Abstract
Objective
Much of the early investigative work on the usefulness of preoperative serum CA-125 levels in identifying patients with early-stage endometrial carcinoma who have occult metastases were carried out in Europe and the United States. This article reviews CA-125 as a possible index for determining the need for full surgical staging, from the results of large medical centers in Asia, particularly Taiwan and Korea.
Methods
A Medline search was performed using CA-125 and endometrial cancer as index words from 1981 to 2012. Those publications felt to be the most important especially from institutions from Asia since 2000 were identified in this review.
Results
Most articles that analyzed the utility of serum CA-125 levels as predictive marker for disease extent or prognosis in uterine cancer used univariate and multivariable logistic regression analysis, and performed receiver operative curves to find the best cut-off values. The main factor of interest was whether clinicians can stratify patients that need lymphadenectomy in early stage disease. Suggested optimal cut-off value ranged from 20 to 210 U/mL. Not only preoperative CA-125 level, but myometrial invasion status by magnetic resonance imaging was the most significant combined parameter for predicting disease extent.
Conclusion
Elevated CA-125 in patients with apparent early-stage disease is clearly a risk factor for the presence of extra-uterine disease although the optimal cut-off levels vary. The evolution of clinical investigations over the past decade, particularly in Asia, suggests employment of the test in a more focused manner to identify high risk patients preoperatively.
Keywords: CA-125 antigen, Neoplasm staging, Uterine neoplasm
Introduction
Measurement of serum CA-125 has been an established part of the management of patients with known or suspected invasive ovarian adenocarcinoma for almost 29 years [1] and has been investigated in patients with invasive endometrial adenocarcinoma for almost as long, first in Europe [2] and then in the United States [3]. Serum CA-125 has proved a useful marker for monitoring the treatment of patients with advanced disease following debulking surgery in both malignancies [4,5] and has similar limitations in patients with both ovarian epithelial and uterine carcinoma: a significant percentage of patients with stage I disease have normal preoperative serum levels [6], and not all cell types express the antigen equally.
Despite these similar limitations, measurement of serum CA-125 levels is an approved and universally accepted component of the preoperative evaluation of patients with an adnexal mass, particularly in the menopausal age group [7] where there is either a high index or suspicion or virtual certainty that invasive cancer is present. The precise role CA-125 should play in the preoperative evaluation of the endometrial cancer patient remains less defined, possibly even controversial. Indeed, the authors of the chapter "Controversies in endometrial cancer screening and diagnosis [8]" in Gershenson's recent authoritative textbook Gynecologic cancer: controversies in management state the issue thusly: "Although the benefit of a preoperative serum CA-125 measurement is unclear, some investigators have suggested obtaining this value to determine preoperatively the risk of advanced endometrial cancer and lymphatic disease."
It is not clear whether this statement is completely accurate, particularly in light of the work which has been done in Asia during the past decade investigating the usefulness of preoperative serum CA-125 levels in uterine cancer. The benefit of preoperative serum CA-125 levels in select uterine cancer patients such as high-risk early stage uterine cancer patients may, on careful analysis, actually be quite clear, particularly in identifying those patients with early stage disease who are more likely to benefit from lymphadenectomy, i.e., from full surgical staging.
The purpose of this article is to review prominent articles from the international literature-United States, European, and Asian-on the usefulness of preoperative serum CA-125 levels in patients with uterine cancer. In particular, the authors were interested in determining if more recent clinical investigations in this area from investigators in Asia provide sufficient evidence to support more specific indications for preoperative use of this test in particular endometrial cancer patients.
Materials and methods
A Medline search was performed using CA-125 and endometrial cancer as the index words from 1981 (the year monoclonal antibody assays were first reported [9] as reactive with human ovarian carcinoma) to 2012, gynecologic oncology literature. Those publications felt to be the most important from Asia were identified and are cited in this review, and findings from the most recent publications from Asia during the past decade are discussed in detail.
Since by definition the subject of this review was the use of serum CA-125 levels in the preoperative evaluation of the patient with uterine carcinoma, publications dealing with either uterine sarcoma or the potential role of CA-125 in the posttreatment surveillance or monitoring of patients who were undergoing consolidation therapy after surgery or who had completed primary therapy were excluded since almost all patient have surgery first. The role for the assay in patients with known or suspected clinically advanced disease (stage III or IV disease under the old International Federation of Obstetrics and Gynecology classification system) was assumed to be different from any role contemplated for serum CA-125 measurement preoperatively in patients whose disease appeared to be confined to the uterus in general (stage I or II disease), and the uterine corpus in particular (stage I disease). However, since some patients first diagnosed with uterine cancer will present with clinical and/or radiological (e.g., X-ray, computed tomography [CT] scan) evidence of metastatic disease at the time they are first evaluated by gynecologic oncologists these patients are included in the discussion.
Results
The literature on the use of serum CA-125 in the preoperative evaluation of patients with clinically early or advanced endometrial cancer over the past quarter century is less than twenty five peer-review publications internationally, of which approximately half might be considered "major" publications. These publications, including author, year, country, number of patients, type of study (retrospective or prospective), and primary conclusion(s) of the investigators are listed in Table 1 [10-19].
Table 1.
Suggested preoperative CA-125 cut-off value for prediction of disease extent in uterine cancer (Asia, 2000-2012)
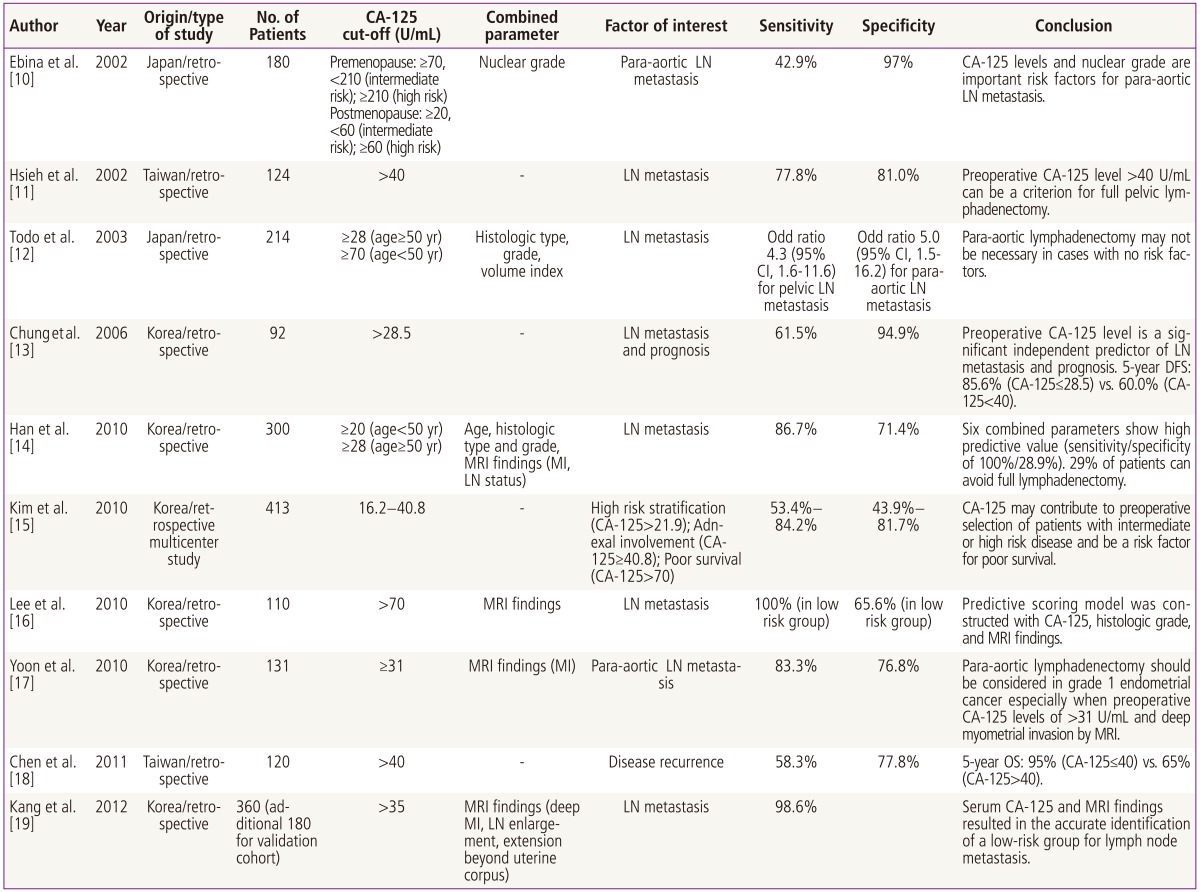
LN, lymph node; CI, confidence interval; DFS, disease free survival; MRI, magnetic resonance imaging; MI, myometrial invasion; OS, overall survival.
The initial data on CA-125 levels in patients with endometrial cancer first appeared in 1984 in Niloff et al. [20]'s work from Boston. More focused investigation correlation serum CA-125 levels with clinical stage of disease at the time of diagnosis soon followed from the Netherlands by Duk et al. [2]. The same year Patsner and Mann published their work on the predictive value of preoperative serum CA-125 levels in patients who presented with clinically localized or advanced endometrial cancer and noted that patients with advanced disease usually had elevated levels (defined at above 35 U/mL) while most patients with disease which appeared to be clinically confined to the uterine corpus had normal levels. Uniform surgical staging was carried out in all patients in this study, unlike prior clinical investigations, and the authors were able to show that elevated preoperative CA-125 levels in patients with stage I clinical disease who had occult metastatic disease had a 75% chance of having an elevated serum CA-125 preoperatively whereas more than 90% of patients with disease surgically confined to the uterine corpus had normal preoperative CA-125s [3]. These results were later confirmed by Soper at al. [21] from Duke although the predictive value of preoperative levels was not quite so good as in Patsner's study. A serum cut-off of 35 U/mL was used in these studies by convention since that was the value which was used in all of the earlier publications on ovarian epithelial, uterine and cervical adenocarcinomas.
Later clinical investigation in the United States strongly suggested that early-stage disease patients with occult metastases were 8.7 times more likely to have elevated preoperative serum CA-125 levels, though a value of 40 U/mL was used as the upper limit of normal in these clinical trials [22]. Other studies by Sood et al. [23] using a lower level of 20 U/mL as the cut-off for normal values along with Histological findings of grade III disease (poorly differentiated adenocarcinoma) on preoperative endometrial biopsy allowed surgeons to predict the need for full surgical staging with lymphadenectomy in 70% of patients as opposed to only 45% of patients if the "standard" cut-off in the literature of 35 U/mL was used. This particular finding has not been uniformly confirmed since that time.
More recent clinical investigation in Asia into the usefulness of preoperative serum CA-125 levels in patients with uterine carcinoma has expanded on the initial work done in the West and has had the benefit of an established approach to patients with early-stage disease which divides patients into either low or high risk for occult metastatic disease based on a combination of factors which may be readily determined before surgery, such as grade, histology, pap smear, and tumor size and depth of invasion on ultrasound or more advanced radiological imaging [10-19]. This current approach has resulted in a pronounced shift in the literature on preoperative use of serum CA-125 which has focused on the ability of the test to allow surgeons to select patients who require lymphadenectomy/full surgical staging rather than on presence of occult metastases per se.
In 2002, Hsieh et al. [11] investigated preoperative serum CA-125 levels in 124 uterine cancer patients who underwent full surgical staging at Chang Gung Memorial Hospital in Taiwan between 1995 and 2000 and in whom preoperative serum CA-125 levels were drawn and compared to other risk prognostic risk factors (tumor size, depth of invasion, cervical invasion, clinical stage, peritoneal cytology and nodal metastases) some of which were available preoperatively and some only postoperatively. Univariate analysis demonstrated that elevated preoperative serum CA-125 levels strongly correlated with other prognostic factors. Multivariate analysis and logistic regression curve demonstrated that lymph node metastases was the single factor which best correlated with elevated preoperative CA-125 levels, and that a cut-off of 40 U/mL had the "best" sensitivity and specificity for the presence of nodal disease with values of 78% and 81% respectively. Based on these results the authors strongly suggested that using a preoperative cut-off of 40 U/mL for serum CA-125 measurements would allow surgeons to select those patients most likely to benefit from lymphadenectomy [1].
A subsequent study from Taiwan a decade later but from a different institution which retrospectively looked at preoperative CA-125 in 120 of 184 cases of endometrial cancer treated between 1998 and 2006 with either simple or radical hysterectomy accompanied by lymph node dissection (not defined) [18]. The authors used both preoperative CA-125 and lymph node metastases to construct a decision matrix to predict risk-free survival and overall survival curves, and for the first time suggested that the presence of both nodal metastases and preoperative serum CA-125 levels above 40 U/mL indicated a group of patients at particularly high risk for lower survival and in whom more aggressive postoperative therapy might be indicated. Although the exact surgical staging was not specified and perhaps differed from that done at Chang Gung Hospital, the take-home point on the usefulness of preoperative values having a predictive value for metastatic disease in the 75% range was confirmed as was the prognostic significance of nodal metastases.
Gynecologic Oncologists from Seoul National University in Korea have published a series of three investigations on the use of preoperative serum CA-125 levels in uterine cancer patients between 2006 and 2011, each with a slightly differ focus. The first paper [13] was a retrospective review of 92 patients with uterine cancer who had both surgical staging and preoperative serum CA-125 levels done between 1999 and 2006 at Seoul National University Hospital. The finding of lymph node metastases in 13% of patients with clinical early stage disease was consistent with other surgical studies. ROC curves were used to determine the best cut-off values of preoperative CA-125 levels for lymph node metastases; using the recommended upper normal value of 40 U/mL recommended by the manufacturer of the assay the investigators determined that a value of 28.5 U/mL yielded the best combination of sensitivity (61.5%) and specificity (94.9%) for prediction of nodal disease. When preoperative serum CA-125 values were less than or equal to 28.5 U/mL, the 5-year disease free survival was also significantly better compared to that of less than 40 U/mL (85.6% vs. 60%, respectively).
A follow-on study from the same institution in 2010 used preoperative CA-125 and preoperative magnetic resonance imaging (MRI) results to attempt to develop a robust preoperative "model" for predicting lymph node metastases in patients newly diagnosed with uterine cancer [16]. The relatively more complicated study design and "scoring system" used by the investigators as well as employment of high-end imaging technology makes it difficult to draw specific conclusions on the usefulness of preoperative CA-125 values alone. The purpose of this study was to allow the combination of preoperative serum CA-125 and MRI along with other factors to provide a predictive model which would identify a group of patients at such low risk for nodal disease that no surgical staging would be required, but the fact the full surgical staging was omitted in the 15% of patients who had no myometrial invasion limits any claims which might be made for the predictive value of the model.
A 2010 collaborative study from six tertiary medical centers in Korea [15] published just prior to the CA-125/MRI modeling study evaluated how preoperative serum CA-125 levels might be used to preoperatively counsel patients with just endometrial adenocarcinoma. The conclusions of the authors of this study-that serum CA-125 levels did not predict "most" prognostic factors nor allow clinicians to predict patients at higher risk for requiring postoperative radiation therapy-must be weighed against the enormous number of patients who did not have preoperative serum CA-125 measurements (346/875 patients) or complete surgical staging (109/875) or variations in surgical staging among different institutions. Given the almost 50% study exclusion rate alone, any conclusions made by the investigators in this study might be considered limited at best.
The most recent study to predict low-risk group for lymph node metastasis using CA-125 level as one of the criterion was conducted by Kang et al. [19] from the Korean Gynecologic Oncology Group study. In their study, serum CA-125 level with three MRI parameters (deep myometrial invasion, lymph node enlargement, and extension beyond uterine corpus) was found to be independent risk factors for nodal metastasis. This combination classified 43% of patients as low-risk, and false negative rate was 1.4% in the validation cohort. Their study was intended to develop a risk criteria that may help patient make informed decisions prior to surgery, unlike previous risk models that were based on intraoperative or final pathologic findings.
Discussion
The recommendations from major clinical investigators on the proper role of preoperative serum CA-125 levels in uterine cancer are not identical, but many of their findings are relatively consistent over time, at least for those studies performed at a single institutions with uniform surgical staging and low patient exclusion rates, and if sets aside the one attempt to integrate preoperative serum CA-125 levels with advanced radiological imaging into a "scoring system."
The figures for the overall positive and negative predictive values for preoperative serum CA-125 levels in endometrial carcinoma have been remarkably consistent over the past 25 years regardless of whether the clinical investigators were working in the United States, Europe, or Asia. That is, the vast majority of patients presenting with clinically advanced adenocarcinoma will have elevated preoperative serum CA-125 levels. As a rule, for patients who present with disease clinically confined to the uterus 75% to 85% of patients will have normal preoperative serum CA-125 levels, and approximately 15% to 25% of patients will have elevated levels of whom approximately 75% will have metastatic disease found on final pathology from surgical staging.
It is within this latter group of higher-risk patients for metastatic disease that more recent work by clinical investigators in Taiwan and Korea has focused on the use of preoperative serum CA-125. Their work seems to consistently indicate that the test is of great value in helping to determine, along with grade, histology (and depth of invasion and tumor size if imaging studies are done) which patients can benefit, and which can likely safely avoid, full staging lymphadenectomy. The usefulness of the test for staging is less necessary for patients with high risk histologies such as clear cell or papillary serous carcinomas since these patients are all staged, monitored, and often treated like ovarian adenocarcinomas so it is a given that the test is routinely measured as a baseline pretreatment value in all of these patients.
Given that approximately 15% of patients with "simple" uterine adenocarinoma will have either adnexal or nodal metastases and that almost all of these will be categorized as "high risk" based on information obtained during preoperative evaluation, roughly one out of every six to seven patients should have serum CA-125 levels drawn preoperatively if other factors indicate that the patient is at higher risk for metastatic disease. Whether this number would be higher if advanced radiological imaging were also routinely performed is unknown.
The gynecologic oncology literature from three continents over a 25-year period of time evaluating the predictive value of preoperative serum CA-125 levels in uterine cancer has consistently shown that elevated levels have good to very good predictive value on identifying those patients with early clinical early-stage disease who are more likely to have occult metastases to regional lymph nodes, the adnexae, or the peritoneum. However, the evolution of clinical investigations over the past decade, particularly in Asia, now allows clinicians to employ the test in a more focused manner than simply drawing serum levels on every patient with uterine cancer prior to surgery.
In particular, routine preoperative testing such as endometrial biopsy histology and grade, cervical examination and cytology, and transvaginal sonography to assess depth of invasion and possible tumor size now allows gynecologic oncologists to classify early stage uterine cancer patients into high or low risk categories for metastatic disease. This categorization allows surgeons to identify preoperatively those patients at higher risk for occult metastases and to select those patients for full staging lymphadenectomy [24]; it is this group of patients, as well as those patients with high-risk histologies such as clear cell or uterine papillary serous carcinomas which tend to spread like ovarian epithelial cancers do who are most likely to benefit from routine measurement of preoperative serum CA-125 levels, both as a baseline and for posttreatment surveillance [25] as some of these patients will require systemic therapy postoperatively and have a significant chance of developing recurrent disease.
1. Further clinical investigations
There is still some disagreement in the literature over what value of preoperative serum CA-125 is the "best" cut-off for predicting the likelihood of finding occult metastatic disease. There is obviously a tradeoff between sensitivity and specificity in any such determination, and preliminary work from Taiwan and Korea suggests that the ideal cut-off may depend on whether the newly diagnosed endometrial cancer patient is pre-menopausal or post-menopausal, i.e., is above or below the age of 50. This topic will be the subject of subsequent review article by one of the authors. At the present time cut-off values of 35 U/mL or 40 U/mL are most commonly used; given a 10% to 15% variation in the assays there may be little difference between these two values from a practical point of view.
If preoperative serum CA-125 is going to be routinely drawn in high-risk early stage uterine cancer patients, it is not yet definitely established whether the serum CA-125 level alone is an independent enough risk factor, based on multivariate analysis to be used to counsel patients that the serum level by itself has independent prognostic significance. While elevated serum CA-125 levels can certainly be used to advise patients that they are at higher risk for having disease spread to regional lymph nodes or the adnexae, and that they require a full surgical staging operation, it is not clear whether the degree of elevation of preoperative serum CA-125 is by itself the type of information which can be used to advise patients of their likelihood of being cured of their disease, as some but not all studies have shown this. Based on the currently available evidence, gynecologic oncologists should not use the test in this way. The presence of nodal metastases has always been shown to be an independent prognostic variable, and telling patients they may require more aggressive postoperative therapy based on this finding alone is warranted, and may be sufficient, without getting into the somewhat inconsistent data on whether the preoperative serum CA-125 level provides the same prognostic information. The suggestion that the combination of an elevated preoperative serum CA-125 and the presence of nodal metastases identifies a group of patients at particularly high risk for recurrent disease warrants further investigation to see if these criteria can be used as a way of stratifying patients in future clinical trials to different treatment arms.
It is also unclear whether serum CA-125 preoperatively should be used to identify those uterine cancer patients who are likely to benefit from advanced radiological imaging, i.e., whether routine preoperative serum CA-125 should be used as a "bridge" test to select high risk, and perhaps select low-risk, patients for more extensive preoperative radiological evaluations with MRI, CT, or positron emission tomography-computed tomography scanning. This is not a question the present review was designed to investigate. Answering this question would, at least in part, require some data on the correlation of metastatic disease sites in patients with elevated serum CA-125s who did go on to have extensive radiological evaluations to see if elevated CA-125 levels in such patients are likely to identify metastatic disease in the same locations as those in follow-on imaging. It would also require a determination of whether those few patients with occult metastatic disease with "false negative" (i.e., normal) preoperative serum CA-125s have their disease detected by radiological imaging. This is clearly an important clinical question worth addressing in future publications.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 2.Duk JM, Aalders JG, Fleuren GJ, de Bruijn HW. CA 125: a useful marker in endometrial carcinoma. Am J Obstet Gynecol. 1986;155:1097–1102. doi: 10.1016/0002-9378(86)90358-3. [DOI] [PubMed] [Google Scholar]
- 3.Patsner B, Mann WJ, Cohen H, Loesch M. Predictive value of preoperative serum CA 125 levels in clinically localized and advanced endometrial carcinoma. Am J Obstet Gynecol. 1988;158:399–402. doi: 10.1016/0002-9378(88)90163-9. [DOI] [PubMed] [Google Scholar]
- 4.Niloff JM. The role of the CA 125 assay in the management of ovarian cancer. Oncology (Williston Park) 1988;2:67–76. [PubMed] [Google Scholar]
- 5.Patsner B, Tenhoppen DJ, Mann WJ. Use of serum CA-125 levels to monitor therapy of patients with advanced or recurrent endometrial carcinoma. Eur J Gynaecol Oncol. 1989;10:322–325. [PubMed] [Google Scholar]
- 6.Mann WJ, Patsner B, Cohen H, Loesch M. Preoperative serum CA-125 levels in patients with surgical stage I invasive ovarian adenocarcinoma. J Natl Cancer Inst. 1988;80:208–209. doi: 10.1093/jnci/80.3.208. [DOI] [PubMed] [Google Scholar]
- 7.Skates SJ, Singer DE. Quantifying the potential benefit of CA 125 screening for ovarian cancer. J Clin Epidemiol. 1991;44:365–380. doi: 10.1016/0895-4356(91)90075-k. [DOI] [PubMed] [Google Scholar]
- 8.Gershenson DM, McGuire WP, Gore M, Quinn M, Thomas G. Gynecologic cancer: controversies in management. Philadelphia (PA): Elsevier Churchill Livingstone; 2004. [Google Scholar]
- 9.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebina Y, Sakuragi N, Hareyama H, Todo Y, Nomura E, Takeda M, et al. Para-aortic lymph node metastasis in relation to serum CA 125 levels and nuclear grade in endometrial carcinoma. Acta Obstet Gynecol Scand. 2002;81:458–465. doi: 10.1034/j.1600-0412.2002.810514.x. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh CH, ChangChien CC, Lin H, Huang EY, Huang CC, Lan KC, et al. Can a preoperative CA 125 level be a criterion for full pelvic lymphadenectomy in surgical staging of endometrial cancer? Gynecol Oncol. 2002;86:28–33. doi: 10.1006/gyno.2002.6664. [DOI] [PubMed] [Google Scholar]
- 12.Todo Y, Sakuragi N, Nishida R, Yamada T, Ebina Y, Yamamoto R, et al. Combined use of magnetic resonance imaging, CA 125 assay, histologic type, and histologic grade in the prediction of lymph node metastasis in endometrial carcinoma. Am J Obstet Gynecol. 2003;188:1265–1272. doi: 10.1067/mob.2003.318. [DOI] [PubMed] [Google Scholar]
- 13.Chung HH, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet Gynecol Scand. 2006;85:1501–1505. doi: 10.1080/00016340601022777. [DOI] [PubMed] [Google Scholar]
- 14.Han SS, Lee SH, Kim DH, Kim JW, Park NH, Kang SB, et al. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand. 2010;89:168–174. doi: 10.3109/00016340903370114. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Park CY, Lee JM, Lee JK, Cho CH, Kim SM, et al. Evaluation of serum CA-125 levels for preoperative counseling in endometrioid endometrial cancer: a multi-center study. Gynecol Oncol. 2010;118:283–288. doi: 10.1016/j.ygyno.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Jung DC, Park SH, Lim MC, Seo SS, Park SY, et al. Preoperative prediction model of lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2010;20:1350–1355. doi: 10.1111/IGC.0b013e3181f44f5a. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JH, Yoo SC, Kim WY, Chang SJ, Chang KH, Ryu HS. Para-aortic lymphadenectomy in the management of preoperative grade 1 endometrial cancer confined to the uterine corpus. Ann Surg Oncol. 2010;17:3234–3240. doi: 10.1245/s10434-010-1199-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen YL, Huang CY, Chien TY, Huang SH, Wu CJ, Ho CM. Value of pre-operative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust N Z J Obstet Gynaecol. 2011;51:397–402. doi: 10.1111/j.1479-828X.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Kang WD, Chung HH, Jeong DH, Seo SS, Lee JM, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean Gynecologic Oncology Group study. J Clin Oncol. 2012;30:1329–1334. doi: 10.1200/JCO.2011.38.2416. [DOI] [PubMed] [Google Scholar]
- 20.Niloff JM, Klug TL, Schaetzl E, Zurawski VR, Jr, Knapp RC, Bast RC., Jr Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol. 1984;148:1057–1058. doi: 10.1016/s0002-9378(84)90444-7. [DOI] [PubMed] [Google Scholar]
- 21.Soper JT, Berchuck A, Olt GJ, Soisson AP, Clarke-Pearson DL, Bast RC., Jr Preoperative evaluation of serum CA 125, TAG 72, and CA 15-3 in patients with endometrial carcinoma. Am J Obstet Gynecol. 1990;163:1204–1209. doi: 10.1016/0002-9378(90)90692-z. [DOI] [PubMed] [Google Scholar]
- 22.Dotters DJ. Preoperative CA 125 in endometrial cancer: is it useful? Am J Obstet Gynecol. 2000;182:1328–1334. doi: 10.1067/mob.2000.106251. [DOI] [PubMed] [Google Scholar]
- 23.Sood AK, Buller RE, Burger RA, Dawson JD, Sorosky JI, Berman M. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstet Gynecol. 1997;90:441–447. doi: 10.1016/s0029-7844(97)00286-x. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer NL, Lurain JR. The role of lymphadenectomy in surgical staging of endometrial cancer. Int J Surg Oncol. 2011;2011:814649. doi: 10.1155/2011/814649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patsner B, Orr JW, Jr, Mann WJ., Jr Use of serum CA 125 measurement in posttreatment surveillance of early-stage endometrial carcinoma. Am J Obstet Gynecol. 1990;162:427–429. doi: 10.1016/0002-9378(90)90400-2. [DOI] [PubMed] [Google Scholar]


