 |
James Duffin is currently a Professor Emeritus in the Departments of Anaesthesia and Physiology at the University of Toronto, and a senior research scientist at Thornhill Research and the University Health Network. His research interests include the physiology of the control of breathing during exercise, and by the respiratory chemoreflexes, the neurophysiology of the generation of respiratory rhythm, the physiological control of cerebral blood flow, and computer simulations of these aspects. Jason H. Mateika is a Professor in the Department of Physiology at Wayne State University and a Research Career Scientist in Research and Development at John D. Dingell VA Medical Center. Dr. Mateika is interested in the control of breathing during quiet wake, sleep and exercise. His research focuses on the role that two forms of respiratory plasticity (i.e. long-term facilitation and progressive augmentation) have in the control breathing during wake and sleep in healthy humans and individuals with sleep apnea.
Introduction
In humans respiratory chemoreceptors are located centrally in the medulla (Nattie, 2010) and peripherally in the carotid bodies (Torrance, 1996; Kumar & Bin-Jaliah, 2007). Does the interaction between hypoxia and carbon dioxide (CO2) occur in the medulla between the central and peripheral chemoreceptor signals or is it within the peripheral chemoreceptors? Several observations are pertinent to this question. Hypoxia does not alter ventilation if the partial pressure of CO2 ( ) is below a threshold (Mohan & Duffin, 1997), and hypoxia is not an independent drive to breathe but depends on the presence of a CO2 stimulus (Torrance, 1996). Experiments with carotid body resected individuals show that hypoxia has little respiratory influence (Dahan et al. 2007), thus hypoxia is sensed by the carotid bodies not the central chemoreceptors. In hyperoxia, the ventilatory response to a step increase in CO2 tension is slow, approximating the time course of central CO2 tension, whereas the response to CO2 in hypoxia is much faster (Pedersen et al. 1999); hence, the influence of hypoxia on the response occurs at a location in rapid equilibration with arterial blood. Similar differences are found in the ventilatory responses to rapid bicarbonate administration (Whitwam et al. 1976), and rapid changes in CO2 (Cunningham et al. 1986). These various experiments support a peripheral location for the interaction of hypoxia and CO2; in hypoxia, the peripheral chemoreceptors are sensitive to CO2.
) is below a threshold (Mohan & Duffin, 1997), and hypoxia is not an independent drive to breathe but depends on the presence of a CO2 stimulus (Torrance, 1996). Experiments with carotid body resected individuals show that hypoxia has little respiratory influence (Dahan et al. 2007), thus hypoxia is sensed by the carotid bodies not the central chemoreceptors. In hyperoxia, the ventilatory response to a step increase in CO2 tension is slow, approximating the time course of central CO2 tension, whereas the response to CO2 in hypoxia is much faster (Pedersen et al. 1999); hence, the influence of hypoxia on the response occurs at a location in rapid equilibration with arterial blood. Similar differences are found in the ventilatory responses to rapid bicarbonate administration (Whitwam et al. 1976), and rapid changes in CO2 (Cunningham et al. 1986). These various experiments support a peripheral location for the interaction of hypoxia and CO2; in hypoxia, the peripheral chemoreceptors are sensitive to CO2.
Therefore, when CO2 increases in isoxic hypoxia both the peripheral and central chemoreceptor responses increase. If the peripheral and central response components add then the ventilatory response has a linear dependence on 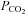 . If the peripheral response interacts with the central response by changing its sensitivity then the ventilatory response becomes non-linear. If the interaction is positive, so that the rising peripheral response increases the central sensitivity, then the combined response will curve upwards, and vice versa if the interaction is negative. Nielsen & Smith (1952) published the first isoxic ventilatory responses to carbon dioxide. They showed linear responses whose slope increased in hypoxia. This finding was quickly verified by others and eventually the responses were characterized as the ‘Oxford’ fan (Cunningham et al. 1986). These steady-state experiments have been replicated by many others and a similar result was found using rebreathing (Mohan & Duffin, 1997). Thus, experimental measurements of the isoxic ventilatory response to CO2 is linear in most individuals (Fig. 1), whether measured using steady-state (Cunningham et al. 1986) or rebreathing methods (Mohan & Duffin, 1997). Most modellers have therefore assumed an additive central–peripheral interaction (e.g. Cheng et al. 2010) and additive models have been used to separate central and peripheral elements of ventilatory responses to CO2. In some individuals the isoxic hypoxic ventilatory responses to CO2 may be characterized by a parabolic fit, indicating a positive interaction, as discussed in Duffin (2010); nevertheless such responses are usually linear in most individuals.
. If the peripheral response interacts with the central response by changing its sensitivity then the ventilatory response becomes non-linear. If the interaction is positive, so that the rising peripheral response increases the central sensitivity, then the combined response will curve upwards, and vice versa if the interaction is negative. Nielsen & Smith (1952) published the first isoxic ventilatory responses to carbon dioxide. They showed linear responses whose slope increased in hypoxia. This finding was quickly verified by others and eventually the responses were characterized as the ‘Oxford’ fan (Cunningham et al. 1986). These steady-state experiments have been replicated by many others and a similar result was found using rebreathing (Mohan & Duffin, 1997). Thus, experimental measurements of the isoxic ventilatory response to CO2 is linear in most individuals (Fig. 1), whether measured using steady-state (Cunningham et al. 1986) or rebreathing methods (Mohan & Duffin, 1997). Most modellers have therefore assumed an additive central–peripheral interaction (e.g. Cheng et al. 2010) and additive models have been used to separate central and peripheral elements of ventilatory responses to CO2. In some individuals the isoxic hypoxic ventilatory responses to CO2 may be characterized by a parabolic fit, indicating a positive interaction, as discussed in Duffin (2010); nevertheless such responses are usually linear in most individuals.
Figure 1.
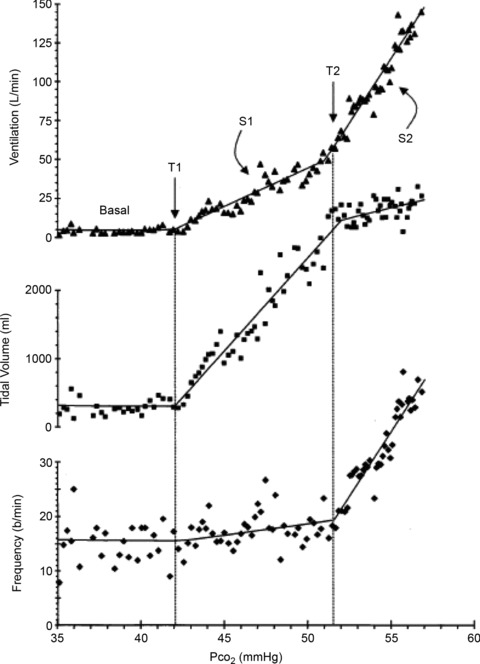
Breath-by breath measurements of ventilation, tidal volume and frequency obtained during a Duffin modified rebreathing test at an isoxic 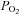 = 40 mmHg from Duffin et al. (2000). T1 is the ventilatory recruitment threshold and T2 a patterning threshold. Although the ventilatory response might be fitted with a parabola indicating a hyperadditive interaction, the tidal volume and frequency measurements show that division into two linear segments is appropriate
= 40 mmHg from Duffin et al. (2000). T1 is the ventilatory recruitment threshold and T2 a patterning threshold. Although the ventilatory response might be fitted with a parabola indicating a hyperadditive interaction, the tidal volume and frequency measurements show that division into two linear segments is appropriate
Interaction experiments
How else could the central–peripheral interaction be tested? Human experiments are often non-invasive. Consequently, differences in chemoreflex response times are frequently used to separate the central and peripheral components. An early experiment of this sort by Robbins (1988) concluded that central–peripheral interaction was more than additive. However, the same protocol was repeated by St Croix et al. (1996), who concluded that in four of the five subjects studied no interaction was evident. In similar experiments involving exercise it was concluded that a central–peripheral interaction was absent (Clement et al. 1992) because the dynamics of the peripheral and central chemoreflex pathways appeared to be largely independent of each other (Macfarlane & Cunningham, 1992), and because the hypoxic–CO2 interaction was mediated solely by the peripheral chemoreceptors (Yang & Khoo, 1994).
Recently Cui et al. (2011) used a different temporal sequence to separate central and peripheral responses. Instead of using a period of sustained central hypercapnia, hyperventilation was used to produce a sustained central hypocapnia. With central CO2 tension below the ventilatory recruitment threshold the central response could be eliminated altogether. After hyperventilation one of two stimuli were used: a step increase in CO2 tension during hypoxia, or normoxia. The difference in responses yielded a peripheral response to hypoxia during a low or absent central ventilatory drive. This hypoxic response during central hypocapnia was compared to the response to hypoxia during central hypercapnia. These hypoxic responses were not different in 10 subjects; central–peripheral interaction was additive.
Long-term facilitation involvement
It therefore appears that experiments in humans to detect a peripheral modulation of central chemoreception were unable to do so. Nevertheless, the finding of possible parabolic isoxic CO2 responses in some individuals (Duffin, 2010) suggests that other factors may alter the central–peripheral interaction, such as long-term facilitation which enhances carotid sinus and phrenic nerve activities. Findings from early work in cats (Eldridge et al. 1981) suggested the central interaction between peripheral and central chemoreflexes, combined with long-term facilitation, is negative; possibly a consequence of saturation of an inter-neuronal pool receiving the convergence of these signals. Indeed, experiments in rats but without long-term facilitation also show a negative central–peripheral interaction (Day & Wilson, 2009). But in contrast to Eldridge's findings, recent studies in rats show that central chemosensitivity and central responses to hypercapnia in the presence of long-term facilitation are enhanced in a multiplicative manner (Molkov et al. 2011).
These animal experiments show that interaction between central and peripheral chemoreceptor signals and long-term facilitation could occur at a number of sites within the medulla (Guyenet & Mulkey, 2010). But whether such interactions are negative, additive or multiplicative in humans requires additional investigation. Nevertheless, Mateika et al. (2004) showed, using the Duffin modified rebreathing technique, that during isoxic hypoxic rebreathing the sensitivity of the response is enhanced after exposure to intermittent hypoxia, a stimulus known to initiate long-term facilitation. Moreover, the response in some individuals was non-linear. Thus, the central–peripheral interaction under these conditions might be multiplicative.
Sympathetic involvement
Such multiplicative interactions as a consequence of long-term facilitation may involve sympathetic responses. Sympathetic excitation occurs with hypercapnia with a central (Pitsikoulis et al. 2008) as well as a peripheral chemoreflex contribution (Shoemaker et al. 2002). Furthermore, Dahan et al. (2007) concluded that the carotid bodies exert a tonic facilitation of the central chemoreflex that is lost after resection. Lastly, Battisti-Charbonney et al. (2011) recently observed that as CO2 tension exceeded a threshold, blood pressure increased; probably due to a sympathetic activation. Nevertheless, it remains unclear whether facilitation of the central and peripheral chemoreceptor interaction is responsible for enhancement of sympathetic nervous system activity or vice versa. Thus, the non-linear increases in ventilation in some individuals mentioned earlier could be the consequence of multiplicative interactions between the central and peripheral chemoreceptors induced by long-term facilitation directly or indirectly via a sympathetic response. Further experimentation is needed to explore this possibility.
Conclusion
We argue that, taken altogether, evidence from human experiments shows that under many circumstances the peripheral and central chemoreflexes have additive effects on ventilation in most individuals, with anything greater than additive occurring only as a consequence of circumstances where other, as yet unknown, factors may apply.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/18/4351
Acknowledgments
The authors note that due to space limits citations of many excellent studies could not be included. There are no conflicts of interest to disclose.
References
- Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011;589:3039–3048. doi: 10.1113/jphysiol.2011.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ivanova O, Fan HH, Khoo MC. An integrative model of respiratory and cardiovascular control in sleep-disordered breathing. Respir Physiol Neurobiol. 2010;174:4–28. doi: 10.1016/j.resp.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement ID, Bascom DA, Conway J, Dorrington KL, O’connor DF, Painter R, Paterson DJ, Robbins PA. An assessment of central–peripheral ventilatory chemoreflex interaction in humans. Respir Physiol. 1992;88:87–100. doi: 10.1016/0034-5687(92)90031-q. [DOI] [PubMed] [Google Scholar]
- Cui Z, Fisher JA, Duffin J. Central–peripheral respiratory chemoreflex interaction in humans. Respir Physiol Neurobiol. 2011;180:126–131. doi: 10.1016/j.resp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Cunningham DJC, Robbins PA, Wolff CB. Integration of respiratory responses to changes in alveolar partial pressures of CO2 and O2 and in arterial pH. In: Fishman AP, Cherniack NS, Widdicombe JG, editors. Handbook of Physiology. Bethesda, MD, USA: American Physiological Society; 1986. pp. 475–528. section 3, The Respiratory System, vol. II. [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Wilson RJA. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. The role of the central chemoreceptors: A modelling perspective. Respir Physiol Neurobiol. 2010;173:230–243. doi: 10.1016/j.resp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respir Physiol. 2000;120:13–26. doi: 10.1016/s0034-5687(00)00095-5. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: More than an oxygen sensor. Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Macfarlane DJ, Cunningham DJ. Dynamics of the ventilatory response in man to step changes of end-tidal carbon dioxide and of hypoxia during exercise. J Physiol. 1992;457:539–557. doi: 10.1113/jphysiol.1992.sp019393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol. 2004;96:1197–1205. doi: 10.1152/japplphysiol.00573.2003. [DOI] [PubMed] [Google Scholar]
- Mohan R, Duffin J. The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respir Physiol. 1997;108:101–115. doi: 10.1016/s0034-5687(97)00024-8. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then … and now. J Appl Physiol. 2010;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M, Smith H. Studies on the regulation of respiration in acute hypoxia. Acta Physiol Scand. 1952;24:293–313. doi: 10.1111/j.1748-1716.1952.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Pedersen ME, Fatemian M, Robbins PA. Identification of fast and slow ventilatory responses to carbon dioxide under hypoxic and hyperoxic conditions in humans. J Physiol. 1999;521:273–287. doi: 10.1111/j.1469-7793.1999.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikoulis C, Bartels MN, Gates G, Rebmann RA, Layton aM, De Meersman RE. Sympathetic drive is modulated by central chemoreceptor activation. Respir Physiol Neurobiol. 2008;164:373–379. doi: 10.1016/j.resp.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Robbins PA. Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man. J Physiol. 1988;401:503–518. doi: 10.1113/jphysiol.1988.sp017175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix CM, Cunningham DA, Paterson DH. Nature of the interaction between central and peripheral chemoreceptor drives in human subjects. Can J Physiol Pharmacol. 1996;74:640–646. doi: 10.1139/cjpp-74-6-640. [DOI] [PubMed] [Google Scholar]
- Shoemaker J, Vovk A, Cunningham D. Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol. 2002;80:1136–1144. doi: 10.1139/y02-148. [DOI] [PubMed] [Google Scholar]
- Torrance RW. Prolegomena. Chemoreception upstream of transmitters. In: Zapata, editor. Frontiers in Arterial Chemoreception. Vol. 410. New York: Plenum Press; 1996. pp. 13–38. [PubMed] [Google Scholar]
- Whitwam JG, Duffin J, Triscott A, Lewin K. Stimulation of the peripheral chemoreceptors with sodium bicarbonate. Brit J Anaesth. 1976;48:853–857. doi: 10.1093/bja/48.9.853. [DOI] [PubMed] [Google Scholar]
- Yang F, Khoo MC. Ventilatory response to randomly modulated hypercapnia and hypoxia in humans. J Appl Physiol. 1994;76:2216–2223. doi: 10.1152/jappl.1994.76.5.2216. [DOI] [PubMed] [Google Scholar]


