Abstract
Endosomal and lysosomal membrane trafficking requires the coordination of multiple signalling events to control cargo sorting and processing, and endosome maturation. The initiation and termination of signalling events in endosomes and lysosomes is not well understood, but several key regulators have been identified, which include small GTPases, phosphoinositides, and Ca2+. Small GTPases act as master regulators and molecular switches in a GTP-dependent manner, initiating signalling cascades to regulate the direction and specificity of endosomal trafficking. Phosphoinositides are membrane-bound lipids that indicate vesicular identities for recruiting specific cytoplasmic proteins to endosomal membranes, thus allowing specificity of membrane fusion, fission, and cargo sorting to occur within and between specific vesicle compartments. In addition, phosphoinositides regulate the function of membrane proteins such as ion channels and transporters in a compartment-specific manner to mediate transport and signalling. Finally, Ca2+, a locally acting second messenger released from intracellular ion channels, may provide precise spatiotemporal regulation of endosomal signalling and trafficking events. Small GTPase signalling can regulate phosphoinositide conversion during endosome maturation, and electrophysiological studies on isolated endosomes have shown that endosomal and lysosomal Ca2+ channels are directly modulated by endosomal lipids. Thus trafficking and maturation of endosomes and lysosomes can be precisely regulated by dynamic changes in GTPases and membrane lipids, as well as Ca2+ signalling. Importantly, impaired phosphoinositide and Ca2+ signalling can cause endosomal and lysosomal trafficking defects at the cellular level, and a spectrum of lysosome storage diseases.
 |
Haoxing Xu (right) is an associate professor at the University of Michigan. He graduated from Peking University, Beijing, China, and received a PhD from Georgia State University, Atlanta, Georgia. He was a postdoctoral fellow in David Clapham's laboratory at Boston Children's Hospital, where he cloned a temperature-sensitive TRP ion channel in the skin. His current research investigates ion flux and Ca2+ signalling mechanisms in the lysosome. As a channel biologist, he has contributed to the initial functional characterization of 10 ion channels. He has received several faculty awards including the Presidential Early Career Award for Scientists and Engineers (PECASE; 2010). Xinran Li (left) received his Bachelor's degree in Biochemistry at the University of Hong Kong. He is a graduate student in the Molecular, Cellular and Developmental Biology program at the University of Michigan. Abigail G. Garrity (middle) received her Bachelor's degree in Neuroscience at Trinity College, Hartford, Connecticut. She is a graduate student in the Neuroscience Program at the University of Michigan.
Introduction
In eukaryotic cells, membrane trafficking through the endocytic pathway (endocytic trafficking) is an ongoing process that requires the cooperation of many proteins, membrane lipids and ions, and defects in trafficking can lead to a number of endosome and lysosome-related human diseases. Endocytic trafficking involves a series of steps including endocytosis, cargo sorting and processing, intracellular membrane fusion and fission, vesicle mobility, and exocytosis (Fig. 1). The purpose of this review is to highlight recent studies and synthesize research findings on how signalling by small GTPases, phosphoinositides, and Ca2+ regulate endosomal and lysosomal trafficking events. We regret that we are unable to cite every paper related to the ideas in this review. As a result, we cite only the most recent review papers and primary research findings to provide an update on the topics discussed. We begin with a brief overview of endocytic trafficking before discussing key regulators of membrane trafficking, including small GTPases, phosphoinositides, and Ca2+ in more depth.
Figure 1. Endosomal trafficking network.
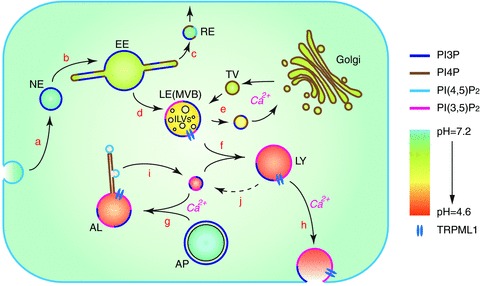
A schematic view of the endosomal trafficking network. Vesicular pH and predominant membrane phosphoinositides on different compartments are represented by different colours. During endocytosis, a piece of the plasma membrane is excised and enters the cytosol in the form of a nascent endosome (NE; a). Nascent endosomes fuse with each other (b) and recruit early endosomal proteins to become early endosomes (EE; b). Membrane receptors are sorted and recycled back to the plasma membrane through recycling endosomes (RE; c). Material destined for degradation is passed on to the late endosomes (LE; d), which are also referred to as multi-vesicular bodies (MVB) due to the intraluminal vesicles (ILVs) that contain membrane proteins sorted for degradation. Hydrolytic enzymes are transported to late endosomes through transport vesicles (TV) from Golgi (e). Membrane receptors carrying the enzymes are shuttled back to Golgi through retrograde transport. Late endosomes mature into lysosomes (LY) either through further acidification, or through fusion with existing lysosomes (f). During starvation or when organelles are damaged, lysosomes also accept cargo from autophagosomes (AP) carrying damaged organelles or cytosolic material for degradation (g). The resulting autophagic lysosomes (AL) are usually larger than endocytic lysosomes. Lysosomes can undergo Ca2+-dependent exocytosis (h). Lysosomal membrane proteins are recycled from autophagic lysosomes by fission processes that happen on tubular structures (i). The mechanism of recycling of membrane proteins from endocytic lysosomes has yet to be established (j).
Early endosomes
Endocytic trafficking begins with the uptake of extracellular substance through the formation of a nascent endocytic vesicle that is excised from the plasma membrane (Fig. 1 (a)). The four types of endocytosis, that is, clathrin-mediated (McMahon & Boucrot, 2011), caveolar (Parton & del Pozo, 2013), phagocytosis (Flannagan et al. 2012) and macropinocytosis (Lim & Gleeson, 2011), have been excellently reviewed elsewhere. With the exception of macropinocytosis, endocytic events are mediated by specific receptors on the cell membrane (for review, see Huotari & Helenius, 2011). Nascent endocytic vesicles then undergo maturation and sorting processes to become early endosomes (Fig. 1 (b)). Early endosomes commonly consist of a large vacuolar domain and multiple tubular domains (Cullen, 2011), through which most plasma membrane receptors are sorted and recycled back to the plasma membrane via the pathway of the recycling endosome (Fig. 1 (c); for review, see Grant & Donaldson, 2009; Hsu & Prekeris, 2010). The cargo destined for further transport and/or degradation is retained inside or on the membranes of early endosomes. Besides cargo taken up through endocytosis, the vacuolar domains of early endosomes are also capable of accepting cytosolic or membrane-bound cargo through a protein complex called endosomal sorting complex required for transport (ESCRT) to sort ubiquitinated cargo proteins into multi-vesicular bodies (MVBs; Babst, 2011).
Late endosomes
After sorting in the early endosomes, endocytic vesicles undergo maturation and further acidification into late endosomes (Fig. 1 (d)). Late endosomes are derived from the vacuolar domain of the early endosome and are often referred to as multi-vesicular bodies (MVBs) because they contain many intraluminal vesicles (Fig. 1; Huotari & Helenius, 2011). Transport vesicles from the Golgi apparatus carry membrane receptors that recognize lysosome-destined hydrolytic enzymes, including mannose 6-phosphate receptor (M6PR), sortilin and lysosomal integral membrane protein 2 (LIMP2), from the Golgi to late endosomes. Upon arrival, the hydrolytic enzymes are dissociated from the receptors due to the acidic environment in late endosomes, while membrane receptors undergo retrograde trafficking back to the Golgi for reuse in the next round of delivery (Fig. 1 (e); Braulke & Bonifacino, 2009; Coutinho et al. 2012).
Lysosomes
Late endosomes can mature into lysosomes through direct acidification by the vacuolar-type H+-ATPase (V-ATPase) proton pump or through fusion with existing lysosomes (Fig. 1 (f)). Proteins found on the membranes of late endosomes and lysosomes are similar. The primary difference between late endosomes and lysosomes is their luminal pH. While late endosomes have a luminal pH of 5.0–6.0, lysosomal pH is 4.5–5.0. The highly acidic pH in the lysosome lumen facilitates efficient hydrolysis of cargo delivered to lysosomes (Mindell, 2012). Extracellular and plasma membrane-derived cargo is delivered to lysosomes via the endocytic pathway. In addition to late endosomes, lysosomes also acquire cytoplasm-derived cargo from autophagosomes during autophagy (Mizushima & Komatsu, 2011). Autophagy, mediated by a set of autophagy-related genes (Atgs; Mizushima et al. 2011), involves the formation of double membrane-bound autophagosomes that contain large cargo such as damaged organelles (Choi et al. 2013). Autophagosomes then fuse with lysosomes to form autolysosomes, in which autophagic substrates are broken down for reutilization (Fig. 1 (g); Mizushima & Komatsu, 2011). Subsequently, the digested products from lysosomes are either released into the cytosol via membrane transporters and channels, or transported to the Golgi via retrograde trafficking for reutilization. However, only a few lysosomal transport proteins have been well characterized to date (Schwake et al. 2013). For example, lipid and cholesterol export from the lysosome is regulated by lysosomal protein NPC1 (Chang et al. 2006). Likewise, proton-assisted amino acid transporters (PATs) on lysosomal membranes couple the H+ gradient, driven by the lysosomal V-ATPase, to amino acid transport into the cytosol for reutilization by the cell (Boll et al. 2004; Thwaites & Anderson, 2011). PAT1, in a complex with Rag GTPases on lysosome membranes, plays important roles in sensing amino acid levels in the lysosome lumen (Ogmundsdottir et al. 2012), and can regulate lysosomal recruitment of mammalian or mechanistic target of rapamycin (mTOR) to promote cell growth (Heublein et al. 2010). There are still many unanswered questions regarding how lysosomal membrane proteins sense and export degraded products, and is a field ripe with opportunity for future research.
Although conventionally believed to be the ‘end point’ of endosomal trafficking, membrane fusion and fission events do occur in lysosomes and autolysosomes. First, lysosomes undergo exocytosis in most, if not all, cell types (Fig. 1 (h); Reddy et al. 2001). The physiological functions of lysosomal exocytosis may include cell migration (Colvin et al. 2010), transmitter release (Dou et al. 2012), large particle phagocytosis (Czibener et al. 2006), membrane repair (Reddy et al. 2001), and release of hydrolytic enzymes into extracellular space (Czibener et al. 2006). Second, with the aid of high-resolution live imaging, lysosomes are also observed to undergo budding off through very dynamic tubular structures (Fig. 1 (i); Yu et al. 2010). This type of tubular fission is most active when autophagy is induced upon prolonged starvation, and is referred to as autophagic lysosome reformation. Lysosome reformation rapidly increases the number of lysosomes when there is a high demand for digestion (Chen & Yu, 2013), and is activated when the digested material in autolysosomes is released from the lumen through membrane transporters into the cytosol (Yu et al. 2010; Rong et al. 2011).
Regulators of endosomal trafficking
Although the detailed mechanisms by which cells regulate endocytic trafficking are still being elucidated, several key regulators have been identified. This review will focus on three key regulators, including small GTPases, phosphoinositides and Ca2+.
Small GTPases
Several subfamilies of the Ras small GTP-binding protein superfamily regulate intracellular membrane trafficking, with the Rab and Arf/Sar subfamilies being the most well studied (Mizuno-Yamasaki et al. 2012). Rab proteins are the largest subfamily of the Ras GTPases, with more than 60 known Rabs in humans (Rojas et al. 2012; see also Galvez et al. 2012). Rab GTPases usually regulate sequential events in the endosome maturation process, including transport of vesicles along the cytoskeleton and vesicle fusion (Mizuno-Yamasaki et al. 2012). Arf proteins are the most divergent subfamily of Ras GTPases (Rojas et al. 2012) and typically control vesicle budding (Mizuno-Yamasaki et al. 2012). Ral GTPases are found only in animal cells and play a role in both endocytosis and exocytosis (Wu et al. 2008). There are more than 20 known Rho GTPases in humans, which function in a variety of processes including vesicle trafficking, as well as cell polarity, cell migration and virus transport (Chi et al. 2013).
The association and dissociation of particular small GTPase proteins with specific vesicle membranes is one method of establishing membrane identity in vesicle trafficking (Pfeffer, 2013). GTPases have two functional states: an active, GTP-bound state, and an inactive, GDP-bound state. Guanine nucleotide exchange factors (GEFs) catalyse the exchange of GDP for GTP which initiates signalling activity. Rab GEFs have been shown to be sufficient to recruit Rab proteins to specific endosomal membranes, suggesting that GEFs play a central role in establishing the localization of Rabs in endosomal trafficking (Blumer et al. 2013). After binding to GTP, GTPases bind to effectors that stimulate their activity and/or recruit them to the appropriate site of action. GTPases can then recruit additional effectors that can help to change the functional identity of the membrane and direct trafficking events (Mizuno-Yamasaki et al. 2012). GTPase-activating proteins (GAPs) mediate inactivation by catalysing the hydrolysis of GTP to GDP, thus terminating signalling (Stenmark, 2009).
The ‘on and off’ nature of GTPases makes them ideal regulators of membrane trafficking (Mizuno-Yamasaki et al. 2012). Indeed, Rab and Arf GTPases are involved in almost all aspects of vesicular transport. The intracellular location of specific members of the Rab protein family is essential to their regulation of membrane trafficking (Hutagalung & Novick, 2011). For example, the switch of Rab5 to Rab7 is a key initiating event that drives maturation from early endosomes to late endosomes (Poteryaev et al. 2010). Rabs also recruit tethering factors that dock vesicles prior to fusion (Fig. 2A). Tethering factors accelerate and increase the efficiency of fusion, and regulate the formation of soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) complexes (Yu & Hughson, 2010). The tethering factors early endosome antigen 1 (EEA1) for early endosomes and the multisubunit homotypic fusion and protein sorting (HOPS) complex for late endosomes are recruited by Rab5 and Rab7, respectively (Yu & Hughson, 2010). Conversely, tethering factors can also act as Rab effectors (Yu & Hughson, 2010).
Figure 2. A proposed model of the phosphoinositide–Ca2+–membrane fusion pathway.
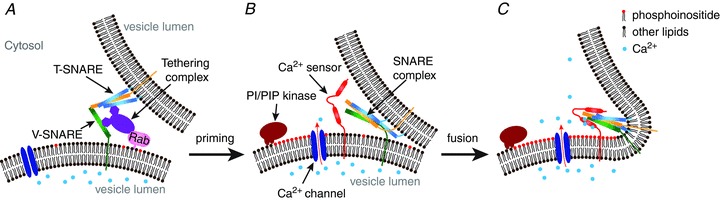
A, the initiation of vesicle fusion is mediated by the cooperation of Rab proteins and tethering complexes, which coordinate the assembly of the SNARE complex. B, after the SNARE complex is assembled, the vesicles are in a ready-to-fuse state. C, an increase in the membrane PI(3,5)P2 concentration activates Ca2+ influx into the cytosol, which acts as a trigger for vesicle fusion.
The coordination of GTPase activity with other intracellular signalling events allows for fine-tuned spatiotemporal regulation of trafficking events. GTPases are networked to one another through coordinated hydrolysis and association/dissociation with vesicle membranes, as well as through common effectors (Mizuno-Yamasaki et al. 2012). Both Rab GTPases and intracellular phosphoinositides (discussed in detail below) help to maintain the identity of intracellular vesicles in order to recruit specific effectors. The coordination of Rab GTPases and phosphoinositides provides additional specificity and directionality in trafficking steps (Jean & Kiger, 2012). First, GTPases can recruit enzymes that regulate phosphoinositides and phosphoinositides can recruit Rab regulators. Secondly, downstream effectors can have co-requirements for specific Rab GTPase and phosphoinositide combinations. Third, enzymes involved in the synthesis and phosphorylation of phosphoinositides have been shown to have GAP and GEF activity for specific Rab GTPases (Jean & Kiger, 2012). For details regarding the function and regulation of small GTPases in membrane traffic, the reader is encouraged to consult recent extensive and excellent reviews (Stenmark, 2009; Hutagalung & Novick, 2011; Jean & Kiger, 2012; Mizuno-Yamasaki et al. 2012; Pfeffer, 2013).
Phosphoinositides
Phosphoinositides are low-abundance membrane lipids that are localized to specific compartments in the endocytic pathway (Di Paolo & De Camilli, 2006). There are seven phosphoinositides based on the combination of phosphorylation at the 3′, 4′ and 5′ positions of the inositol head group: phosphatidylinositol 3-phosphate or PI(3)P, PI(4)P, PI(5)P, PI(3,4)P2, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P3. PI(3)P is found on the cytoplasmic sides of early and late endosomes and newly formed phagosomes and autophagosomes (Poccia & Larijani, 2009). PI(4)P is mainly localized on the cytoplasmic leaflet of Golgi membranes (Santiago-Tirado & Bretscher, 2011). The localization of PI(5)P is the least well studied; however, it has been suggested that it plays a role in nuclear signalling and membrane dynamics (Mayinger, 2012). PI(3,4)P2 and PI(3,4,5)P3 are generated transiently upon activation of plasma membrane receptors. PI(4,5)P2 is relatively abundant on the plasma membrane and participates in a variety of plasma membrane-specific functions (Falkenburger et al. 2010). Finally, PI(3,5)P2 is produced in late endosomes and lysosomes using PI(3)P as the substrate (Y. Zhang et al. 2012; Zolov et al. 2012).
Phosphoinositides regulate compartment-specific membrane trafficking via at least two distinct mechanisms. First, they recruit a spectrum of cytoplasmic effector proteins that function on specific membrane compartments (Falkenburger et al. 2010). Rapid production and elimination of phosphoinositides by their specific regulatory enzymes could recruit phosphoinositide effector proteins necessary for the initiation and termination of membrane trafficking events in a sequential manner. For example, the recruitment of early endosomal tethering factor EEA1 onto early endosomes requires the recognition of PI(3)P by EEA1's FYVE domain (Poccia & Larijani, 2009). Second, phosphoinositides can directly regulate the activity of membrane proteins such as ion channels and transporters (X. Zhang et al. 2012). Many plasma membrane ion channels have been shown to be activated or positively regulated by the plasma membrane phosphoinositide PI(4,5)P2 (Falkenburger et al. 2010). Two families of late endosomal and lysosomal cation channels, the transient receptor potential cation channels, mucolipin subfamily (TRPML) and two-pore channels (TPC), both implicated in endosomal and lysosomal membrane trafficking, are activated by the late endosomal and lysosomal phosphoinositide PI(3,5)P2 (Dong et al. 2010; Wang et al. 2012). Furthermore, TRPML1 is inactivated by the plasma membrane phosphoinositide PI(4,5)P2 (X. Zhang et al. 2012). Thus, phosphoinositides may have dual functions in recruiting cytoplasmic proteins and providing compartment-specific regulation of membrane proteins in intracellular vesicular compartments.
Genetically encoded fluorescent phosphoinositide probes, constructed from phosphoinositide-binding domains of a variety of proteins, have been generated for at least four of the seven phosphoinositides (Balla, 2007). Phosphoinositide probes allow for the visualization of real-time changes in both the abundance and localization of phosphoinositides, revealing novel aspects of phosphoinositide-mediated regulation of membrane trafficking. For example, a transient, localized increase in the endosomal PI(3,5)P2 level may induce Ca2+ release, which could trigger a membrane fusion event (see Fig. 2). Likewise, localized production of PI(4,5)P2 on tubular structures of lysosomes may recruit clathrin in a microdomain, which initiates clathrin-mediated membrane fission (Rong et al. 2012). Live imaging with phosphoinositide probes to monitor phosphoinositide levels in vivo could directly test such hypotheses. If phosphoinositides also act as triggers, then it is expected that the levels of phosphoinositides will undergo local increases that directly precede or even coincide with membrane fusion/fission events. Optogenetics will allow very precise manipulation of phosphoinositide levels both spatially and temporally, providing a powerful means towards decoding the functions of phosphoinositides in membrane trafficking (Idevall-Hagren et al. 2012).
PI(3)P dynamics in early and late endosomes
PI(3)P is the predominant phosphoinositide on early endosomes and autophagosomes, and is responsible for the localization of a spectrum of otherwise cytosol-localized proteins to these compartments (Noda et al. 2010). PI(3)P is produced from phosphatidylinositol (PI) by the class III phosphatidylinositol 3-kinase (class III PI3K, also known as Vps34) complex (Lindmo & Stenmark, 2006). PI(3)P can be converted back to PI by lipid phosphatases of the myotubularin/myotubularin-related-protein family (MTM/MTMR; Shen et al. 2011). Alternatively, PI(3)P can be converted into PI(3,5)P2 by the phosphatidylinositol 5-kinase PIKfyve complex (Gillooly et al. 2000). PI(3)P effector proteins, for example, EEA1 and hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs), contain the PI(3)P-binding FYVE domains, which have been used to generate PI(3)P probes (Gillooly et al. 2000). FYVE-containing PI(3)P probes exhibit vesicular membrane localization that is consistent with early endosomal markers such as EEA1 (Fig. 3A).
Figure 3. Fluorescently tagged phosphoinositide probes.
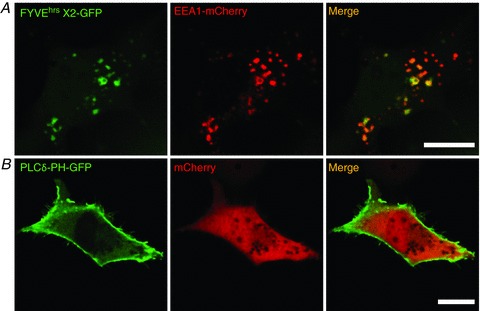
Cos7 cells are transfected with green fluorescent protein (GFP)-tagged phosphoinositide-binding domains. A, a PI(3)P probe, FYVEhrsX2-GFP, is concentrated in EEA1-positive vesicles. mCherry-EEA1 is co-transfected to visualize early endosomes. B, the PI(4,5)P2 probe, PLCδ-PH-GFP, is mainly localized in the plasma membrane at rest. mCherry is co-transfected to define cell morphology. Scale bars represent 20 μm.
PI(3)P probes are recruited to the membranes of newly formed endosomal compartments (including nascent phagosomes, macropinosomes, and autophagosomes) within minutes after endocytosis, but are also visible on lysosomal-associated membrane protein 1 (LAMP1)-positive compartments, suggesting that PI(3)P may play a role in the entire endosomal maturation process (Kerr et al. 2010). Vsp34 knockout (KO) mice, which lack the enzyme to make PI(3)P, are defective in autophagy and normal physiological functions in a spectrum of tissues and organs, indicating the importance of PI(3)P signalling for normal cell functions (Jaber et al. 2012). Hence PI(3)P signalling may carry important information for the maturation of endosomes.
PI(3,5)P2 dynamics in late endosomes and lysosomes
PI(3,5)P2 is produced from PI(3)P by PIKfyve (Zolov et al. 2012). PIKfyve exists in a complex with the scaffold protein Vac14 and the phosphoinositide 5-phosphatase Fig4, and is the only enzyme found in mammalian cells to produce PI(3,5)P2 (Duex et al. 2006b; Zhang et al. 2007). Knockout of PIKfyve in mice results in embryonic lethality (Ikonomov et al. 2011), and knockout of Vac14 or Fig4, which results in a roughly 50% decrease in total PI(3,5)P2 levels, leads to severely enlarged vacuoles of late-endocytic origin (Chow et al. 2007; Zhang et al. 2007). It is still unclear how PI(3,5)P2 regulates late endocytic trafficking, however. Atg18 is the first identified PI(3,5)P2 effector protein, but is localized in the autophagic compartments (Obara et al. 2008), suggesting that it may not be responsible for the primary effect of PI(3,5)P2 in late endosomes and lysosomes. Patch-clamp recordings of endolysosomes have demonstrated that PI(3,5)P2 in a nanomolar range robustly activates TRPML1, a late-endosomal and lysosomal channel (Dong et al. 2010). In addition, cells that lack the TRPML1 channel share a similar vacuolar phenotype to Vac14 and Fig4 KO cells (Dong et al. 2010). Taken together, these findings suggest that PI(3,5)P2 may regulate endosomal trafficking through endolysosomal ion channels. However, the cellular and animal phenotypes of TRPML1 KO mice are much less severe than PI(3,5)P2-deficient Vac14 or Fig4 KO mice, suggesting that there are additional unidentified PI(3,5)P2 effector proteins that regulate late endosomes and lysosomes (Shen et al. 2011).
The level of PI(3,5)P2 and its recruitment dynamics in the late endosomes and lysosomes is poorly understood, largely due to the lack of a widely-accepted PI(3,5)P2 probe. Biochemical studies have revealed that the PI(3,5)P2 level in the endosome and lysosome is regulated by serum factors, insulin and osmotic stress in some mammalian cell types (Poccia & Larijani, 2009; Zolov et al. 2012). However, the connection of these conditions to endosomal and lysosomal trafficking has not been established. The most well-understood example of PI(3,5)P2 dynamics is from yeast studies. Deletion mutants of Vac14 or Fab1 (the yeast orthologues of mammalian Vac14 and PIKfyve, respectively) lead to a single huge vacuole in yeast cells (Odorizzi et al. 1998; Bonangelino et al. 2002; Dove et al. 2002), which could result from increased fusion or decreased fission of vacuoles. Additionally, a rapid, more than 10-fold increase in PI(3,5)P2 levels in yeast cells caused by hyperosmotic shock is required for the synchronized vacuole fission in response to hyperosmolarity, because in deletion mutants fission is impaired (Duex et al. 2006a,b). These findings suggest that the production of PI(3,5)P2 is required for vesicle membrane fission, at least in yeast.
PI(4)P and PI(4,5)P2 dynamics in lysosomes
Current findings suggest that PI(4,5)P2 is localized mainly on the plasma membrane (Fig. 3B). PI(4)P is most abundant on the Golgi. Recent studies have demonstrated that both PI(4)P and PI(4,5)P2 are also probably generated on lysosomal membranes, in particular on lysosomes and autolysosomes with tubular structures (Yu et al. 2010; Rong et al. 2012; Sridhar et al. 2013). Upon completion of autophagy, degradation of the cargo delivered by autophagosomes to lysosomes triggers the reactivation of mTOR. mTOR reactivation in turn triggers the formation of proto-lysosomal tubules from lysosomes that reform to regenerate functional lysosomes (Yu et al. 2010). The tubular structures undergo very quick elongation and retraction, and proto-lysosomes have been observed to bud off (membrane fission) from tubular structures (Rong et al. 2012). Lysosomal tubular structures are infrequently observed in healthy, well-fed cells, but their number greatly increases in conditions like prolonged serum starvation, when there is a high demand for digestion. The tubular domains of lysosomes contain many lysosomal membrane proteins, while cargo and luminal enzymes are restrained in the vacuolar domains of the lysosomes to complete degradation (Yu et al. 2010; Sridhar et al. 2013). At least three phosphoinositide kinases have been suggested to regulate this lysosomal reformation process. In cells lacking phosphatidylinositol 4-phosphate 5-kinase 1A (PI4P5K1A) or PI4P5K1B, two enzymes catalysing the generation of PI(4,5)P2 (Rong et al. 2012), or phosphatidylinositol 4-kinase IIIβ (PI4K-IIIβ), an enzyme catalysing the generation of PI(4)P (Sridhar et al. 2013), the lysosome reformation process is defective. Using the PI(4)P probe and PI(4,5)P2 antibody, it was shown that the levels of PI(4)P and PI(4,5)P2 are elevated in the tubular structures of autolysosomes (Rong et al. 2012). The production of PI(4,5)P2 on tubular structures is considered to be important for the initiation of clathrin-mediated membrane fission (Rong et al. 2012), but exactly how PI(4)P and PI(4,5)P2 coordinate the tubular-lysosomal fission process is still unclear. It seems likely that the conversion of PI(4)P into PI(4,5)P2 on autophagic lysosomes can initiate tubule formation, while the production of PI(4,5)P2 on elongated tubules can promote final fission (Rong et al. 2012; Sridhar et al. 2013).
Calcium
Ca2+ serves as a multifunctional signal in a variety of intracellular processes. Ca2+ signalling can occur in a wide range of spatial (highly localized vs. synchronized release) and temporal (transient vs. sustained release; microseconds to hours) ways (Berridge et al. 2003). The endoplasmic reticulum (ER) is appreciated as a significant source of Ca2+ in the cell, and ER-mediated Ca2+ signalling is integral to numerous intracellular processes (Berridge, 2002), including autophagy and membrane trafficking (Smaili et al. 2013). Lysosomes are beginning to be appreciated as an additional store of Ca2+ (Morgan et al. 2011), and some recent reports suggest that Ca2+ signalling can occur between the ER and lysosomes (Kilpatrick et al. 2013; Lopez-Sanjurjo et al. 2013; Morgan et al. 2013). However, while the mechanisms by which the ER maintains its Ca2+ stores are beginning to be elucidated (Lewis, 2011), less is currently known about how lysosomal Ca2+ stores are regulated. After endocytosis, early endosomes rapidly lose their Ca2+ as endosomes mature and acidify (Gerasimenko et al. 1998), a process driven by the V-ATPase on the lysosome (Gerasimenko et al. 2001). Conversely, increasing lysosomal pH results in a significant loss of lysosomal Ca2+ (Christensen et al. 2002), which suggests that the ionic balance in the lysosome is important in maintaining lysosomal Ca2+ stores. Thus, the high proton gradient (pHlumen 4.6 vs. pHi 7.2) in the lysosome probably drives a putative Ca2+/H+ pump to regulate Ca2+ in the lysosome. Furthermore, it is likely that the concentration of various intracellular ions including Na+, Zn2+ and Ca2+ in endosomes and lysosomes changes throughout endosome maturation and that the balance of these ions is important; however, this possibility remains to be explored. The role of Ca2+ in trafficking and signalling is the most well understood and will be discussed here; however, other ions such as Na+ (Wang et al. 2012) and Zn2+ (Aballay et al. 1995) may also be involved in trafficking. For example, Na+ efflux may regulate the fusogenic potential of the lysosome by causing membrane depolarization (Wang et al. 2012), or affecting Ca2+ uptake (Morgan et al. 2011). However, further studies are necessary to test these possibilities.
Neurotransmitter exocytosis as a model for membrane fusion events
The relationship between Ca2+ and membrane trafficking has been best characterized in the case of neurotransmitter release, which serves as a reference model for the less well understood case of intracellular membrane trafficking. Neurotransmitter release involves very rapid (<1 ms) membrane fusion (exocytosis) between synaptic vesicles and the plasma membrane of synaptic terminals. Membrane fusion requires the binding of a variety of SNARE proteins found on the vesicle (v-SNAREs) and target (t-SNAREs) membranes (Sudhof & Rothman, 2009). SNAREs are a protein superfamily sharing a coiled-coil homology domain (Jahn & Fasshauer, 2012). Fluorescence resonance energy transfer (FRET) at synapses has allowed for precise visualization of the assembly, rearrangements and disassembly of SNARE proteins (Degtyar et al. 2013). Fusion of a synaptic vesicle requires the binding, or ‘zippering’, of a vesicle-associated membrane protein (VAMP, also known as synaptobrevin) with plasma membrane-bound syntaxin and SNAP-25 to form a SNARE complex (Gao et al. 2012). SNARE complexes are competent to form a fusion pore between membranes (Domanska et al. 2009), but fast membrane fusion requires the interaction between the SNARE complex and the Ca2+ sensor synaptotagmin, and is triggered by the binding of Ca2+ ions to the cytoplasmic C2 domains of synaptotagmin (Tucker et al. 2004). During an action potential, Ca2+ enters the synaptic terminal through voltage-gated Ca2+ channels and binds to synaptotagmin on the pre-docked synaptic vesicles (Xu et al. 2007). Synaptotagmin undergoes a conformational change upon Ca2+ binding and the C2 domains interact directly with the SNARE complex (van den Bogaart et al. 2011) and phospholipids on the plasma membrane to trigger SNARE-mediated membrane fusion.
Intracellular membrane trafficking
Membrane traffic-king involves the fusion and fission of endosomes and lysosomes, as well as lysosomal exocytosis. Like neurotransmitter release, intracellular vesicle fusion requires SNARE proteins. After membrane tethering, membrane fusion is mediated by endosomal SNARE proteins, which include v-SNAREs such as VAMP4 (Tran et al. 2007) and VAMP7 (Fraldi et al. 2010), and t-SNAREs such as syntaxin6 (Jung et al. 2012) and syntaxin7 (Ward et al. 2000; Fig. 2A). Hence, similar machinery is employed for general trafficking and neurotransmitter release.
Ca2+ as a trigger for membrane fusion
Similar to neurotransmitter release, the Ca2+ release that mediates endosomal trafficking events is likely to come from a local source, as membrane trafficking steps can be efficiently blocked by the fast, membrane-permeable Ca2+ chelator BAPTA-AM, but not the slow chelator EGTA-AM (Chen et al. 2002; Czibener et al. 2006; Shen et al. 2011). In late endosomes and lysosomes, there are two potential known candidates: TRPMLs (Cheng et al. 2010) and TPC channels (Grimm et al. 2012). TRPML1 is a Ca2+-permeable cation channel that is ubiquitously expressed in most cell types. TRPML1 is primarily localized in the late endosome and lysosome and has been shown to mediate the release of Ca2+ from the lysosome lumen to the cytosol (Dong et al. 2009, 2010). Mammalian cells lacking TRPML1 exhibit multiple membrane trafficking defects in the late-endocytic pathway including altered autophagosome–lysosome fusion and lysosome-to-Golgi trafficking (Shen et al. 2011, 2012). TPC channels are a family of ion channels that reside on endocytic compartments, and were reported to mediate the nicotinic acid adenine dinucleotide phosphate (NAADP)-evoked Ca2+ release from late endosomes and lysosomes (Zong et al. 2009). However, whole-endolysosome patch-clamp analyses indicate that TPC channels exhibit little Ca2+ permeability and are essentially PI(3,5)P2-activated sodium-selective channels (Wang et al. 2012). Therefore, TPCs may not be the direct targets of NAADP. Instead, NAADP may bind to a unidentified 23 kDa protein (Lin-Moshier et al. 2012) to regulate the activity of a variety of Ca2+ release channels (Guse, 2012). However, the role of NAADP is still debated. Collectively, these findings suggest that TRPML1 plays a role in lysosomal membrane trafficking similar to voltage-gated Ca2+ channels in neurotransmission. Interestingly, overexpression of both TRPML1 and TPC channels leads to enlarged endolysosomes, indicating that both channels could be up-regulating fusion events (Dong et al. 2010; Wang et al. 2012). TFEB, a transcription factor and master regulator for lysosome biogenesis and autophagy, has been suggested to induce lysosomal exocytosis in a TRPML1-dependent manner (Medina et al. 2011). Hence, TRPML1 is a strong candidate in mediating Ca2+ release in general vesicular trafficking events in late endosomes and lysosomes.
The majority of the aforementioned evidence supporting Ca2+ as a triggering event in membrane trafficking remains inconclusive. Cell-free vesicle fusion assays do not fully mimic in vivo situations. Intracellular vesicle fusion has been mainly studied using Ca2+ chelators such as BAPTA-AM (Chen et al. 2002), which also chelates other cations in in vitro assays and may have additional actions that are not specific to Ca2+ (Starai et al. 2005). While EGTA-AM has a slower time course than BAPTA-AM and probably does not chelate transient Ca2+ increases (making it a good negative control for BAPTA-AM), many studies would benefit from the direct measurement of Ca2+ release from Ca2+ channels located in endosomal membranes. The use of high resolution, genetically encoded Ca2+ indicators (GECIs) has aided the study of local Ca2+ release from ion channels in endosomal membranes (Tian et al. 2009). For example, fusing the GECI GCaMP3 to the cytoplasmic amino acid terminus of TRPML1 has enabled the direct measurement of Ca2+ release from the lysosome using Ca2+ imaging (Fig. 4; Shen et al. 2012). Furthermore, ratiometric lysosome-targeted GECIs may prove useful in monitoring Ca2+ dynamics in moving vesicles (McCue et al. 2013). Whether Ca2+ acts as a trigger for trafficking events, or is more upstream from direct vesicle fusion and fission is unknown. If Ca2+ is the trigger for vesicle fusion, then the local Ca2+ increase revealed by vesicularly targeted Ca2+ indicators should coincide with, or directly precede, membrane fusion or fission (Fig. 2C).
Figure 4. A lysosome-targeted genetically encoded Ca2+ sensor.
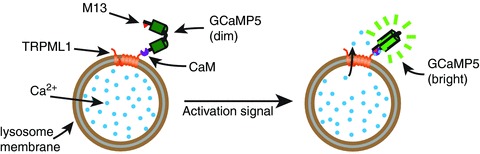
GCaMP5 contains a circularly permutated enhanced GFP (EGFP). The N-terminal of the fragment containing residues 149–238 is linked to the M13 peptide, while the C-terminal of the fragment containing residues 1–144 is linked to calmodulin (CaM). The binding of Ca2+ ions to calmodulin causes a conformational change and calmodulin then binds to the M13 peptide, bringing the two parts of the permutated EGFP together, reconstituting a functional EGFP. When GCaMP5 is attached to a lysosomal membrane protein (e.g. TRPML1), it works as a sensor for local Ca2+ release.
Ca2+ sensors in membrane trafficking
Distinct Ca2+ sensors are implicated in different membrane fusion and fission events (Fig. 2B; Ghislat & Knecht, 2013). Lysosomal exocytosis is mediated by synaptotagmin-VII with VAMP7 as the major v-SNARE (Reddy et al. 2001; Czibener et al. 2006). It is not known whether synaptotagmin-VII also regulates the fusion between lysosomes and other endocytic vesicles. There are at least 16 members of the synaptotagmin family, and many of them sense and respond to different ranges of Ca2+ (Bhalla et al. 2005). This suggests that different synaptotagmins may be expressed in different cell types and may reside on different intracellular vesicles to promote vesicle fusion and fission. Apart from synaptotagmins, calmodulin (CaM) has been shown to be important for vesicle fusion in yeast studies and in vitro reconstitution systems (Peters & Mayer, 1998; Pryor et al. 2000), but its involvement in intact mammalian cells is still unclear. Additionally, other Ca2+-binding proteins such as ALG-2 may serve as Ca2+ sensors in the late endosomal and lysosomal membranes. Importantly, ALG-2 binds to the N-terminal cytosolic tail of TRPML1, probably to modulate its function in trafficking events (Vergarajauregui et al. 2009). Future studies examining the recruitment kinetics of Ca2+ sensors in vesicular membranes will provide a greater understanding of Ca2+ signalling in membrane trafficking.
Concluding remarks
With the recent development of high-resolution live imaging methods, we are just beginning to understand some aspects of membrane signalling and trafficking. The coordinated interactions between small GTPases, lipids and Ca2+ signalling are essential; however, other signalling events should not be underestimated. For example, luminal pH is known to affect endosomal and lysosomal membrane trafficking, and acidification is tightly associated with endosome maturation. In addition, the lysosome has a high Na+ content, and Na+-selective TPC channels are found in lysosomal membranes. This suggests that endosomal membrane potential may undergo large regulatory changes; however, this possibility has yet to be explored. Continued advances in live imaging, molecular probes and electrophysiological methods to measure endosomal membrane potential will allow us to continue to elucidate how membrane trafficking is regulated.
Acknowledgments
We thank Dr Harald Stenmark for the FYVE*2-GFP construct. We appreciate the encouragement and helpful comments from other members of the Xu laboratory.
Glossary
- Atg
autophagy-related gene
- EEA1
early endosome antigen 1
- ER
endoplasmic reticulum
- GAP
GTPase-activating protein
- GECIs
genetically encoded Ca2+ indicators
- GEF
guanine nucleotide exchange factor
- KO
knockout
- mTOR
mammalian or mechanistic target of rapamycin
- NAADP
nicotinic acid adenine dinucleotide phosphate
- PATs
proton-assisted amino acid transporters
- PI
phosphatidylinositol
- SNARE
soluble N-ethylmaleimide-sensitive fusion attachment protein receptor
- TRPML
transient receptor potential cation channel, mucolipin subfamily
- TPC
two-pore channel
- V-ATPase
vacuolar-type H+-ATPase
- VAMP
vesicle-associated membrane protein
Additional information
Competing interests
None declared.
Funding
This work in the author's laboratory is supported by NIH grants (NS062792, MH096595 and AR060837 to H.X.).
References
- Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Zn2+ depletion blocks endosome fusion. Biochem J. 1995;312:919–923. doi: 10.1042/bj3120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23:452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. Imaging and manipulating phosphoinositides in living cells. J Physiol. 2007;582:927–937. doi: 10.1113/jphysiol.2007.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bhalla A, Tucker WC, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol Biol Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer J, Rey J, Dehmelt L, Mazel T, Wu YW, Bastiaens P, Goody RS, Itzen A. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200:287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflugers Arch. 2004;447:776–779. doi: 10.1007/s00424-003-1073-4. [DOI] [PubMed] [Google Scholar]
- Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Chen JL, Ahluwalia JP, Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J Biol Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu L. Autophagic lysosome reformation. Exp Cell Res. 2013;319:142–146. doi: 10.1016/j.yexcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1–3 channels. FEBS Lett. 2010;584:2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X, Wang S, Huang Y, Stamnes M, Chen JL. Roles of rho GTPases in intracellular transport and cellular transformation. Int J Mol Sci. 2013;14:7089–7108. doi: 10.3390/ijms14047089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Means TK, Diefenbach TJ, Moita LF, Friday RP, Sever S, Campanella GS, Abrazinski T, Manice LA, Moita C, Andrews NW, Wu D, Hacohen N, Luster AD. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. 2010;11:495–502. doi: 10.1038/ni.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho MF, Prata MJ, Alves S. A shortcut to the lysosome: the mannose-6-phosphate-independent pathway. Mol Genet Metab. 2012;107:257–266. doi: 10.1016/j.ymgme.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Cullen PJ. Phosphoinositides and the regulation of tubular-based endosomal sorting. Biochem Soc Trans. 2011;39:839–850. doi: 10.1042/BST0390839. [DOI] [PubMed] [Google Scholar]
- Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174:997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyar V, Hafez IM, Bray C, Zucker RS. Dance of the SNAREs: Assembly and rearrangements detected with FRET at neuronal synapses. J Neurosci. 2013;33:5507–5523. doi: 10.1523/JNEUROSCI.2337-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Domanska MK, Kiessling V, Stein A, Fasshauer D, Tamm LK. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J Biol Chem. 2009;284:32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Shen D, Chen S, Liu M, Wang Y, Mills E, Cheng X, Delling M, Xu H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J, Lou HF, Chen Z, Li XM, Luo QM, Duan S. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012;22:1022–1033. doi: 10.1038/cr.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, McEwen RK, Mayes A, Hughes DC, Beggs JD, Michell RH. Vac14 controls PtdIns(3,5)P2 synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol. 2002;12:885–893. doi: 10.1016/s0960-9822(02)00891-6. [DOI] [PubMed] [Google Scholar]
- Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006a;5:723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duex JE, Tang F, Weisman LS. The Vac14p–Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol. 2006b;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B. Phosphoinositides: lipid regulators of membrane proteins. J Physiol. 2010;588:3179–3185. doi: 10.1113/jphysiol.2010.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Fraldi A, Annunziata F, Lombardi A, Kaiser HJ, Medina DL, Spampanato C, Fedele AO, Polishchuk R, Sorrentino NC, Simons K, Ballabio A. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010;29:3607–3620. doi: 10.1038/emboj.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Galvez T, Gilleron J, Zerial M, O'Sullivan GA. SnapShot: Mammalian Rab proteins in endocytic trafficking. Cell. 2012;151:234–234 e2. doi: 10.1016/j.cell.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zorman S, Gundersen G, Xi Z, Ma L, Sirinakis G, Rothman JE, Zhang Y. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science. 2012;337:1340–1343. doi: 10.1126/science.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Gerasimenko OV, Petersen OH. Membrane repair: Ca2+-elicited lysosomal exocytosis. Curr Biol. 2001;11:R971–974. doi: 10.1016/s0960-9822(01)00577-2. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- Ghislat G, Knecht E. Ca2+-sensor proteins in the autophagic and endocytic traffic. Curr Protein Pept Sci. 2013;14:97–110. doi: 10.2174/13892037112139990033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hassan S, Wahl-Schott C, Biel M. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J Pharmacol Exp Ther. 2012;342:236–244. doi: 10.1124/jpet.112.192880. [DOI] [PubMed] [Google Scholar]
- Guse AH. Linking NAADP to ion channel activity: a unifying hypothesis. Sci Signal. 2012;5:pe18. doi: 10.1126/scisignal.2002890. [DOI] [PubMed] [Google Scholar]
- Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VW, Prekeris R. Transport at the recycling endosome. Curr Opin Cell Biol. 2010;22:528–534. doi: 10.1016/j.ceb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P. Optogenetic control of phosphoinositide metabolism. Proc Natl Acad Sci U S A. 2012;109:E2316–2323. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, Rappolee D, Shisheva A. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem. 2011;286:13404–13413. doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S, Kiger AA. Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat Rev Mol Cell Biol. 2012;13:463–470. doi: 10.1038/nrm3379. [DOI] [PubMed] [Google Scholar]
- Jung JJ, Inamdar SM, Tiwari A, Choudhury A. Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci Rep. 2012;32:383–391. doi: 10.1042/BSR20120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Wang JT, Castro NA, Hamilton NA, Town L, Brown DL, Meunier FA, Brown NF, Stow JL, Teasdale RD. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J. 2010;29:1331–1347. doi: 10.1038/emboj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci. 2013;126:60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011;3:a003970. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–614. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sanjurjo CI, Tovey SC, Prole DL, Taylor CW. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J Cell Sci. 2013;126:289–300. doi: 10.1242/jcs.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue HV, Wardyn JD, Burgoyne RD, Haynes LP. Generation and characterization of a lysosomally targeted, genetically encoded Ca2+-sensor. Biochem J. 2013;449:449–457. doi: 10.1042/BJ20120898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Mayinger P. Phosphoinositides and vesicular membrane traffic. Biochim Biophys Acta. 2012;1821:1104–1113. doi: 10.1016/j.bbalip.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- Noda T, Matsunaga K, Taguchi-Atarashi N, Yoshimori T. Regulation of membrane biogenesis in autophagy via PI3P dynamics. Semin Cell Dev Biol. 2010;21:671–676. doi: 10.1016/j.semcdb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Obara K, Sekito T, Niimi K, Ohsumi Y. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem. 2008;283:23972–23980. doi: 10.1074/jbc.M803180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Ogmundsdottir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One. 2012;7:e36616. doi: 10.1371/journal.pone.0036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. Biochem J. 2009;418:233–246. doi: 10.1042/BJ20082105. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, Cao Z, Li Y, Ren H, Zhang C, Li L, Chen S, Xi J, Yu L. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat Cell Biol. 2012;14:924–934. doi: 10.1038/ncb2557. [DOI] [PubMed] [Google Scholar]
- Rong Y, McPhee CK, Deng S, Huang L, Chen L, Liu M, Tracy K, Baehrecke EH, Yu L, Lenardo MJ. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci U S A. 2011;108:7826–7831. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Bretscher A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011;21:515–525. doi: 10.1016/j.tcb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Schröder B, Saftig P. Lysosomal membrane proteins and their central role in physiology. Traffic. 2013;14:739–748. doi: 10.1111/tra.12056. [DOI] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: Phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays. 2011;33:448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaili SS, Pereira GJ, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu YT. The role of calcium stores in apoptosis and autophagy. Curr Mol Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Patel B, Aphkhazava D, Macian F, Santambrogio L, Shields D, Cuervo AM. The lipid kinase PI4KIIIβ preserves lysosomal identity. EMBO J. 2013;32:324–339. doi: 10.1038/emboj.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Thorngren N, Fratti RA, Wickner W. Ion regulation of homotypic vacuole fusion in Saccharomyces cerevisiae. J Biol Chem. 2005;280:16754–16762. doi: 10.1074/jbc.M500421200. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CM. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br J Pharmacol. 2011;164:1802–1816. doi: 10.1111/j.1476-5381.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Zeng Q, Hong W. VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes. J Cell Sci. 2007;120:1028–1041. doi: 10.1242/jcs.03387. [DOI] [PubMed] [Google Scholar]
- Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- van den Bogaart G, Thutupalli S, Risselada JH, Meyenberg K, Holt M, Riedel D, Diederichsen U, Herminghaus S, Grubmuller H, Jahn R. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat Struct Mol Biol. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol Biol Cell. 2000;11:2327–2333. doi: 10.1091/mbc.11.7.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li X, Xu H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A. 2012;109:11384–11389. doi: 10.1073/pnas.1202194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCartney AJ, Zolov SN, Ferguson CJ, Meisler MH, Sutton MA, Weisman LS. Modulation of synaptic function by VAC14, a protein that regulates the phosphoinositides PI(3,5)P2 and PI(5)P. EMBO J. 2012;31:3442–3456. doi: 10.1038/emboj.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A. 2007;104:17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolov SN, Bridges D, Zhang Y, Lee WW, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, Saltiel AR, Meisler MH, Russell MW, Weisman LS. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A. 2012;109:17472–17477. doi: 10.1073/pnas.1203106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


