 |
Richard J. A. Wilson holds a BSc in Neurobiology from the University of Sussex. Recipient of a Wellcome Price Studentship, his PhD at the University of Glasgow investigated neuronal circuits. Following postdocs at UCSD, NYMC and the University of Calgary, and supported by the Parker B Francis Foundation and Alberta Innovates Health Solutions (AIHS), he established a laboratory at the University of Calgary in 2002. Richard is an AIHS Senior Scholar and co-directs a graduate department consisting of 90 neuroscience students. His research program, spanning from genes to behaviour and from fish to humans, focuses on neuronal circuits involved in cardiorespiratory control. Trevor A. Day obtained a B.Sc. in Neuropsychology from the University of Calgary. Funded by CIHR, he obtained a Ph.D. in Respiratory Neurobiology, also from the University of Calgary, studying the control of breathing in animal preparations with Dr. Wilson. He is now an Associate Professor of Physiology at Mount Royal University in Calgary, Canada, where his research program focuses on integrative cardiorespiratory and cerebrovascular physiology in humans. He also has an active interest in science communication, particularly how scientists can better engage the public in the importance and relevance of the scientific endeavour.
Introduction
Breathing is all-important for survival, yet key aspects of the control system remain hidden from the gaze of consensus, not least the issue of central and peripheral chemoreceptor interaction. Activation (or inactivation) of either chemoreceptor alone will increase (or decrease) ventilation, but it remains unclear how the activation state of one chemoreceptor modality affects the chemoreflex magnitude of the other (i.e. how inputs interact). Recent investigations consider three possibilities (e.g. Day & Wilson, 2009; Blain et al. 2010; Forster & Smith, 2010; Smith et al. 2010; Cui et al. 2012; Tin et al. 2012). Below, we consider four possibilities, three of which implicate some degree of hypoadditive interaction.
Additive interaction
Most models of respiratory control assume central and peripheral chemoreceptor inputs simply sum (e.g. Heeringa et al. 1979). Simple addition is supported by several animal studies (e.g. van Beek et al. 1983, Daristotle & Bisgard, 1989) and compelling evidence from characterization of human ventilatory response dynamics that assume a fast (peripheral) and slow (central) component (e.g. Clement et al. 1992; St Croix et al. 1996; Cui et al. 2012). However, conclusions of simple addition must take into account that (a) accuracy of using temporal dynamics to define contribution of chemoreceptors is limited to rapid changes (e.g. slow peripheral chemoreflex responses may be wrongly attributed to the central component), (b) systemic changes in blood gases affect multiple physiological systems beyond direct effects on chemoreceptors (including possible baroreflex-chemoreflex interactions; e.g. McMullan & Pilowsky, 2010) and (c) algebra dictates that simple addition observed in minute ventilation must necessitate a hypoadditive interaction in tidal volume and/or frequency (Mitchell, 1990).
Hyperadditive interaction
A second possibility is hyperadditive (i.e. multiplicative) interaction, whereby activating (or removing) one chemoreceptor augments (or reduces) the response of the other. An example is provided by the well-established O2–CO2 interaction mediated by the carotid body, which translates to ventilation (e.g. Lahiri & DeLaney, 1975). A strong case for hyperadditive central–peripheral interaction was made recently by elegant experiments in awake dogs by Blain et al. (2010). Using a similar preparation to that used in awake goats to show an additive interaction (Daristotle & Bisgard, 1989), a single extracorporeally perfused carotid body (other carotid body denervated) was maintained at different levels of steady-state activation and inactivation, while systemic (assumed central chemoreflex) responses to increases in inspired 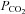 were tested. This stimulus order is reversed to that of intact animals where the peripheral chemoreflex has a faster response than the central, leading to suggestions that presentation order accounts for the observed hyperadditivity (Tin et al. 2012). In addition, in order to retrogradely perfuse the carotid sinus region, perfusion pressure was elevated above systemic blood pressure, apparently without arterial baroreflex activation, as systemic blood pressure was unchanged.
were tested. This stimulus order is reversed to that of intact animals where the peripheral chemoreflex has a faster response than the central, leading to suggestions that presentation order accounts for the observed hyperadditivity (Tin et al. 2012). In addition, in order to retrogradely perfuse the carotid sinus region, perfusion pressure was elevated above systemic blood pressure, apparently without arterial baroreflex activation, as systemic blood pressure was unchanged.
This required the authors to argue that any retrogradely perfused blood reaching brainstem and aortic chemoreceptors was negligible. Other concerns, also largely placated by the authors, include (a) having one perfused and one denervated carotid body is functionally different to having two intact carotid bodies (e.g. Fatemian et al. 2003) and (b) ventilatory responses to changes in inspired 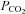 may not be solely via direct effects on central chemoreceptors (e.g. McMullan & Pilowsky, 2010).
may not be solely via direct effects on central chemoreceptors (e.g. McMullan & Pilowsky, 2010).
Hypoadditive interaction
A third possibility is hypoadditive (i.e. negative) interaction, in which the sum of responses from each chemoreceptor compartment is less than the mathematical sum of independent responses. This is akin to a system with a high degree of redundancy, where the power of one chemoreceptor is most apparent when the influence of the other is reduced or eliminated. Redundancy is ubiquitous in biological systems crucial for homeostasis (Poon & Siniaia, 2000; Joyner, 2013).
Using the dual-perfused rat preparation (DPP; Day & Wilson, 2005), we demonstrated a robust hypoadditive interaction between carotid body and brainstem chemoreceptors. Reducing steady-state brainstem 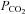 increased phrenic responses to changes in specific carotid body
increased phrenic responses to changes in specific carotid body 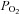 or
or 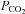 , and increasing brainstem
, and increasing brainstem 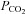 had the opposite effect (Day & Wilson, 2007, 2009).
had the opposite effect (Day & Wilson, 2007, 2009).
Unique among preparations used in attempts to resolve the interaction controversy, the DPP allows the environment of the carotid body and brainstem chemoreceptors to be artificially perfused without non-specific ventilatory effects of anaesthetic or gas challenges on systemic circulation, descending influences, vagal input, or other nervous and endocrine effects. To accomplish this, the DPP is decerebrate and vagotomized, two key caveats that compromise sensory and descending inputs (including influence of hypothalamic chemoreceptors; Nattie, 2011), potentially changing the dynamic range of the system. However, data from other groups using conscious and anaesthetized rat preparations corroborate the existence of a hypoadditive interaction with the hallmark of redundancy. Tin et al. (2012) report that increasing systemic CO2 blunts hypoxic responses in conscious and anaesthetized rats despite the known hyperadditive O2–CO2 interaction within the carotid body (Tin et al. 2012). Similarly, recent data from several groups suggest rat carotid bodies are not involved in systemic CO2 chemosensitivity above eupnoea (da Silva et al. 2011; Mouradian et al. 2012), yet the fact that carotid body afferents are CO2 sensitive appears unequivocal. Also consistent with a hypoadditive system, and reminiscent of observations of the dog extracorporeally perfused carotid body model (Smith et al. 2007), we recently found that increasing carotid body stimulation in the DPP translated to a powerful drive to breath during central hypocapnia (Fiamma et al. 2013). When stimulated with hypercapnic hypoxia, peripheral chemoreceptor activation was capable of maintaining phrenic activity even as brainstem 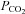 approached zero (Fiamma et al. 2013).
approached zero (Fiamma et al. 2013).
Similar observations have been made in anaesthetized cats (Berkenbosch et al. 1984). These observations are difficult to reconcile with a hyperadditive interaction, whereby if activation of one modality is miniscule, the influence of the other modality should also be miniscule.
Hybrid model
A fourth possible form of interaction is a hybrid model, whereby additive, hyperadditive and hypoadditive interactions are all possible, with the form of interaction dependent upon behaviour and/or metabolism. For example, sleep, arousal, temperature, inflammation, exercise, experience and a host of other factors working through autocrine, paracrine and endocrine modulators likely have the ability to differentially affect chemoreflexes and how they are integrated. We note that a hybrid model is consistent with the principle of redundancy and a system that requires a large dynamic range. Thus, below eupnoea when the system is most vulnerable, central and peripheral chemoreceptors might form a redundant system to protect from and respond to apnoea, whereas above eupnoea they may act more synergistically, expanding the overall range of responses in time and magnitude domains to help maintain blood gases during diverse metabolic and behavioural demands (Fig. 1). We suggest this possibility may be a useful paradigm to design and interpret experiments.
Figure 1.
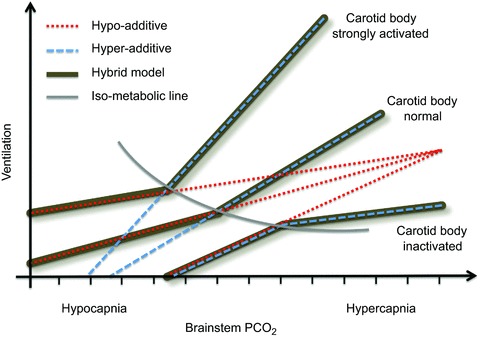
Using the dual-perfused rat preparation, we found evidence for a hypoadditive interaction between central and peripheral chemoreceptors, which was most pronounced when the brainstem was hypocapnic (converging red dotted lines; Day & Wilson, 2007, 2009; Fiamma et al. 2013). Using the extracorporeally perfused carotid body awake dog preparation, Blain et al. (2010) found evidence for a hyperadditive interaction when systemic 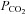 was above eucapnic levels (diverging blue dashed lines). Superimposing these findings (thick dark lines) yields a hybrid system, whereby the breaking point between interaction types is approximately the iso-metabolic line (grey arc). This hybrid model may offer new hope in resolving the interaction controversy.
was above eucapnic levels (diverging blue dashed lines). Superimposing these findings (thick dark lines) yields a hybrid system, whereby the breaking point between interaction types is approximately the iso-metabolic line (grey arc). This hybrid model may offer new hope in resolving the interaction controversy.
Conclusion
The observed interaction between chemoreceptors may depend upon factors such as species differences, preparation utilized (e.g. afferents intact or removed) and experimental protocol (e.g. order and duration of compartment stimulated). In addition, temporal domains (fast vs. slow), chemoreceptor stimulation or inhibition and the fact that physiological responses will have both a threshold and an asymptote (i.e. saturation), may also contribute to the range of observed responses. However, notwithstanding the importance of these considerations when interpreting or planning experimental work, we suspect that the solution to the interaction controversy lies in a hybrid model that may favour hypoadditive interaction below eupnoea and hyperadditive interaction above eupnoea.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/18/4355
Acknowledgments
This work was supported by Alberta Innovates-Health Solutions and Canadian Institutes of Health Research (R.J.A.W.) and a Mount Royal University sabbatical (T.A.D.).
References
- Berkenbosch A, van Beek JH, Olievier CN, De Goede J, Quanjer PH. Central respiratory CO2sensitivity at extreme hypocapnia. Respir Physiol. 1984;55:95–102. doi: 10.1016/0034-5687(84)90119-1. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement ID, Bascom DA, Conway J, Dorrington KL, O’Connor DF, Painter R, Paterson DJ, Robbins PA. An assessment of central–peripheral ventilatory chemoreflex interaction in humans. Respir Physiol. 1992;88:87–100. doi: 10.1016/0034-5687(92)90031-q. [DOI] [PubMed] [Google Scholar]
- Cui Z, Fisher JA, Duffin J. Central–peripheral respiratory chemoreflex interaction in humans. Respir Physiol Neurobiol. 2012;180:126–131. doi: 10.1016/j.resp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Daristotle L, Bisgard GE. Central–peripheral chemoreceptor ventilatory interaction in awake goats. Respir Physiol. 1989;76:383–391. doi: 10.1016/0034-5687(89)90078-9. [DOI] [PubMed] [Google Scholar]
- da Silva GSF, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LGS, Glass ML. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflügers Arch. 2011;462:407–418. doi: 10.1007/s00424-011-0990-x. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Specific carotid body chemo-stimulation is sufficient to elicit phrenic post-stimulus frequency decline in a novel in situ dual perfused rat preparation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R532–R544. doi: 10.1152/ajpregu.00812.2004. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Brainstem PCO2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol. 2007;578:843–857. doi: 10.1113/jphysiol.2006.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Wilson RJA. A negative interaction between central and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemian M, Nieuwenhuijs DJ, Teppema LJ, Meinesz S, van der Mey AG, Dahan A, Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol. 2003;549:965–973. doi: 10.1113/jphysiol.2003.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiamma MN, O’Connor ET, Roy A, Zuna I, Wilson RJ. The essential role of peripheral respiratory chemoreceptor inputs in maintaining breathing revealed when CO2stimulation of central chemoreceptors is diminished. J Physiol. 2013;591:1507–1521. doi: 10.1113/jphysiol.2012.247304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ J Appl Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa J, Berkenbosch A, DeGoede J, Olievier CN. Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respir Physiol. 1979;37:365–379. doi: 10.1016/0034-5687(79)90082-3. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Physiology and redundancy. Physiol (Bethesda) 2013;28:136–137. doi: 10.1152/physiol.00015.2013. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol. 1975;24:267–286. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- McMullan S, Pilowsky PM. The effects of baroreceptor stimulation on central respiratory drive: a review. Respir Physiol Neurobiol. 2010;174:37–42. doi: 10.1016/j.resp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Phrenic nerve responses to lung inflation and hypercapnia in decerebrate dogs. Pflugers Arch. 1990;416:580–585. doi: 10.1007/BF00382693. [DOI] [PubMed] [Google Scholar]
- Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol. 2012;590:3335–3347. doi: 10.1113/jphysiol.2012.234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then … and now. J Appl Physiol. 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CS, Siniaia MS. Plasticity of cardiorespiratory neural processing: classification and computational functions. Respir Physiol. 2000;122:83–109. doi: 10.1016/s0034-5687(00)00152-3. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Cunningham DA, Paterson DH. Nature of the interaction between central and peripheral chemoreceptor drives in human subjects. Can J Physiol Pharmacol. 1996;74:640–646. doi: 10.1139/cjpp-74-6-640. [DOI] [PubMed] [Google Scholar]
- Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol. 2007;103:578–586. doi: 10.1152/japplphysiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin C, Song G, Poon CS. Hypercapnia attenuates inspiratory amplitude and expiratory time responsiveness to hypoxia in vagotomized and vagal-intact rats. Respir Physiol Neurobiol. 2012;181:79–87. doi: 10.1016/j.resp.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek JH, Berkenbosch A, de Goede J, Olievier CN. Influence of peripheral O2tension on the ventilatory response to CO2in cats. Respir Physiol. 1983;51:379–390. doi: 10.1016/0034-5687(83)90030-0. [DOI] [PubMed] [Google Scholar]


