Abstract
Studies performed at the beginning of the last century revealed the importance of carbohydrate as a fuel during exercise, and the importance of muscle glycogen on performance has subsequently been confirmed in numerous studies. However, the link between glycogen depletion and impaired muscle function during fatigue is not well understood and a direct cause-and-effect relationship between glycogen and muscle function remains to be established. The use of electron microscopy has revealed that glycogen is not homogeneously distributed in skeletal muscle fibres, but rather localized in distinct pools. Furthermore, each glycogen granule has its own metabolic machinery with glycolytic enzymes and regulating proteins. One pool of such glycogenolytic complexes is localized within the myofibrils in close contact with key proteins involved in the excitation–contraction coupling and Ca2+ release from the sarcoplasmic reticulum (SR). We and others have provided experimental evidence in favour of a direct role of decreased glycogen, localized within the myofibrils, for the reduction in SR Ca2+ release during fatigue. This is consistent with compartmentalized energy turnover and distinctly localized glycogen pools being of key importance for SR Ca2+ release and thereby affecting muscle contractility and fatigability.
 |
Niels Ørtenblad is Associate Professor based at the University of Southern Denmark. His research is focused on basic mechanisms linking cell energy production with energy utilization, with special reference to mechanisms of muscle fatigue. In his research he has used various techniques from the whole body level to mechanically skinned muscle fibres, and organelles. HåkanWesterblad is professor in cellular muscle physiology at the Karolinska Institutet in Stockholm, Sweden. He and his colleagues developed techniques to study contractile function and Ca2+ handling in isolated, intact fibres from mammalian skeletal muscle. A major focus of his research has been on cellular mechanisms of skeletal muscle fatigue. Joachim Nielsen is post doc at the Institute of Sports Science and Clinical Biomechanics, University of Southern Denmark. His major research focus has been to elucidate the role and regulation of local storage of glycogen molecules in specific micro-domains within muscle fibres.
Introduction
Glycogen is a complex glucose polymer found in most species in the animal kingdom. It serves as a storage form for glucose found in a variety of tissues, although quantitatively mainly in skeletal muscles and the liver. The wide occurrence of glycogen in skeletal muscles indicates that it is essential in providing a mechanism by which ATP rapidly can be produced in muscle cells, which display a high and rapidly fluctuating energy turnover. The responses of muscle glycogen to such perturbations as exercise and diet are very well documented in studies of humans as well as various animal models (Bergström et al. 1967; Jensen & Richter, 2012). In addition, it has been understood for more than half a century that the ability of muscle to exercise is seriously compromised when the glycogen store is reduced to low levels, even when there is an abundance of other fuel sources (Bergström et al. 1967). Thus, Hultman and co-workers demonstrated a strong correlation between muscle glycogen content and endurance capacity during prolonged cycling exercise (Bergström et al. 1967) and an inability to continue such exercise when the glycogen stores were exhausted (Hermansen et al. 1967). These observations have subsequently been confirmed in numerous studies and it is now well established that there is a close relationship between muscle glycogen content and fatigue resistance, both during prolonged (more than 1 h) and during high-intensity intermittent exercise (Pernow & Saltin, 1971; Gollnick et al. 1972; Bangsbo et al. 1992; Hargreaves et al. 1995). However, the precise mechanism linking glycogen to muscle function remains elusive. The fundamental aspects of glycogen regulation are also very clearly documented in any biochemistry context, with most single enzymatic and regulatory steps well described. Thus, it is noteworthy that we cannot explain precisely why muscle function is impaired when muscle glycogen is low and how the glycogen stores affect basic cell function. The relationship between low muscle glycogen stores and fatigue is generally considered to be explained by a compromised rate of ATP regeneration and this aspect has been covered in other reviews (Sahlin et al. 1998; Allen et al. 2008; Jensen & Richter, 2012). In this review we put forward evidence that in skeletal muscle, glycogen should not only be considered as a form of global carbohydrate storage but also a dynamic molecule regulating distinct and spatially restricted cellular functions.
Role of glycogen in muscle fatigue
The most recognized theory for the association between low muscle glycogen levels and impaired contractile function is that glycogen is an essential substrate, the depletion of which results in a reduction in the rate of ATP regeneration. As a consequence, the muscle is unable to maintain an adequate global energy supply to one or more of the processes involved in excitation and contraction, leading to an inability to translate the motor drive into an expected force, i.e. fatigue develops. This is supported by observations of phosphocreatine (PCr) decreases along with an increase in free ADP and IMP (inositol monophosphate) following prolonged glycogen-depleting exercise (Norman et al. 1988; Sahlin et al. 1997). However, the energy deficiency theory in skeletal muscle fatigue is challenged by both in vitro and in vivo studies demonstrating a strong association between low glycogen and decreased muscle function even after recovery periods, where ATP levels would be normal (Bangsbo et al. 1992; Chin & Allen, 1997). However, observations on whole body experiments or intact muscle preparations will not provide direct evidence for a specific role of glycogen on muscle function, because changes in total muscle glycogen content bring about other metabolic changes, which may affect the excitation–contraction coupling (E-C coupling; Stephenson et al. 1999; Allen et al. 2008). Thus, our knowledge of the mechanism responsible for glycogen modulation of muscle function mainly originates from studies at the single fibre or organelle level.
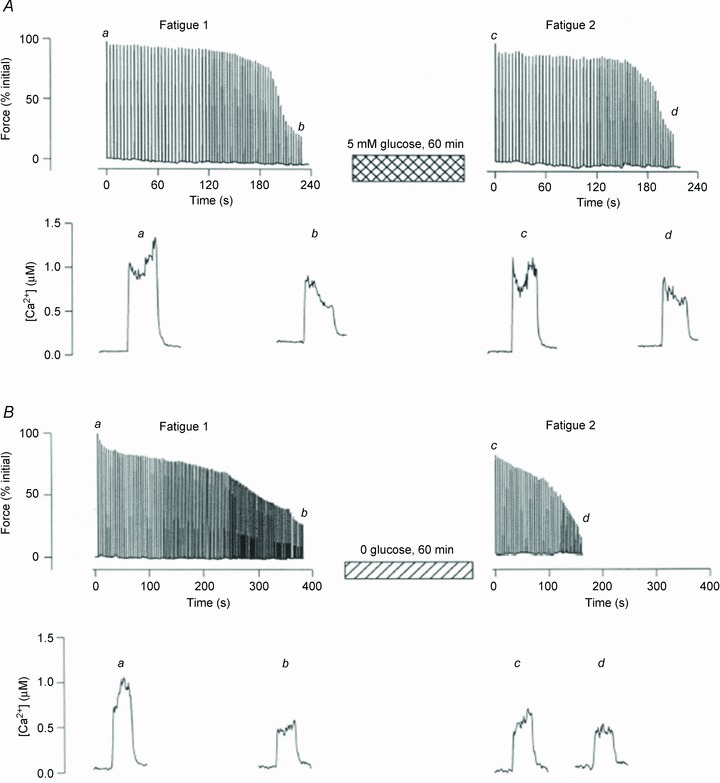
Studies on both rodent single fibres and human studies have pointed to a modulating role of glycogen availability on sarcoplasmic reticulum (SR) Ca2+ handling (Chin et al. 1997; Duhamel et al. 2006b; Nielsen et al. 2009; Ørtenblad et al. 2011). Experiments with simultaneous measurements of force and intracellular free [Ca2+] ([Ca2+]i) have been used to identify the cellular mechanisms linking low glycogen to fatigue. By using both rat and toad fibre bundles it has been demonstrated that when recovery after glycogen-reducing contractions occurs in the absence of glucose, glycogen does not recover and fibre bundles fatigue more rapidly and show reduced tetanic [Ca2+]i transients in a subsequent fatigue run (Chin et al. 1997; Kabbara et al. 2000; Helander et al. 2002). Thus, a reduced level of glycogen is associated with a faster decrease of tetanic [Ca2+]i during repeated contractions (Fig. 1). Studies on SR vesicles from human muscle support a link between reduced muscle glycogen and decreased tetanic [Ca2+]i (Duhamel et al. 2006a,b; Ørtenblad et al. 2011). Duhamel et al. (2006b) examined the relationship between muscle glycogen content and SR vesicle Ca2+ release rate during a prolonged fatiguing cycling session at 70%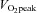 . To manipulate muscle glycogen concentrations, exercise was preceded by a glycogen-depleting exercise session followed by 4 days of either low or high carbohydrate (CHO) diet. Muscle glycogen content was markedly reduced at the initiation of exercise with the low CHO diet and deteriorations in SR Ca2+ release occurred earlier during exercise in this condition compared to the high CHO diet (Duhamel et al. 2006b). We have recently demonstrated an association between muscle glycogen and SR vesicle Ca2+ release rate by manipulating muscle glycogen levels in the recovery period following fatiguing cross country skiing exercise (Ørtenblad et al. 2011). Skiers were provided with either a CHO-enriched diet or water during the initial 4 h following exercise. After 4 h of recovery with CHO, SR vesicle Ca2+ release rate was normalized and muscle glycogen content was markedly increased compared to post exercise, whereas both SR release and glycogen remained depressed in the group which did not receive CHO. Together, data from SR vesicle experiments indicate a mechanistic role of glycogen on SR Ca2+ release. A reduced SR Ca2+ release rate will per se cause a decrease in tetanic [Ca2+]i, which is very much in line with the studies on isolated fibres demonstrating a faster decrease of tetanic [Ca2+]i in fibres with low levels of glycogen.
. To manipulate muscle glycogen concentrations, exercise was preceded by a glycogen-depleting exercise session followed by 4 days of either low or high carbohydrate (CHO) diet. Muscle glycogen content was markedly reduced at the initiation of exercise with the low CHO diet and deteriorations in SR Ca2+ release occurred earlier during exercise in this condition compared to the high CHO diet (Duhamel et al. 2006b). We have recently demonstrated an association between muscle glycogen and SR vesicle Ca2+ release rate by manipulating muscle glycogen levels in the recovery period following fatiguing cross country skiing exercise (Ørtenblad et al. 2011). Skiers were provided with either a CHO-enriched diet or water during the initial 4 h following exercise. After 4 h of recovery with CHO, SR vesicle Ca2+ release rate was normalized and muscle glycogen content was markedly increased compared to post exercise, whereas both SR release and glycogen remained depressed in the group which did not receive CHO. Together, data from SR vesicle experiments indicate a mechanistic role of glycogen on SR Ca2+ release. A reduced SR Ca2+ release rate will per se cause a decrease in tetanic [Ca2+]i, which is very much in line with the studies on isolated fibres demonstrating a faster decrease of tetanic [Ca2+]i in fibres with low levels of glycogen.
Figure 1. Fatigue occurs more rapidly after recovery without glucose.
Original data traces of force (upper panels) and [Ca2+]i (lower panels) obtained in single fast-twitch mouse muscle fibres during fatigue induced by repeated tetanic contractions (100 Hz, 350 ms). This type of fatiguing stimulation resulted in marked decrease in glycogen to ∼30% of the control value (Chin & Allen, 1997). Force and [Ca2+]i were well maintained during a second fatigue run after 60 min of recovery in 5.5 mm glucose (A), which restored glycogen to pre-fatigue levels. Conversely, force and [Ca2+]i were not fully restored and fatigue occurred more rapidly after 60 min of recovery without glucose (B), where glycogen stores remained depleted. Adapted from Figs 5 and 6 of Chin & Allen (1997).
Furthermore, in mechanically skinned muscle fibres, where global ATP can be kept high and constant, low glycogen content is associated with an irreversible force depression during repeated tetanic contractions (Stephenson et al. 1999; Barnes et al. 2001; Nielsen et al. 2009). In this preparation the extensive transverse tubular system (t-system), which represents the greater part of the plasma membrane, reseals and becomes normally polarized when placed in a medium mimicking the cytosolic environment of the intact cell (Lamb et al. 1995; Stephenson, 2006). With this preparation it is possible to measure fibre excitability and force production while at the same time having direct access to the intracellular environment. This makes it possible to estimate the effect of muscle fibre glycogen content per se without changes in other metabolites, i.e. keeping PCr and ATP high and constant. In mechanically skinned toad muscle fibres the ability to respond to t-tubular depolarizations by ion substitution correlated with muscle glycogen content (Stephenson et al. 1999). This was later both confirmed (Barnes et al. 2001) and disproved (Goodman et al. 2005) using rat muscle fibres. In a later experiment, mechanically skinned rat muscle fibres were activated by electric field stimulation and glycogen content was estimated by electron microscopy, also providing an estimate of the subcellular localization of glycogen (Nielsen et al. 2009). Here, the fatigability (number of tetanic contractions until 50% force reduction) correlated only with the distinct deposition of glycogen located within the myofibrils (intramyofibrillar glycogen, see next section; Nielsen et al. 2009). These results demonstrate that the muscle glycogen content affects muscle function in contracting single muscle fibres under conditions where the global myoplasmic ATP level is kept high and constant. Thus, glycogen affects the E-C coupling despite global ATP being held constant, which argues against a direct metabolic effect of low glycogen levels at the whole cell level. However, this does not exclude a role of glycogen on compartmentalized energy transfer in the cell. To sum up, depletion of glycogen during prolonged, exhausting exercise may contribute to fatigue by causing decreased SR Ca2+ release and the underlying mechanism seems to be independent of the overall energy level of muscle fibres.
The glycogen granule and glycogen localization
Glycogen is often interpreted as if it is uniformly distributed in the cell, providing an average concentration of the cell. Interestingly, electron microscopy has revealed that glycogen is found as discrete glycogen particles located in distinct pools within the fibres (Wanson & Drochman, 1968; Friden et al. 1989; Marchand et al. 2002, 2007; Nielsen et al. 2010, 2011). Furthermore, each glycogen granule has its own metabolic machinery with glycolytic enzymes and regulating proteins (Wanson & Drochman, 1972; Graham et al. 2010). Based on two-dimensional electron microscopy images, three distinct intracellular pools of glycogen have been identified: (1) subsarcolemmal glycogen, just beneath the sarcolemma; (2) intermyofibrillar glycogen, located between the myofibrils, mainly at the level of the I-band close to mitochondria and SR; and (3) intramyofibrillar glycogen, in the myofibril, mainly near the z-line (Fig. 2). Estimates of the relative distribution of glycogen in the distinct pools in humans have revealed that the major glycogen pool is the intermyofibrillar one, constituting roughly 75% of the total glycogen store, whereas intramyofibrillar and subsarcolemmal glycogen accounts for 5–15% each. The relative distribution is, however, to a large degree dependent on fibre type, training status, immobilization, exercise and species (rat, human; see Nielsen & Ørtenblad, 2013).
Figure 2. Glycogen content in three subcellular localizations in muscle before and after approximately 1 h of exhaustive exercise.
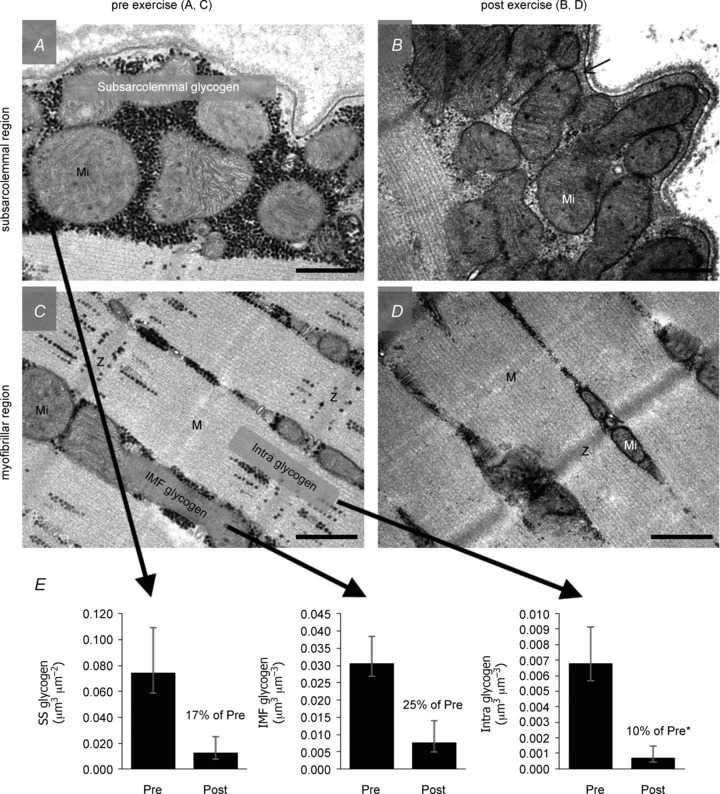
A–D, overview showing the typical localization pattern of glycogen particles in the subsarcolemmal region (A, B) and the myofibrillar region (C, D) of a muscle fibre, pre (A, C) and post (B, D) approximately 1 h of exhaustive exercise. Representative images originate from an arm (m. triceps brachii) type I fibre from trained subjects. Glycogen particles are visualized as black dots, with the intermyofibrillar (IMF) glycogen located between the myofibrils and the intramyofibrillar (Intra) glycogen within the myofibrils, mainly located in the I-band. Subsarcolemmal (SS) glycogen is located between the sarcolemma and the outermost myofibril. E, geometric mean glycogen content in the three localizations, with a significantly higher relative utilization of the Intra glycogen (5–15% of total glycogen) during the exercise compared to SS and IMF glycogen (5–15 and 75% of total glycogen, respectively). Mi, mitochondria; Z, Z-line; M, M-band. Scale bars = 0.5 μm. Original ×40,000 magnification. Adapted from Fig. 4 of Nielsen et al. (2011).
Role of glycogen localization during exercise
Early studies of the subcellular localization-dependent utilization of glycogen during exercise were performed in the 1980s by Ekblom and colleagues (Sjöström et al. 1982; Fridén et al. 1985, 1989). More recently, quantitative methods following unbiased stereological rules have demonstrated a larger relative utilization of intramyofibrillar glycogen during exercise compared to intermyofibrillar and subsarcolemmal glycogen (Marchand et al. 2007; Nielsen et al. 2011). In the study by Marchand et al. (2007), recreationally active subjects completed an exhaustive glycogen-depletion cycling exercise at 70% of 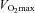 before biopsies were obtained during a 48 h recovery period. Immediately after the cycling exercise, glycogen levels were very low [36 mmol (kg dw)−1] and the relative contribution of intramyofibrillar glycogen was only 3% compared to 12–14% in resting controls (Marchand et al. 2002) or after 48 h recovery (Marchand et al. 2007). In the study by Nielsen et al. (2011), muscle biopsies were obtained both before and after exercise consisting of completion of a approximately 1 h cross country skiing time trial performed by elite skiers. In type I fibres before exercise, glycogen was distributed with 77% in the intermyofibrillar space, 12% in the intramyofibrillar space and 11% in the subsarcolemmal space. After exercise, intramyofibrillar glycogen was reduced by 90% compared to reductions by only 75% in intermyofibrillar glycogen and 83% in subsarcolemmal glycogen (Fig. 2); thus, the relative content of intramyofibrillar glycogen was decreased from 11% before exercise to only 6% after exercise. Interestingly, after exercise there was a correlation between the decreased rate of Ca2+ release in SR vesicles and the reduction in the intramyofibrillar glycogen content, corroborating the results from rat single fibres (Nielsen et al. 2009). In the latter study (Nielsen et al. 2009), a fast partial recovery of force might indicate that the force reduction was due to some loss of excitability, hence suggesting that decreased intramyofibrillar glycogen may also be related to reduced Na,K-ATPase activity. An interesting perspective is that intramyofibrillar glycogen may be related to both SR Ca2+ release and t-system Na,K-ATPase activity, both processes located in the restricted space of the triadic junction, which ensures conversion of the action potential to SR Ca2+ release and subsequently muscle contraction. Together the t-system and the SR form a triadic junction, around 12 nm wide, with a diffusional restricted space for more than 90% of the t-system length, and with a high metabolic activity (Dulhunty et al. 1984; Han et al. 1992). In line with this, it has been shown that Na,K-ATPase preferential uses glycolytically derived ATP (Lynch & Paul, 1987; James et al. 1999; Dutka & Lamb, 2007).
before biopsies were obtained during a 48 h recovery period. Immediately after the cycling exercise, glycogen levels were very low [36 mmol (kg dw)−1] and the relative contribution of intramyofibrillar glycogen was only 3% compared to 12–14% in resting controls (Marchand et al. 2002) or after 48 h recovery (Marchand et al. 2007). In the study by Nielsen et al. (2011), muscle biopsies were obtained both before and after exercise consisting of completion of a approximately 1 h cross country skiing time trial performed by elite skiers. In type I fibres before exercise, glycogen was distributed with 77% in the intermyofibrillar space, 12% in the intramyofibrillar space and 11% in the subsarcolemmal space. After exercise, intramyofibrillar glycogen was reduced by 90% compared to reductions by only 75% in intermyofibrillar glycogen and 83% in subsarcolemmal glycogen (Fig. 2); thus, the relative content of intramyofibrillar glycogen was decreased from 11% before exercise to only 6% after exercise. Interestingly, after exercise there was a correlation between the decreased rate of Ca2+ release in SR vesicles and the reduction in the intramyofibrillar glycogen content, corroborating the results from rat single fibres (Nielsen et al. 2009). In the latter study (Nielsen et al. 2009), a fast partial recovery of force might indicate that the force reduction was due to some loss of excitability, hence suggesting that decreased intramyofibrillar glycogen may also be related to reduced Na,K-ATPase activity. An interesting perspective is that intramyofibrillar glycogen may be related to both SR Ca2+ release and t-system Na,K-ATPase activity, both processes located in the restricted space of the triadic junction, which ensures conversion of the action potential to SR Ca2+ release and subsequently muscle contraction. Together the t-system and the SR form a triadic junction, around 12 nm wide, with a diffusional restricted space for more than 90% of the t-system length, and with a high metabolic activity (Dulhunty et al. 1984; Han et al. 1992). In line with this, it has been shown that Na,K-ATPase preferential uses glycolytically derived ATP (Lynch & Paul, 1987; James et al. 1999; Dutka & Lamb, 2007).
In support for a role of an essential use of glycogen for Na,K-ATPase, it has been demonstrated that, in astrocytes, glycogen is a critical energy source for neurons with significant implications in brain function, i.e. synaptic activity and memory formation (Obel et al. 2012). Taken together, the distinct pool of intramyofibrillar glycogen may be important for energy status in the triad junction and low amount of glycogen in this restricted area between t-tubules and the SR may decrease SR Ca2+ release rate and Na,K-ATPase activity.
Mechanisms of glycogen modulating E-C coupling
What is the basis of the mechanism responsible for glycogen modulation of Ca2+ release from the SR? A consequence of microenvironments with high ATPase activity and the limited diffusability in the muscle cell is the need to compartmentalize metabolic pathways. In other words, the main ATPases depend on a structural organization of substrate and metabolic enzymes in the direct vicinity and cannot rely on simple diffusion (Ovadi & Saks, 2004). Thus, distinct channelling of the intermediates from the substrate through enzymatic pathways and transfer of ATP to the metabolically active sites will ensure a sufficient metabolic power without the need of free diffusion. In line with this, glycogen particles are found in distinct locations within this organization including just beneath the plasma membrane, close to SR and mitochondria, and within the myofibrils between the contractile filaments. The CK does channel ATP from sites of production to buffer free ADP and Pi (free phosphate) concentrations. CK (creatine kinase) is localized at the mitochondrial membrane, on the M- and I-band, and on the SR membrane (Rossi et al. 1990; Wegmann et al. 1992; Wallimann et al. 2011). CK may be present in the triad, but to our knowledge this has not been demonstrated. From a functional aspect, there is a clear association of muscle glycogen contents and muscle function in experiments with the mechanically skinned fibre (Stephenson et al. 1999; Nielsen et al. 2009). This association has been established under conditions where ATP and PCr are kept high and constant. Furthermore, the t-tubular Na,K-ATPase function is improved by addition of phospho(enol)pyruvate (supported by endogenous pyruvate kinase bound within the triad) even with 40 mm PCr present (Dutka & Lamb, 2007). Together, these results show that the triad junction is a highly restricted microenvironment, which may not be adequately buffered by CK channelling.
The observed effect of low glycogen on mechanically skinned fibres, where global ATP can be kept high and constant, might be taken as an argument for a non-metabolic role of glycogen (Stephenson et al. 1999; Nielsen et al. 2009). However, as discussed above, an alternative explanation is that restricted areas in the muscle cell (e.g. the triadic junction) with a limited access and high metabolic activity may still be metabolically affected by low glycogen levels. The association between the specific pool of intramyofibrillar glycogen and SR Ca2+ release rate combined with the preferential depletion of this glycogen pool during most forms of exercise argues for a specific role of this pool to secure a sufficient metabolic milieu in the triads. This idea supports the energy-deficient theory, stating that low glycogen levels may compromise ATP synthesis and importantly this may be exacerbated in the triads, which are out of diffusion equilibrium with global ATP (Han et al. 1992; Ovadi & Saks, 2004). However, at this stage a non-metabolic (e.g. structural) role of glycogen cannot be discarded. Furthermore, it is possible that the connection between glycogen and SR Ca2+ release is not related to glycogen as an energy source, but to the action of enzymes associated with the glycogen particles, which themselves might modulate the function of the SR Ca2+ release channels in their proximity, e.g. by phosphorylation or de-phosphorylation of proteins involved in E-C coupling (Hamilton & Serysheva, 2009; Sharma et al. 2012).
Conclusion
In this review we present evidence that the subcellular localization of glycogen has to be considered to fully understand the role of glycogen metabolism for skeletal muscle function in general and especially in relation to fatigue. Studies performed from the whole-body to organelle level suggest that the pool of intramyofibrillar glycogen has a key role during repeated contractions by counteracting contractile impairments caused by defective SR Ca2+ release (Fig. 3).
Figure 3. Tentative glycogen-dependent components in muscle fatigue.
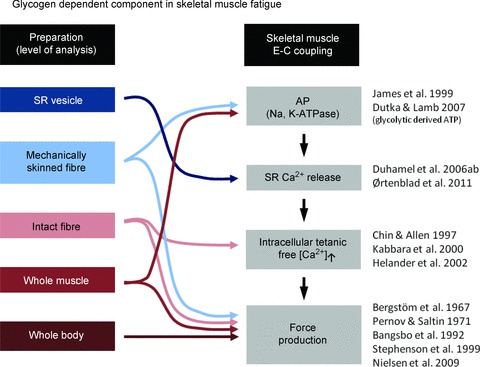
Measurements at the level from organelles (SR vesicles) to whole body experiments indicate a glycogen-dependent role in the E-C coupling failure leading to muscle fatigue. Arrows indicate the level in the series of events in the E-C coupling, where glycogen has been demonstrated to play a significant role.
Glossary
- CHO
carbohydrate
- E-C coupling
excitation–contraction coupling
- SR
sarcoplasmic reticulum
- t-system
transverse tubular system
Additional information
Competing interests
None.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy-production during exhaustive exercise in man. J Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M, Gibson LM, Stephenson DG. Increased muscle glycogen content is associated with increased capacity to respond to T-system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflugers Arch. 2001;442:101–106. doi: 10.1007/s004240000510. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hermansen L, Hultman E, Saltin B. Diet muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Norman B, Sollevi A, Jansson E. Increased IMP content in glycogen-depleted muscle fibres during submaximal exercise in man. Acta Physiol Scand. 1988;133:97–100. doi: 10.1111/j.1748-1716.1988.tb08385.x. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol. 1997;498:17–29. doi: 10.1113/jphysiol.1997.sp021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Balnave CD, Allen DG. Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol. 1997;272:C550–C559. doi: 10.1152/ajpcell.1997.272.2.C550. [DOI] [PubMed] [Google Scholar]
- Duhamel TA, Green HJ, Perco JG, Ouyang J. Effects of prior exercise and a low-carbohydrate diet on muscle sarcoplasmic reticulum function during cycling in women. J Appl Physiol. 2006a;101:695–706. doi: 10.1152/japplphysiol.00052.2006. [DOI] [PubMed] [Google Scholar]
- Duhamel TA, Perco JG, Green HJ. Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am J Physiol Regul Integr Comp Physiol. 2006b;291:R1100–R1110. doi: 10.1152/ajpregu.00858.2005. [DOI] [PubMed] [Google Scholar]
- Dulhunty A, Carter G, Hinrichsen C. The membrane capacity of mammalian skeletal-muscle fibers. J Muscle Res Cell Motil. 1984;5:315–332. doi: 10.1007/BF00713110. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol Cell Physiol. 2007;293:C967–C977. doi: 10.1152/ajpcell.00132.2007. [DOI] [PubMed] [Google Scholar]
- Fridén J, Seger J, Ekblom B. Implementation of periodic acid-thiosemicarbazide-silver proteinate staining for ultrastructural assessment of muscle glycogen utilization during exercise. Cell Tissue Res. 1985;242:229–232. doi: 10.1007/BF00225582. [DOI] [PubMed] [Google Scholar]
- Fridén J, Seger J, Ekblom B. Topographical localization of muscle glycogen – an ultrahistochemical study in the human vastus lateralis. Acta Physiol Scand. 1989;135:381–391. doi: 10.1111/j.1748-1716.1989.tb08591.x. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert CW, Saltin B, Piehl K. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972;33:421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- Goodman C, Blazev R, Stephenson G. Glycogen content and contractile responsiveness to T-system depolarization in skinned muscle fibres of the rat. Clin Exp Pharmacol Physiol. 2005;32:749–756. doi: 10.1111/j.1440-1681.2005.04260.x. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yuan Z, Hill AK, Wilson RJ. The regulation of muscle glycogen: the granule and its proteins. Acta Physiol (Oxf) 2010;199:489–498. doi: 10.1111/j.1748-1716.2010.02131.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SL, Serysheva II. Ryanodine receptor structure: progress and challenges. J Biol Chem. 2009;284:4047–4051. doi: 10.1074/jbc.R800054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Thieleczek R, Varsányi M, Heilmeyer LMG. Compartmentalized ATP synthesis in skeletal-muscle triads. Biochemistry. 1992;31:377–384. doi: 10.1021/bi00117a010. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose-uptake during exercise in humans. J Appl Physiol. 1995;78:288–292. doi: 10.1152/jappl.1995.78.1.288. [DOI] [PubMed] [Google Scholar]
- Helander I, Westerblad H, Katz A. Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Physiol Cell Physiol. 2002;282:C1306–C1312. doi: 10.1152/ajpcell.00490.2001. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fischer JE. Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol. 1999;277:E176–E186. doi: 10.1152/ajpendo.1999.277.1.E176. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590:1069–1076. doi: 10.1113/jphysiol.2011.224972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA, Nguyen LT, Stephenson GMM, Allen DG. Intracellular calcium during fatigue of cane toad skeletal muscle in the absence of glucose. J Muscle Res Cell Motil. 2000;21:481–489. doi: 10.1023/a:1005650425513. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular Ca2+ abolishes excitation–contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Paul RJ. Compartmentation of carbohydrate-metabolism in vascular smooth-muscle. Am J Physiol. 1987;252:C328–C334. doi: 10.1152/ajpcell.1987.252.3.C328. [DOI] [PubMed] [Google Scholar]
- Marchand I, Chorneyko K, Tarnopolsky M, Hamilton S, Shearer J, Potvin J, Graham TE. Quantification of subcellular glycogen in resting human muscle: granule size, number, and location. J Appl Physiol. 2002;93:1598–1607. doi: 10.1152/japplphysiol.00585.2001. [DOI] [PubMed] [Google Scholar]
- Marchand I, Tarnopolsky M, Adamo KB, Bourgeois JM, Chorneyko K, Graham TE. Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol. 2007;580:617–628. doi: 10.1113/jphysiol.2006.122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Holmberg HC, Schrøder HD, Saltin B, Ørtenblad N. Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol. 2011;589:2871–2885. doi: 10.1113/jphysiol.2010.204487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Schrøder HD, Rix CG, Ørtenblad N. Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol. 2009;587:3679–3690. doi: 10.1113/jphysiol.2009.174862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Suetta C, Hvid LG, Schrøder HD, Aagaard P, Ørtenblad N. Subcellular localization-dependent decrements in skeletal muscle glycogen and mitochondria content following short-term disuse in young and old men. Am J Physiol Endocrinol Metab. 2010;299:E1053–E1060. doi: 10.1152/ajpendo.00324.2010. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Ørtenblad N. Physiological aspects of the subcellular localization of glycogen in skeletal muscle. Appl Physiol Nutr Metab. 2013;38:91–99. doi: 10.1139/apnm-2012-0184. [DOI] [PubMed] [Google Scholar]
- Norman B, Sollevi A, Jansson E. Increased IMP content in glycogen-depleted muscle fibres during submaximal exercise in man. Acta Physiol Scand. 1988;133:97–100. doi: 10.1111/j.1748-1716.1988.tb08385.x. [DOI] [PubMed] [Google Scholar]
- Obel LF, Müller MS, Walls AB, Sickmann HM, Bak LK, Waagepetersen HS, Schousboe A. Brain glycogen – new perspectives on its metabolic function and regulation at the subcellular level. Front Neuroenergetics. 2012;4:3. doi: 10.3389/fnene.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B, Holmberg HC. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol. 2011;589:711–725. doi: 10.1113/jphysiol.2010.195982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadi J, Saks V. On the origin of intracellular compartmentation and organized metabolic systems. Mol Cell Biochem. 2004;256:5–12. doi: 10.1023/b:mcbi.0000009855.14648.2c. [DOI] [PubMed] [Google Scholar]
- Pernow B, Saltin B. Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol. 1971;31:416–422. doi: 10.1152/jappl.1971.31.3.416. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Eppenberger HM, Volpe P, Cotrufo R, Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem. 1990;265:5258–5266. [PubMed] [Google Scholar]
- Sahlin K, Söderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol. 1997;273:C172–C278. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M, Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162:261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- Sharma P, Ishiyama N, Nair U, Li WP, Dong AP, Miyake T, Wilson A, Ryan T, MacLennan DH, Kislinger T, Ikura M, Dhe-Paganon S, Gramolini AO. Structural determination of the phosphorylation domain of the ryanodine receptor. FEBS J. 2012;279:3952–3964. doi: 10.1111/j.1742-4658.2012.08755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström M, Fridén J, Ekblom B. Fine structural details of human muscle fibers after fibre type specific glycogen depletion. Histochemistry. 1982;76:425–438. doi: 10.1007/BF00489899. [DOI] [PubMed] [Google Scholar]
- Stephenson DG. Tubular system excitability: an essential component of excitation–contraction coupling in fast-twitch fibres of vertebrate skeletal muscle. J Muscle Res Cell Motil. 2006;27:259–274. doi: 10.1007/s10974-006-9073-6. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Nguyen LT, Stephenson GMM. Glycogen content and excitation–contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol. 1999;519:177–187. doi: 10.1111/j.1469-7793.1999.0177o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–96. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson JC, Drochman P. Rabbit skeletal muscle glycogen – a morphological and biochemical study of glycogen beta-particles isolated by precipitation-centrifugation method. J Cell Biol. 1968;38:130–150. doi: 10.1083/jcb.38.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson JC, Drochman P. Role of sarcoplasmic reticulum in glycogen metabolism – binding of phosphorylase, phosphorylase kinase, and primer complexes to sarcovesicles of rabbit skeletal-muscle. J Cell Biol. 1972;54:206–224. doi: 10.1083/jcb.54.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann G, Zanolla E, Eppenberger HM, Wallimann T. In situ compartmentation of creatine kinase in intact sarcomeric muscle: the acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil. 1992;13:420–35. doi: 10.1007/BF01738037. [DOI] [PubMed] [Google Scholar]


