Duffin & Mateika (2013) state that their rebreathing data could also be fitted to a parabola. If they consider a parabolic shape an indication of hyper-additive interaction, it is surprising that they did not compare the quality of the fits of linear regression vs. parabolic or hyperbolic fits in order to decide which interaction mode would fit their data best. Apart from this, we have doubts about several assumptions underlying the modified rebreathing technique: (1) the absence of carotid body activity in hyperoxia; (2) that the hypoxic response would be a modified acidic response; (3) due to variable changes in CBF during the manouevre, the tissue–arterial 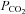 relationship cannot be constant (Battisti-Charbonney et al. 2011); and (4) absence of cortical influences on ventilation following 5 min of voluntary hyperventilation.
relationship cannot be constant (Battisti-Charbonney et al. 2011); and (4) absence of cortical influences on ventilation following 5 min of voluntary hyperventilation.
As explained by Robbins (1988), a linear 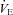 –
–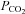 relationship is not inconsistent with multiplicative interaction. As outlined previously (Teppema & Berendsen, 2012), we do not agree with the claim of simple addition in humans by Cui et al. (2012) because they ignored the O2–CO2 interaction within the carotid bodies.
relationship is not inconsistent with multiplicative interaction. As outlined previously (Teppema & Berendsen, 2012), we do not agree with the claim of simple addition in humans by Cui et al. (2012) because they ignored the O2–CO2 interaction within the carotid bodies.
The fact that increasing carotid body output decreases the central CO2 threshold (Wilson & Day, 2013) is not inconsistent with (hyper)addition. By itself, it does not indicate hypoaddition.
In the dog model, blood pressure changes in the carotid sinus did not change ventilation despite potential cross-talk between brainstem sympathetic and respiratory neurons (Saupe et al. 1995). There was no evidence of retrogradely perfused blood affecting ventilation via the brainstem.
In dogs and goats, unilateral CBD has no functional implications (Smith et al. 1995). Reversing the stimulus order in steady-state conditions has no effect (Adams et al. 1978). The presentation order of changes in stimuli is not always peripheral and then central. Ischaemia or changes in CBF will first increase brain 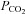 . During an apnoea,
. During an apnoea, 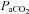 will rise first but thereafter it no longer depends on ventilation and tissue
will rise first but thereafter it no longer depends on ventilation and tissue 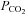 will rise faster than
will rise faster than 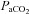 . Changes in metabolism may also cause changes in tissue
. Changes in metabolism may also cause changes in tissue 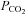 /pH, to be followed later by changes in the arterial blood.
/pH, to be followed later by changes in the arterial blood.
The hybrid model (Wilson & Day, 2013) is inconsistent with data from dogs during the hyperventilation secondary to hypoxic exposure in non-REM sleep which showed an increased propensity for apnoea due to a steeper, not shallower, ventilatory response slope (Nakayama et al. 2002).
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/18/4367
References
- Adams JM, Attinger FM, Attinger EO. Medullary and carotid chemoreceptor interaction for mild stimuli. Pflugers Arch. 1978;374:39–45. doi: 10.1007/BF00585695. [DOI] [PubMed] [Google Scholar]
- Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011;589:3039–3048. doi: 10.1113/jphysiol.2011.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Fisher JA, Duffin J. Central–peripheral respiratory chemoreflex interaction in humans. Respir Physiol Neurobiol. 2012;180:126–131. doi: 10.1016/j.resp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Duffin J, Mateika JH. CrossTalk opposing view: Peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol. 2013;591:4351–4353. doi: 10.1113/jphysiol.2013.256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–1260. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- Robbins PA. Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man. J Physiol. 1988;401:503–518. doi: 10.1113/jphysiol.1988.sp017175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory and cardiovascular responses to increased and decreased carotid sinus pressure in sleeping dogs. J Appl Physiol. 1995;78:1688–1698. doi: 10.1152/jappl.1995.78.5.1688. [DOI] [PubMed] [Google Scholar]
- Smith CA, Saupe KW, Henderson KS, Dempsey JA. Ventilatory effects of specific carotid body hypocapnia in dogs during wakefulness and sleep. J Appl Physiol. 1995;79:689–699. doi: 10.1152/jappl.1995.79.3.689. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Berendsen RR. Response to the reply of J. Duffin to our letter entitled ‘Acetazolamide and cerebrovascular function at high altitude’. J Physiol. 2012;590:3625–3626. doi: 10.1113/jphysiol.2012.233569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJA, Day TA. CrossTalk proposal: Peripheral and central chemoreceptors have hypoadditive effects on respiratory motor output. J Physiol. 2013;591:4355–4357. doi: 10.1113/jphysiol.2013.256578. [DOI] [PMC free article] [PubMed] [Google Scholar]


