Abstract
Malignant mixed müllerian tumor (MMMT) arising from female internal genitalia is rare with the uterine corpus being the most prevalently affected site. It is even more rare when it occurs on both uterus and ovary at the same time. We describe a case of synchronized occurrence of MMMT on ovary and uterine adenosarcoma with review of literature.
Keywords: Malignant mixed müllerian tumor, Ovary, Uterus
Introduction
Malignant mixed müllerian tumor (MMMT) arising from female internal genitalia is rare with the uterine corpus being the most prevalently affected site. Ovarian MMMT contains malignant epithelial and stromal elements comprising less than 1% of ovarian malignancies [1]. Uterine adenosarcoma is a rare variant of uterine mixed müllerian tumor the prevalence of which is only 8% of uterine sarcoma [2]. It is characterized by a mixture of benign glandular epithelium and a malignant sarcomatous stroma resembling that of endometrial stromal sarcoma or mixed müllerian tumors, which are regarded as low-grade sarcoma [3].
We describe a case of synchronized occurrence of MMMT on both ovary and uterus with review of literature.
Case Report
A 57-year-old woman was admitted on January 2011 because of irregular vaginal bleeding for the last 10 months and recently aggravating in amount. She experienced the menopause on her age of 50. She had visited other clinic on June 2010 and was advised to follow up for the uterine tumor. Her family history was not contributory. Vital signs were within normal range and consciousness was clear. On physical examination, no abdominal tenderness or rebound tenderness was identified. Necrotic tissue covered with blood clot was noticed on cervix observed through vaginal speculum. The results of complete blood count, urinalysis, serologic tests, electrocardiogram, and chest X-ray were within normal range. Serum CA-125 and CA-19-9 levels were 12 U/mL and 2 U/mL, respectively. On abdominopelvic computed tomography (CT) examination, a mass of 8 cm in diameter arising from uterine corpus and 5.5 cm sized cystic and solid mass on right ovary were identified (Fig. 1).
Fig. 1.
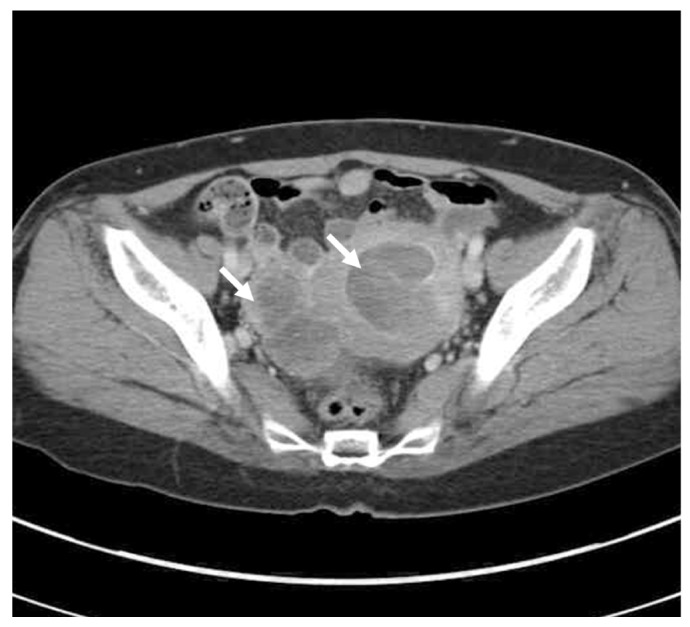
Computed tomography showing a huge sized mass in uterine cavity. And 5.5-cm sized cystic and solid mass in right adnexa (white arrows).
Explo-laparotomy was performed on February 2011 under the impression of endometrial cancer with ovarian metastasis. Low midline incision was placed under general anesthesia. Right ovarian mass was fixed on the posterior aspect of uterus and rectal wall by dense adhesive band and surrounded by necrotic tissue debris and blood clot. Extrafascial hysterectomy and bilateral adnexectomy was performed. Right ovarian mass was ruptured during the manipulation. Poorly differentiated sarcoma was suspected from frozen biopsy of both uterus and right ovary. Left adnexa was grossly free of tumor. However, multiple metastases of diameter 0.5 to 2 cm were identified on greater omentum. Partial omentectomy and appendectomy was performed. Small metastatic nodules were identified and all removed from right pelvic wall and mesentery of small intestine. No gross residual tumor was left.
On gross examination, huge polypoid mass of 8×4 cm was identified in uterine cavity and two myomatous nodule in myometrium. Cervix and both fallopian tubes were free of tumor (Fig. 2A). On microscopic examination, endometrial tumor was composed of mostly invasive cells with large and polymorphic nucleus with pale and distinct plasmid. Sarcomatous component was overwhelming and included small area of adenomatous component which was attributed as adenosarcoma of MMMT (Fig. 2B). Immunohistochemical staining with CD10 of both ovary and uterus was positive. The tumor arising from ovary was mostly composed of sarcoma while the individual cells looked different from those of uterine tumor and did not include adenomatous component (Fig. 2C). The metastatic nodules showed the histologic feature that are similar to that of the ovarian tumor suggesting the metastases originated from the ovary. This case was diagnosed as primary MMMT arising from uterus and from ovary as well.
Fig. 2.
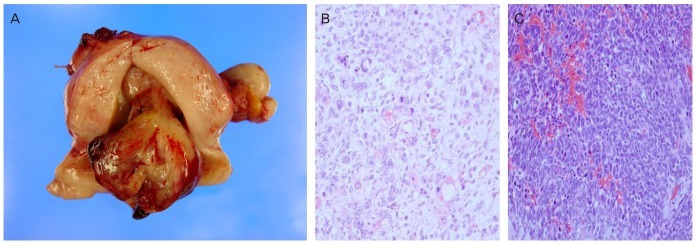
Gross and microscopic findings. (A) Gross findings after surgery: 8×5-cm sized polypoid mass in uterine cavity. (B) The uterine mass shows mainly diffuse sheets of anaplastic sarcomatous components with scattered benign looking glandular components (H&E, ×200). (C) The ovarian mass shows diffuse sheets of mixed adenocarcinoma and anaplastic sarcomatous component (H&E, ×200).
Oral intake was initiated on postoperative day 3 and no significant complication was noticed. She was discharged on day 9 and three cycles of chemotherapy with paclitaxel 175 mg/m2 plus carboplatin with area under the curve (AUC) 5. The follow-up was lost because she refused further treatment.
Discussion
MMMT arising from female genital organ is a very rare tumor and affects mostly uterine corpus followed by cervix, vagina, ovary, and fallopian tube [2]. MMMT comprises 10% of ovarian tumors and 5% of uterine tumors and it is even more rare to be found in both uterus and ovary [4]. In 1985, Ulbright and Roth [5] firstly suggested the pathological criteria to classify the independent malignancies arising concurrently from individual sites. The relationship between the tumors in individual site is not always clear. However, it is evident that the tumors are independent such as this case when the histologic findings of the tumors are different.
Uterine adenosarcoma develops mostly in postmenopausal women. However, one third affects premenopausal women including the teens [3]. Eighty percent of ovarian MMMT also affects postmenopausal women majority of which lies on 50 to 70 of age [1]. Abnormal uterine bleeding is the most frequent clinical presentation followed by low abdominal pain, cervical mass, and increase in uterine size. Cervical MMMT typically resembles polyp or submucosal myoma filling uterine cavity and extends into the cervix [3]. MMMT is usually diagnosed at early stage that stage III or IV comprises only 11% of all cases [6]. However, the recurrence and death rate is reported to be 30% to 40% and 20% to 55%, respectively. The death rate of uterine adenosarcoma is 65% and tends to diagnosed in earlier stage than carcinosarcoma [6]. The symptoms of ovarian MMMT is identical to those of advanced epithelial ovarian cancer such as abdominal distention, pelvic mass, and belching [1]. Peritoneal seeding is a common finding in ovarian MMMT accompanying ascites in 67% to 100% [6,7]. Ovarian MMMT is diagnosed as stage III or IV in 75% and metastasize to extragonadal sites in over 90% [1,7,8].
The prognostic factors of uterine adenosarcoma include stage, myometrial invasion, mitotic index, presence of heterotopic tissue, and tissue necrosis [9]. Deep myometrial invasion and extrauterine metastasis affects more adversely and deep myometrial invasion is the only prognostic factor for the recurrence of uterine adenosarcoma. Tumor is limited to endometrium in most cases and myometrial invasion is observed in 15% while deep myometrial invasion is reported only in 4%. Extrauterine metastasis occurs in less than 50% [3].
The prognostic factors of ovarian MMMT are reported to be the age at presentation, insufficient surgical removal, and the stage. The recurrence rate is 50% in stage I and up to 90% to 100% in stage II or greater [7].
Preoperative diagnosis is possible for uterine adenosarcoma by endometrial biopsy. However, ovarian MMMT is not diagnosed before surgery and the incidence is extremely rare. Thus the treatment strategy for ovarian MMMT is not established to date and maximal surgical resection and adjuvant chemotherapy is generally performed [1,10]. Optimal cytoreduction resulted in longer recurrence-free interval and median survival [1]. Duska et al. [11] reported that 14 out of 24 who received optimal cytoreduction were associated with increased time to recurrence. Silasi et al. [12] reported that median survival was 46 months in optimally debulked patients and 27 months in suboptimally debulked. The treatment is not established for uterine adenosarcoma either but surgical resection and adjuvant chemotherapy is generally performed as well. Radiotherapy for uterine adenosarcoma may effectively control local disease without significant increase in survival rate [6].
Three to six cycles with doxorubicin, dacarbazine, and vincristine was suggested for adjuvant chemotherapy for uterine adenosarcoma but the data are limited to determine efficacy [13]. Platinum based monotherapy or combination chemotherapy with platinum plus paclitaxel or ifosphamide was reported for ovarian MMMT [1,14]. Tate Thigpen et al. [14] reported that complete response was reached in 1 out of 44 who received cisplatin monotherapy while partial response in 8, stable disease in 10, and progression in 25 was observed. Overall response was 20%, which is identical to the repose rate of uterine carcinosarcoma. Progression-free survival was 5.2 months and median survival was 11.7 months. Duska et al. [11] reported the experience of doublet with paclitaxel and carboplatin. Complete response was reached in 16 out of 28 patients while 6 patients showed partial response and 5 patients progressed. The overall response rate was 72% and median survival was 27.1 months. Of the 16 patients of complete response, 10 patients received optimal cytoreduction and had significantly longer recurrence-free survival [11]. The doublet with ifosfamide and cisplatin showed median survival of 23 months, which is improved over paclitaxel and carboplatin doublet. However, digestive complications such as nausea and vomiting and hematopoietic complications such as neutropenia and anemia were more severe in patients who treated with ifosfamide+cisplatin doublet [15]. Chemotherapies with vincristine+adriamycin+cyclophosphamide (VAC), ifosfamide+mesna, and adriamycin were reported, however, the response rate was not comparable [1,11].
We performed optimal cytoreduction and adjuvant chemotherapy with paclitaxel 175 mg/m2 and carboplatin with AUC 5. However the follow-up was lost after three cycles of chemotherapy because the patient refused further treatment. The uterine adenosarcoma and ovarian MMMT are both rare tumors and treatment strategy is not settled to date. Proper management might be established when more cases are cumulated in the future.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cantrell LA, Van Le L. Carcinosarcoma of the ovary a review. Obstet Gynecol Surv. 2009;64:673–680. doi: 10.1097/OGX.0b013e3181b8aff3. [DOI] [PubMed] [Google Scholar]
- 2.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of ten cases of a distinctive type of mullerian mixed tumor. Cancer. 1974;34:1138–1149. doi: 10.1002/1097-0142(197410)34:4<1138::aid-cncr2820340425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990;21:363–381. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- 4.Zaino R, Whitney C, Brady MF, DeGeest K, Burger RA, Buller RE. Simultaneously detected endometrial and ovarian carcinomas: a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83:355–362. doi: 10.1006/gyno.2001.6400. [DOI] [PubMed] [Google Scholar]
- 5.Ulbright TM, Roth LM. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Hum Pathol. 1985;16:28–34. doi: 10.1016/s0046-8177(85)80210-0. [DOI] [PubMed] [Google Scholar]
- 6.Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, et al. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol. 2010;119:305–308. doi: 10.1016/j.ygyno.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Brown E, Stewart M, Rye T, Al-Nafussi A, Williams AR, Bradburn M, et al. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004;100:2148–2153. doi: 10.1002/cncr.20256. [DOI] [PubMed] [Google Scholar]
- 8.Silverman JF, Gardner J, Larkin EW, Finley JL, Norris HT. Ascitic fluid cytology in a case of metastatic malignant mixed mesodermal tumor of the ovary. Acta Cytol. 1986;30:173–176. [PubMed] [Google Scholar]
- 9.Zaloudek CJ, Norris HJ. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer. 1981;48:354–366. doi: 10.1002/1097-0142(19810715)48:2<354::aid-cncr2820480222>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Sood AK, Sorosky JI, Gelder MS, Buller RE, Anderson B, Wilkinson EJ, et al. Primary ovarian sarcoma: analysis of prognostic variables and the role of surgical cytoreduction. Cancer. 1998;82:1731–1737. [PubMed] [Google Scholar]
- 11.Duska LR, Garrett A, Eltabbakh GH, Oliva E, Penson R, Fuller AF. Paclitaxel and platinum chemotherapy for malignant mixed mullerian tumors of the ovary. Gynecol Oncol. 2002;85:459–463. doi: 10.1006/gyno.2002.6645. [DOI] [PubMed] [Google Scholar]
- 12.Silasi DA, Illuzzi JL, Kelly MG, Rutherford TJ, Mor G, Azodi M, et al. Carcinosarcoma of the ovary. Int J Gynecol Cancer. 2008;18:22–29. doi: 10.1111/j.1525-1438.2007.00948.x. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Liu Z, Peng Z, Liu H, Yang K, Yao X. The diagnosis and treatment of Mullerian adenosarcoma of the uterus. Aust N Z J Obstet Gynaecol. 2008;48:596–600. doi: 10.1111/j.1479-828X.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 14.Tate Thigpen J, Blessing JA, DeGeest K, Look KY, Homesley HD Gynecologic Oncology Group. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;93:336–339. doi: 10.1016/j.ygyno.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Sutton GP, Blessing JA, Homesley HD, Malfetano JH. A phase II trial of ifosfamide and mesna in patients with advanced or recurrent mixed mesodermal tumors of the ovary previously treated with platinum-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 1994;53:24. doi: 10.1006/gyno.1994.1081. [DOI] [PubMed] [Google Scholar]


