even in the context of low AF WBC counts (<19 cells/mm3) (Table 2). Moreover, all except two of patients with IAI were delivered within 4 days of amniocentesis even among cases without detectable AF WBC (Table 4). This result suggests only a documentation of low AF WBC count cannot give a guarantee of the prolongation of pregnancy without the aid of other tests in patients with PTL.
Our data demonstrated that near half (47.2%) of patients with IAI (defined as AF MMP-8≥23 ng/mL) had acute-HCA while 10.8% of cases without IAI did in the context of low AF WBC counts (Table 2). Moreover, IAI was a better independent predictor for acute-HCA than any other potential contributing factors (Table 3). However, it should be noted that total grade of acute-HCA was less than 4 in all 25 cases with acute-HCA (this data is not shown). These findings indicate that patients with PTL and low AF WBC counts may have only a low grade placental inflammation though patients of this group have acute-HCA. Therefore, one may expect that AF MMP-8 can be an appropriate tool for the identification of low grade placental inflammation among acute-HCA.
Romero et al. [4] previously demonstrated that an elevated AF MMP-8 concentration was associated with chorio-decidua activation for accelerated catabolism of collagen type I, the main collagen of chorio-amnion. Indeed, our data shows that only 11% (6/74) of patients had an inflammation in chorio-decidua and no patients had an inflammation in the anatomical region beyond chorio-decidua (i.e., amnion, umbilical cord and chorionic plate) when AF MMP-8 was not elevated (Table 2). Moreover, in spite of AF WBC count zero, more than half (52.9%) of patients had an inflammation in chorio-decidua in the context of IAI (defined as AF MMP-8≥23 ng/mL) (Table 4). Our results suggest that an elevated AF MMP-8 may develop without the significant recruitment of neutrophils in the amniotic cavity in the case of an inflammation in chorio-decidua, that is extra-amniotic inflammation.
Major strengths of this study are: 1) its large cohort (n = 220) of singleton gestation with PTL in the context of low AF WBC counts; 2) this study analyzed the grade as well as the presence of inflammation in each anatomical region of placenta; 3) there was no difference in any clinical characteristics according to the presence or absence of IAI, and therefore the two groups according to the presence or absence of IAI could be comparable groups for analyzing adverse pregnancy outcomes by virtue of obstetric management. One potential weakness of the study is that patients who had PTL and delivered shortly after admission before amniocentesis, could not be enrolled in the study and therefore there was a possibility of bias in the inclusion of study population. However, this may be uncommon because as soon as all patients were admitted with the diagnosis of PTL, amniocentesis was routinely offered to them for the assessment of intra-amniotic infection or inflammation and fetal lung maturity at our institution.
A novel finding of this study is that one-fifth of patients with PTL even in the context of low AF WBC counts, have an evidence of IAI and are at risk for impending preterm delivery and acute-HCA. Therefore, we propose that patients with IAI (defined as AF MMP-8>23 ng/mL) should be separated from those without IAI among cases with PTL and low AF WBC counts in future clinical practice. Moreover, we should try to find the hidden pathophysiology of preterm birth in patients who did not have IAI but delivered shortly after amniocentesis.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2009-0080429).
References
- 1.Tschesche H. Human neutrophil collagenase. Methods Enzymol. 1995;248:431–449. doi: 10.1016/0076-6879(95)48028-5. [DOI] [PubMed] [Google Scholar]
- 2.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H, Werb Z, Welgus H, Van Wart H. Matrix metalloproteinases and inhibitors [Matrix Suppl No.1] Stuttgart: Gustav Fischer; 1992. [Google Scholar]
- 4.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder MG, Lamont RL, Romero R, editors. Preterm labor. New York: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 5.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara S, Yamada T, Minakami H, Watanabe T, Takizawa T, Sato I. Polymorphonuclear leukocytes in the fetal membranes are activated in patients with preterm delivery: ultrastructural and enzyme-histochemical evidence. Placenta. 1999;20:185–188. doi: 10.1053/plac.1998.0366. [DOI] [PubMed] [Google Scholar]
- 7.Winkler M, Fischer DC, Ruck P, Marx T, Kaiserling E, Oberpichler A, et al. Parturition at term: parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum Reprod. 1999;14:1096–1100. doi: 10.1093/humrep/14.4.1096. [DOI] [PubMed] [Google Scholar]
- 8.Been JV, Rours IG, Kornelisse RF, Lima Passos V, Kramer BW, Schneider TA, et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol. 2009;201:587.e1–587.e8. doi: 10.1016/j.ajog.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Bersani I, Thomas W, Speer CP. Chorioamnionitis: the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(Suppl 1):12–16. doi: 10.3109/14767058.2012.663161. [DOI] [PubMed] [Google Scholar]
- 10.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–343. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 12.Greig PC, Murtha AP, Jimmerson CJ, Herbert WN, Roitman-Johnson B, Allen J. Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstet Gynecol. 1997;90:465–469. doi: 10.1016/s0029-7844(97)00294-9. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–F135. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186:1062–1068. doi: 10.1067/mob.2002.122293. [DOI] [PubMed] [Google Scholar]
- 17.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 18.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 19.Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94:1000–1005. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292.e1–292.e5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 22.Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–1030. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 25.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 26.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 27.Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–1155. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 28.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–1148. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 30.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 31.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–1040. doi: 10.1016/s0029-7844(96)00339-0. [DOI] [PubMed] [Google Scholar]
- 32.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87:231–237. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]