 |
Luc J. Teppema is associate professor of Physiology at the Department of Anaesthesiology Leiden University Medical Centre, Leiden The Netherlands. His research is focused on the effects of hypercapnia and hypoxia on the control of breathing and the effects of anaesthetics, analgesics and carbonic anhydrase inhibitors. Curtis A. Smith is a research professor in the John Rankin Laboratory of Pulmonary Medicine in the School of Medicine and Public Health, University of Wisconsin-Madison. His research focuses on peripheral/central chemoreceptor interaction as it relates to sleep apnea and the ventilatory responses to hypoxia.
Since the discovery of the O2 and CO2 respiratory chemoreceptors there has been a long debate as to their relative contributions to eupnoea and the ventilatory responses to hypoxia and hypercapnia. Recent evidence suggests that attempting to assign relative contributions to the central and peripheral chemoreceptors may not be a useful approach (e.g. Teppema & Dahan, 2010; Smith et al. 2010) if the two sets of chemoreceptors interact in other than a simply additive way and are thus capable of modulating the responsiveness of one another. This means that neural signals arising from stimuli at both sets of chemoreceptors have the potential to interact; indeed, such interdependence is a pre-condition for hypo- or hyperadditive interaction (Adams & Severns, 1982). In this ‘pro–con’ debate three potential interaction modes are discussed: hypoadditive, additive and hyperadditive. The literature reports a broad spectrum of results and opinions between the extremes of hypo- and hyperaddition (reviewed in Blain et al. 2010; Smith et al. 2010; Teppema & Dahan, 2010). Here we focus on recent evidence that supports a hyperadditive or multiplicative (synergistic) interaction; we will not discuss the well-known O2–CO2 interaction at the level of the carotid bodies (Fitzgerald & Parks, 1971; Lahiri & DeLaney, 1975).
Neuroanatomy and physiology
Central chemoreceptors may be widespread throughout the medulla, with the nucleus tractus solitarius (NTS), locus coeruleus, raphe nuclei and the retrotrapezoid nucleus (RTN) as major sites. Other structures, such as the lateral parabrachial nucleus and the dorso-lateral pontine respiratory group are probably also involved in integrative and chemoreceptive pathways (references in Smith et al. 2010). The RTN is considered to be the predominant location where the integration of the central chemoreceptor drive takes place (Mulkey et al. 2004; Guyenet et al. 2008). Carotid body afferents mainly terminate on NTS neurons that have reciprocal connections not only with the RTN but also with many other neurons within the respiratory network, some of which are also chemosensitive (Rosin et al. 2006; Takakura et al. 2006; Nuding et al. 2009). With its numerous reciprocal anatomical and functional connections, the network provides a plausible substrate for interdependence between peripheral and central chemoreceptors.
Evidence for hyperaddition
Carotid body denervation
Immediately or shortly after carotid body denervation (CBD) in unanesthetized goats, dogs, rats, and humans (Pan et al. 1998; Rodman et al. 2001; Hodges et al. 2005; Dahan et al. 2007, 2008), remaining central CO2 sensitivity was markedly decreased, even when the CO2 was presented in a hyperoxic background, and this effect persisted for days to months (central chemosensory plasticity in the long term following CBD is a distinct question and will not be discussed here). In goats, CBD also reduced the ventilatory response to focal acidosis of medullary raphe chemosensitive areas (Hodges et al. 2005).
Isolated and perfused carotid body
In unanaesthetized dogs undergoing selective perfusion of the isolated right carotid body with an extracorporeal circuit, central CO2 sensitivity was substantially reduced when the carotid bodies were per-fused with hyperoxic–hypocapnic blood. Conversely, perfusion with hypoxic–nor-mocapnic blood considerably augmented central CO2 sensitivity above normoxic–eucapnic perfused control (Fig. 1; Blain et al. 2010).
Figure 1.

Top panel, ventilatory responses of a representative dog to central hypercapnia in a background of normal (filled squares), inhibitory (open squares), and stimulatory (filled triangles) CB perfusion. Note the increase in response slope with increased CB stimulation (redrawn from Blain et al. 2010, with permission). Bottom panel, influence of bilateral carotid body resection on the outputs of the central and peripheral chemoreflex loops in a single patient with bilateral carotid body tumours. The peripheral and central ventilatory CO2 sensitivities were derived from a multi-frequency binary sequence in  . Note the marked decrease in central CO2 sensitivity in the first few weeks following CBR (redrawn from Dahan et al. 2008, with permission)
. Note the marked decrease in central CO2 sensitivity in the first few weeks following CBR (redrawn from Dahan et al. 2008, with permission)
Other methods of central–peripheral separation
In humans after an intravenous bicarbonate infusion, Teppema et al. (2010) found an almost twofold larger hypoxic sensitivity at a higher mean arterial  (45.2 mmHg) than at a lower
(45.2 mmHg) than at a lower  (38.6 mmHg) before infusion. The arterial pH was virtually the same in both conditions. Teppema et al. concluded that the higher central
(38.6 mmHg) before infusion. The arterial pH was virtually the same in both conditions. Teppema et al. concluded that the higher central  after the bicarbonate infusion resulted in a central multiplication of a hypoxia-induced carotid body response.
after the bicarbonate infusion resulted in a central multiplication of a hypoxia-induced carotid body response.
Robbins (1988) presented step decreases in end-tidal  (
( ) to human subjects after several minutes of increased
) to human subjects after several minutes of increased  (that had been returned abruptly to near eucapnia) and relied on temporal separation to dissociate the central from peripheral stimuli. Robbins reported an increased acute hypoxic ventilatory response at a time when the brain and central chemoreceptors were thought to be hypercapnic.
(that had been returned abruptly to near eucapnia) and relied on temporal separation to dissociate the central from peripheral stimuli. Robbins reported an increased acute hypoxic ventilatory response at a time when the brain and central chemoreceptors were thought to be hypercapnic.
Why is the literature so inconsistent?
Nature of the stimulus
An important consideration is that the carotid body is a polymodal sensory organ (Kumar & Bin-Jaliah, 2007) and, among other factors, the type of peripheral–central interaction could depend on the nature of the peripheral stimulus. Two studies in unanaesthetized humans (cited above) are consistent with increased carotid body chemoreceptor output increasing the central gain and the converse, central CO2 augmenting a carotid body-induced response. This suggests that the observed hyperaddition is symmetrical (or commutative, i.e. central stimulation increases peripheral sensitivity and vice versa) but this may not be the case for all stimuli.
Experimental limitations
Most, but not all, studies showing additive or hypoadditive interaction required the use of anaesthetics, sensory denervation, and/or decerebration (reviewed in Smith et al. 2010; Teppema & Dahan, 2010). In addition, several different species were used. Apart from potential species differences, the use of anaesthetics, non-blood perfusates, or reduced preparations may explain why results from experiments utilizing separate carotid body and brainstem perfusion did not observe hyperaddition (references in Day & Wilson, 2007; Teppema & Dahan, 2010; Smith et al. 2010). Such preparations may be prone to the non-specific phenomenon of neural saturation described by Eldridge et al. (1981), which would manifest as an apparent hypoaddition. Here, we have summarized evidence from organisms that were always unanaesthetized and, with the exception of some that underwent bilateral carotid body denervation/excision, intact. We must recognize that there are limitations to both denervation studies and studies relying on separation of central and peripheral chemoreceptor stimulation/inhibition using differences in central vs. peripheral response times or by manipulation of acid/base status. In the former, the neural control system is no longer intact and the loss of carotid sinus afferents may alter the behaviour of the remaining elements of the control system in unpredictable ways. In the latter, the major concern is uncertainty about complete separation of stimuli.
We think that the best evidence to date in support of hyperaddition comes from studies in both the intact and CBD human (Teppema et al. 2010; Dahan et al. 2007, 2008), the unanaesthetized, CBD goat with focal acidosis applied at the medullary raphé (Hodges et al. 2005), and the unanaesthetized canine isolated and perfused carotid body model (Blain et al. 2010). The canine model demonstrates clearly that the gain of the CNS CO2–H+ chemoreceptors is critically dependent on CB afferent activity and that CNS–CB interaction under these conditions results in hyperadditive ventilatory responses. Whether hyperaddition holds true for all stimuli sensed by the carotid and central chemoreceptors, and/or whether or not the primary stimulus is central or peripheral, will require further study.
Implications
Studying the peripheral–central interaction mode not only provides us with fundamental insight into the mechanisms by which the respiratory control system responds to acute and chronic changes in arterial 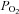 and
and  but also into the pathophysiology of breathing disorders. For example, a synergistic action of the peripheral and central chemoreceptors might explain the finding that carotid body hypocapnia is a prerequisite for the occurrence of central apnoea induced by central hypocapnia (Smith et al. 2010).
but also into the pathophysiology of breathing disorders. For example, a synergistic action of the peripheral and central chemoreceptors might explain the finding that carotid body hypocapnia is a prerequisite for the occurrence of central apnoea induced by central hypocapnia (Smith et al. 2010).
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/18/4359
References
- Adams JM, Severns ML. Interaction of chemoreceptor effects and its dependence on the intensity of stimuli. J Appl Physiol. 1982;52:602–606. doi: 10.1152/jappl.1982.52.3.602. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of Central Chemoreceptors: Effect of Bilateral Carotid Body Resection on Central CO2 Sensitivity. PLoS Medicine. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L. Plassticity in the brain: Influence of bilateral carotid body resection (bCBR) on centgral CO2 sensitivity. Adv Exp Med Biol. 2008;605:312–316. doi: 10.1007/978-0-387-73693-8_54. [DOI] [PubMed] [Google Scholar]
- Day TA, Wilson RJ. Brainstem PCO2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol. 2007;578:843–857. doi: 10.1113/jphysiol.2006.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS, Parks DC. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respir Physiol. 1971;12:218–229. doi: 10.1016/0034-5687(71)90054-5. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol. 2005;98:1234–1242. doi: 10.1152/japplphysiol.01011.2004. [DOI] [PubMed] [Google Scholar]
- Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor. Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975;24:249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Robbins PA. Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man. J Physiol. 1988;401:503–518. doi: 10.1113/jphysiol.1988.sp017175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, van Dorp EL, Dahan A. Arterial [H+] and the ventilatory response to hypoxia in humans: influence of acetazolamide-induced metabolic acidosis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L89–L95. doi: 10.1152/ajplung.00255.2009. [DOI] [PubMed] [Google Scholar]


