Abstract
We have previously demonstrated a principal role for nitric oxide (NO) in the endothelium/shear-dependent regulation of contractility in rat thoracic duct (TD). In this study we tested the hypothesis that cyclic guanosine monophosphate (cGMP) and the dependent protein kinase (PKG) are central to the intrinsic and extrinsic flow-dependent modulation of lymphatic contractility. Lymphatic diameters and indices of pumping in isolated, cannulated and pressurized segments of rat TD were measured. The influences of increased transmural pressure (1–5 cmH2O) and imposed flow (1–5 cm H2O transaxial pressure gradients) on lymphatic function were studied before and after: (1) inhibition of guanylate cyclase (GC) with and without a NO donor; (2) application of stable cGMP analogue; and (3) inhibition of the cGMP activation of PKG. Additionally, Western blotting and immunofluorescent tissue staining were used to analyse the PKG isoforms expressed in TD. We found that the GC inhibitor ODQ induced changes in TD contractility similar to NO synthase blockade and prevented the relaxation induced by the NO donor S-nitroso-N-acetylpenicillamine. The cGMP analogue, 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate sodium salt (8pCPTcGMP), mimicked the extrinsic flow-induced relaxation in a dose-dependent manner, whereas treatment with the cGMP/PKG inhibitor, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio)-, Rp-isomer, triethylammonium salt (Rp-8-Br-PETcGMPS), eliminated intrinsic flow-dependent relaxation, and largely inhibited extrinsic flow-dependent relaxation. Western blotting demonstrated that both PKG-Iα and -Iβ isoforms are found in TD, with ∼10 times greater expression of the PKG-Iα protein in TD compared with the aorta and vena cava. The PKG-Iβ isoform expressed equally in TD and vena cava, both being ∼2 times higher than that in the aorta. Immunofluorescent labelling of PKG-Iα protein in the wall of rat thoracic duct confirmed its localization inside TD muscle cells. These findings demonstrate that cGMP is critical to the flow-dependent regulation of TD contractility; they also indicate an important involvement of PKG, especially PKG-Iα in these processes and identifies PKG protein as a potential therapeutic target.
Key points
Nitric oxide plays a principal role in the lymphatic endothelium/shear-dependent regulation of contractility in rat thoracic duct.
In this study we tested the hypothesis that cyclic guanosine monophosphate (cGMP) and the dependent protein kinase (PKG) are central to the intrinsic and extrinsic flow-dependent modulation of lymphatic contractility.
Both PKG-Iα and -Iβ isoforms are found in the thoracic duct, with ∼10 times greater expression of the PKG-Iα protein compared with the aorta and vena cava.
Functional data demonstrate that cGMP is critical to the flow-dependent regulation of thoracic duct contractility.
These findings indicate an important role for PKG, especially PKG-Iα in these processes and identifies the PKG protein as a potential therapeutic target.
Introduction
Previous studies demonstrated that wall shear stress generated by intrinsic and extrinsic lymph flow in lymphangions is an important factor that regulates lymphatic contractility and thus lymphatic function (Gashev et al. 2002; Gasheva et al. 2006; Gashev, 2008, 2010; Gashev & Zawieja, 2010). Extrinsic flow is dependent on the action of driving forces that originate outside the observed lymphatic section to develop the axial pressure gradients that result in lymph flow. Such flow is generated by numerous forces and may be increased during various physiological events (increased gut absorption, skeletal muscle activity, etc.) as well as during the increases of lymph formation driven by pathologic processes (inflammation, tissue oedemagenic stress of different origins). Lymphangions are highly sensitive to increases in imposed flow (Gashev et al. 2002, 2004); and even low, steady imposed flows in isolated lymphatics (which imitates the influences of extrinsic flow) produced a NO-dependent inhibition of both phasic and tonic contractile activity. Additionally, lymphangions are also influenced by the intrinsic flow generated by their own phasic contractions. We have shown that the NO pathway is responsible for the reduction of lymphatic tone and the self-regulatory modulation of thoracic duct (TD) pumping elicited by changes in the intrinsic pump flow (Gasheva et al. 2006). Furthermore, by direct in vivo measurements of NO concentrations inside and outside of the lymphatic wall, we confirmed that phasic contractions generate local spikes of NO, and that extrinsic flow can greatly alter the basal NO in lymph, both of which play important roles in the regulation of lymphatic contractility (Bohlen et al. 2009, 2011).
While investigating the nature of the endothelium-dependent regulation of contractility in rat TD we determined that neither potassium channels (personal observations) nor the cyclooxygenase pathway (Gasheva et al. 2006) have significant roles in the shear-dependent modulation of its contractility, which appears solely NO-dependent (Gashev et al. 2002; Gasheva et al. 2006). In the blood vasculature, shear stress induces the biosynthesis of NO from l-arginine by endothelial NO synthase (eNOS). Subsequently NO diffuses from endothelial to vascular smooth muscle cells where it activates soluble guanylate cyclase (sGC), which catalyses the production of cyclic guanosine monophosphate (cGMP) (Friebe & Koesling, 2003). cGMP can then activate cGMP-dependent protein kinase (PKG), cyclic nucleotide-gated channels, phosphodiesterases and the cross-activates (cAMP)-dependent protein kinase (PKA). As a result, activation of the NO/cGMP pathway leads to blood vascular smooth muscle cell relaxation through multiple PKG-dependent effects: the reduction of cytoplasmic Ca2+; dephosphorylation of myosin light chain; and reduction of Ca2+ sensitization of the contractile apparatus. However, there is no evidence in the literature regarding direct studies that link measured changes of flow inside lymphatic vessels to involvement of PKG in flow-dependent regulation of lymphatic contractility, despite strong evidence for a lymphatic endothelium/NO-dependent modulation of lymphatic contractility (Ohhashi & Takahashi, 1991; Yokoyama & Ohhashi, 1993; Ohhashi & Yokoyama, 1994; Mizuno et al. 1998; von der Weid et al. 2001; Tsunemoto et al. 2003; Ohhashi et al. 2005; Gasheva et al. 2006; Bohlen et al. 2009; Gashev, 2010).
Cyclic guanosine monophosphate-dependent protein kinases are serine/threonine kinases. A wide variety of cells contain at least one of the three cGMP-dependent PKG isoforms: PKG-Iα, PKG-Iβ, or PKG-II that are involved in the regulation of different cellular functions (Godfrey & Schwarte, 2003, 2010; Murad, 2006; Godfrey et al. 2007; Rastaldo et al. 2007). The PKG-Iα isoform is found predominantly in the lung, heart, dorsal root ganglia and cerebellum and has a sensitivity to cGMP, which is about 10-fold greater than PKG-Iβ. Together with the PKG-Iα, the PKG-Iβ isoform is highly expressed in smooth muscle cells, including those in blood vessels, uterus, intestine and trachea. Platelets, hippocampal neurons and olfactory bulb neurons contain mainly the PKG-Iβ isoform. However, the expression, localization and specific characterization of the PKG isoforms have not been reported in lymphatic smooth muscle.
In this study, we tested the hypothesis that the cGMP/PKG-mediated pathway is central to the intrinsic and extrinsic shear stress/flow-dependent modulation of lymphatic contractility. To test this hypothesis we used rat TD, which is known for its well-developed flow/NO-dependent regulatory mechanisms (Gashev et al. 2002, 2004; Gasheva et al. 2006; Gashev, 2008; Gashev & Zawieja, 2010). Lymph vessels appear to have greatly enhanced sensitivity to fluid flow/shear in terms of its production of NO as well as its contractile responses compared to blood vessels, given the very low levels of shear found in lymphatic vessels (Dixon et al. 2006; Bohlen et al. 2009, 2011). We used the sGC inhibitor (ODQ, 30 μm) (Godfrey et al. 2007; Ying et al. 2012) to test if it can alter TD contractility similar to NO synthase blockade and if it can prevent NO donor-induced relaxation [S-nitroso-N-acetylpenicillamine (SNAP), 100 μm] (Terluk et al. 2004; Mochizuki et al. 2005). Additionally we attempted to mimic the changes in TD contractility seen with extrinsic flow by the application of the cGMP analogue (8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate sodium salt (8pCPTcGMP), 1–100 μm) (Russo et al. 2004; Williams et al. 2006; Nimmegeers et al. 2008). We used a cGMP/PKG inhibitor [guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio)-, Rp-isomer, triethylammonium salt (Rp-8-Br-PET-cGMPS), 10–50 μm] (Elmedal Laursen et al. 2006; Godfrey et al. 2007; Qin et al. 2007; Shukla et al. 2012) to inhibit the NO-mediated flow-dependent relaxation of TD. To verify and quantify the expression of PKG isoforms in the lymphatics, we performed Western blot analysis of the PKG-Iα and PKG-Iβ in rat TD and compared it to the expressions in nearby major blood vessels, the aorta and vena cava. To determine the location of PKG protein expression we performed immunohistochemistry of frozen, fixed sections of the TD with co-staining of PKG in conjunction with either α-vascular smooth muscle actin protein, known to be present in lymphatic muscle cells (Muthuchamy et al. 2003), or for eNOS, protein, known to be present in lymphatic endothelial cells (Bohlen et al. 2011).
Methods
Animals and surgery
We examined the contractile activity of TDs from 20 male Sprague–Dawley rats (weighing between 300 and 400 g). The animal facilities used for these studies have been accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (International) and adheres to the regulations, policies and principles detailed in Public Health Service Policy for the Humane Care and Use of Laboratory Animals (PHS Policy, 1996) and the United States Department of Agriculture's Animal Welfare Regulations (Animal Welfare Act, AWA, 9CFR, 1985, 1992). All animal procedures performed for this study were reviewed and approved by our institutional animal care and use committee, the Texas A&M University Laboratory Animal Care Committee.
To isolate the TD, rats were killed with pentobarbital (120 mg kg−1 body weight i.p.). Then the animal was positioned on its back; the ventral chest wall was opened by lateral incision; the sternum and approximately half of the ribs were excised. The inferior vena cavae was ligated and cut close to the diaphragm. The lungs and heart were set to the left side of the animal to expose the TD between the aorta and vertebral column. The TD was then carefully cleared of all surrounding tissues using a dissecting microscope. Extreme caution was used to not hold or pinch the TD at any time, thereby reducing the likelihood of damage. The area of interest was kept moist for the period of dissection using the standard Dulbecco's phosphate-buffered saline (Invitrogen Corp., Carlsbad, CA, USA, Catalog # 14040–133). Sections of TD 1–2 cm long were dissected and used for experiments. Throughout the experiments, we measured the diameters of the TD sections used for these studies. At a transmural pressure of 3 cm H2O, the average diastolic diameters were 720 ± 28 μm.
For Western blot analyses, we used separate animals (n= 9). In addition to TD isolation, we subsequently ligated (1–2 cm long) sections of inferior vena cavae and descending part of thoracic aorta. Blood vessels were then carefully cleaned from surrounding tissues and isolated. The vessel specimens (TD, vena cavae and aorta) assigned for Western blotting were transferred to separate 35 mm Petri dishes filled by Dulbecco's phosphate-buffered saline, sutures were cut and blood and lymph remains were flushed out of vessel lumens.
Isolated thoracic duct procedures, experimental techniques and protocol for functional tests
Once the TD was exteriorized, the lymphatic segment was transferred to an isolated vessel chamber (modified Living Systems Instrumentation single vessel chamber model CH/1) filled with room temperature albumin-physiological salt solution (APSS) (in mm: 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, 3.0 Mops and 10 g l−1 bovine serum albumin) pH adjusted to 7.36 at 38°C. The isolated TD segment was cannulated and tied on to two carefully matched glass pipettes (600–650 μm). Great care was used to prepare and select pairs of resistance-matched pipettes for these experiments as described in previous studies (Gashev et al. 2004; Gasheva et al. 2006). The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with APSS. Care was taken to ensure that there were no air bubbles in the tubing or the pipettes. Once the vessel was cannulated, a slight positive transmural pressure (2–3 cm H2O) was applied to detect leaks and to ensure that the vessel was undamaged and untwisted. The vessel was set to its approximate in situ length and positioned just above the glass coverslip comprising the chamber bottom. The chamber was transferred to the stage of a microscope. The vessel was set to an equilibration transmural pressure of 3 cm H2O and warmed to 38°C for over 15–20 min. Once tone and spontaneous contractions were observed, the vessel was allowed to equilibrate at 3 cm H2O for another 30 min. A video camera, monitor and DVD/HDD recorder were used to observe and record the lymphatic segments continuously in all experiments.
At the beginning of every experiment, we evaluated the pressure-induced contractile responses of the TD. Lymphatic segments were exposed to a range of transmural pressures, where inlet and outlet pressure were set equally: 1, 3 and 5 cm H2O for 5 min at each pressure. We chose this set of transmural pressures because we have shown that the TD displays maximal active pumping at 3 cm H2O (Gashev, 2002; Gashev et al. 2004, 2006). As we have previously shown that imposed flow inhibits the active lymph pump (Gashev, 2002; Gashev et al. 2002, 2004, 2006), we constantly monitored the levels of input and output pressure to prevent any imposed flow during this experimental period. Thus, lymph flow and shear were only generated by phasic contractions of TD during this experimental period.
To determine the imposed flow-induced responses of the TD, vessel segments were exposed to sets of imposed flow gradients, which were generated using techniques previously employed (Gashev, 2002; Gashev et al. 2002, 2004, 2006). Because lymphatics are very sensitive to changes in transmural pressure, it was important to maintain a constant net transmural pressure at any given set of imposed flows. As described before, this was accomplished by raising the pressure on the inflow end of the isolated vessel and lowering the pressure on the outflow end of the isolated vessel by identical amounts to create a pressure gradient of 0–5 cm H2O across the input to output ends of the vessel (Gashev, 2002; Gashev et al. 2002, 2004, 2006).
After the completion of the transmural pressure and imposed flow ranges in APSS (control), we replaced the APSS in the chamber with APSS containing different activators of inhibitors of the NO/cGMP/PKG pathway. In different experiments, we used a sGC inhibitor (ODQ at 30 μm; Sigma-Aldrich Corp., St. Louis, MO, USA, catalogue no. O3636), NO donor (SNAP at 100 μm; Sigma-Aldrich Corp., St. Louis, MO, USA, catalogue no. N3398), the cGMP analogue (8pCPTcGMP at 1, 10 and 100 μm; Sigma-Aldrich Corp., St. Louis, MO, USA, catalogue no. C5438) or the cGMP/PKG inhibitor (Rp-8-Br-PET-cGMPS at 10 and 50 μm; EMD Chemicals, Gibbstown, NJ, USA, catalogue no. 370677). The transmural pressure and imposed flow ranges were repeated in the presence of the various treatments (see Results). At the end of each experiment in every segment, the passive diameter was measured at each level of transmural pressure after the vessels were exposed for 15 min to a nominally calcium-free, EDTA (3.0 mm) supplemented APSS.
Data analysis and statistics for isolated vessel experiments
The lymphatic diameters were tracked from the DVD records of experiments using vessel track software developed and generously provided by Professor Michael J. Davis (Davis, 2005; Davis et al. 2006). Briefly, the outer lymphatic diameters were measured from the DVD record with a tracking frequency of ∼30 times s−1. We used cardiac pump analogies to define systolic and diastolic lymphatic diameters in reference to the lymphatic contractile cycle (Granger et al. 1977; Benoit et al. 1989; Zawieja et al. 1991; Gashev et al. 2004) and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5 min interval for each set of pressures and imposed flow with or without drugs used. From the lymphatic end-diastolic and end-systolic diameters (EDD and ESD), the following lymph pump parameters were calculated: lymphatic tone index (the difference between the passive lymphatic diameter in calcium-free APSS and EDD, expressed as a percentage of the passive lymphatic diameter in calcium-free APSS), contraction amplitude (the difference between EDD and ESD), contraction frequency, ejection fraction [EF, the fraction of end-diastolic volume ejected during the single lymphatic contraction, calculated using formula EF = (EDD2– ESD2)/EDD2 (Benoit et al. 1989)], lymphatic pump flow [LPF, an index of lymph flow, calculated using formula LPF =π× 250 × 10−6× (EDD2– ESD2) (Benoit et al. 1989)] and expressed in nl min−1) and fractional pump flow (an index of lymph pump flow, calculated as EF × contraction frequency). To compare changes in diameters during the lymphatic contractile cycle, EDD and ESD were normalized to the passive lymphatic diameters in calcium-free APSS at the corresponding transmural pressure because of the anatomical variations between lymphatic vessels.
Statistical differences were determined by ANOVA, regression analysis and Student's t test (JMP software version 5.0.1.2. for Windows, SAS Institute Inc., Cary, NC, USA) and considered significant at P < 0.05. In Results, the numbers of lymphatic segments used in the reported data are shown separately for each group of experiments, where n depicts the number of TD segments used for each experimental protocol. In all cases, one vessel segment from a single animal was used for each experiment.
Western blot analyses of cyclic guanosine monophosphate-dependent protein kinase isoforms
Isolated and carefully cleaned (isolation technique used as described above) segments of TD, vena cavae and aorta were used for Western blot analyses. The specimens of vessels frozen by liquid nitrogen were homogenized manually and protein was extracted using RIPA lysis buffer (25 mm 0.5 m Tris, pH 7.6, 150 mm NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Lysis buffer also contained a protease inhibitor cocktail (Calbiochem, EMD Chemicals, Gibbstown, NJ, USA, catalogue no. 539131). The tissue homogenate was sonicated in an ultrasonic bath followed by placing in liquid nitrogen three times. After homogenization, the samples were centrifuged for 15 min at 14 000 g. Supernatants were separated, and protein concentrations were determined by Pierce BCA Protein Assay Kit (ThermoScientific, Rockford, Il, USA, catalog # 23227). Uniform samples, prepared in NuPAGE LDS sample buffer (Invitrogen Corp., Carlsbad, CA, USA) with added disulphide bond NuPAGE reducing agent 10× dithiothreitol were warmed up to 70°C for 10 min and then loaded on to a NuPage 4–12% Bis-Tris gel (Invitrogen Corp., Carlsbad, CA, USA) and electrophoresed at 200 V until the marker front reached the limit. The separated proteins were transferred to nitrocellulose membranes at 30 V over 1 h using Xcell II blot module (Invitrogen Corp., Carlsbad, CA, USA). Membranes were blocked with blocking solution (5% non-fat milk in Tween-supplemented Tris-buffered saline (TTBS): 20 mm Tris, pH 7.4, 137 mm NaCl, 0.1% Tween 20) and incubated overnight at 4°C with primary antibodies that were appropriately diluted in the blocking solution.
PKG-Iα and PKG-Iβ were individually detected with isoform-specific antibodies [(goat polyclonal anti-PKG-Iα at a dilution of 1:200, Santa Cruz Biotechnology, Inc., Dallas, TX, USA, N-16, catalogue no. sc-10335); (rabbit polyclonal anti-PKG-Iβ at a dilution of 1:800; Novus Biologicals Inc., Littleton, CO, USA; catalogue no. NB100-1989)]. The membrane was then washed four times with TTBS and incubated with secondary horseradish peroxidase-linked antibody (for PKG-Iα, 1:25,000 donkey antigoat IgG, Santa Cruz, catalogue no. sc-2020; for PKG-Iβ 1:50,000 goat antirabbit IgG; Jackson ImmunoResearch, West Grove, PA, USA) for 2 h at room temperature. Membranes were then washed 6× with TTBS and protein expression detected using the Pierce detection system (SuperSignal West Dura Extended Duration Substrate; Pierce). Membranes were exposed to KODAK BioMax Light Films (Pierce) and the films were processed in a Futura 3000 SV developer (Fischer Industries Inc., Geneva, IL, USA). Densitometry on the resulting bands was performed using the software Image Gauge v.4.22. To verify equal loading of each sample, membranes were stripped using Restore Stripping Buffer (Pierce) and then re-probed with mouse monoclonal anti-GAPDH primary antibody (1:4000; Abcam Inc., Cambridge, MA, USA). The resulting PKG-Iα/GAPDH or PKG-Iβ/GAPDH ratios ± SEM were calculated and used for quantitative analyses. Western blot analyses of lymphatic and blood vessel proteins followed by quantification were performed with the number of samples as shown in Fig. 4. The statistical differences were determined by Student's t test and considered significant at P≤ 0.05.
Figure 4. Influence of the cyclic guanosine monophosphate-dependent protein kinase inhibitor Rp-8-Br-PET-cGMPS (10–50 μm) on the active lymph pump in rat thoracic duct at different levels of imposed flow pressure gradients (inlet pressure > outlet pressure).
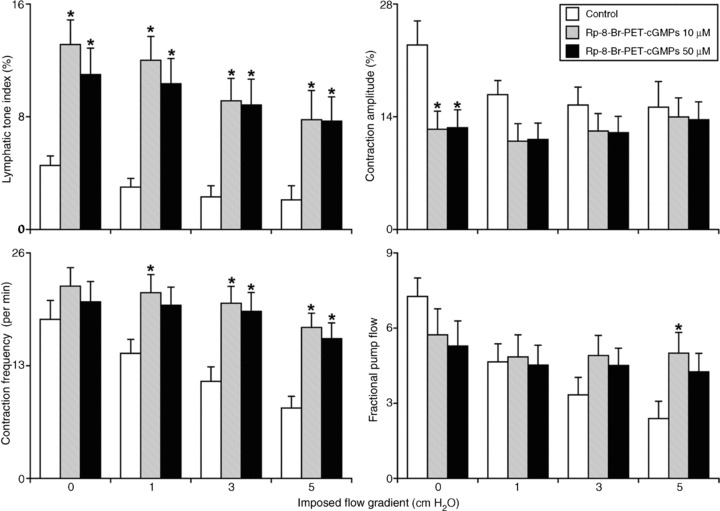
*Significant differences (P < 0.05) between control and Rp-8-Br-PET-cGMPS treatment within each level of imposed flow pressure gradients. Rp-8-Br-PET-cGMPS, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-isomer, triethylammonium salt.
Immunohistochemical labelling of cyclic guanosine monophosphate-dependent protein kinase protein in rat thoracic duct
Freshly isolated TD was rinsed in standard phosphate-buffered saline (PBS) and immediately placed in OCT and frozen at −80°C. Frozen sections (10 μm thick) of the TD were prepared using a cryomicrotome, then mounted on glass slides, fixed in 4% paraformaldehyde for 1 h, and permeabilized in methanol (−20°C for 5 min), following rinsing with PBS. Subsequent antigen retrieval was performed with trypsin (0.01% for 5 min). Then unspecific binding sites were blocked with 1% bovine serum albumin and 5% normal goat serum in PBS for 1 h. The sections were then incubated (4°C, overnight) with primary antibodies: combinations of PKG-Iα (LifeSpan Biosciences, Inc., Seattle, WA, USA, catalog # LS-B2219; 1:50) and eNOS (BD Biosciences, San Jose, CA USA, catalog # 610297; 1:100) antibodies, or PKG-Iα and α-vascular smooth muscle actin (Sigma-Aldrich Corp., St. Louis, MO, USA, Catalog # A5228; 1:200) antibodies. Incubations with the corresponding fluorescently tagged secondary antibodies were conducted for 1 h at room temperature. The negative controls for all experiments were produced using similar procedures except that the samples were not incubated with the primary antibodies but with immunoglobulins of the same host species. After incubation with the secondary antibody conjugated to a fluorescent dyes, the specimens were imaged confocally to determine the intensity and location of the signal in cells of the lymphatic wall. The sections were observed using 40× WLSM and 60× Plano Apo WLSM objectives on an Olympus FluoView 300 confocal microscope (Olympus America Inc, Center Valley, PA, USA). Confocal images were acquired at 0.75 μm intervals in the z-axis using 488 and 647 nm peak excitation wavelengths, and 520 and 668 nm peak emission wavelengths correspondingly. The corresponding negative controls were scanned at the same instrument settings as the unknowns for valid comparison of relative fluorescence intensities. Projection averages of the 3D images stacks were prepared and used for analysis.
Results
Effects of the cyclic guanosine monophosphate inhibitor (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) on contractility of the rat thoracic duct in control conditions and after nitric oxide-induced contractile inhibition using SNAP
In a series of experiments (n= 9), we evaluated the role of sGC in the NO-induced relaxation of the rat TD. Initially we exposed the TD to the NO donor SNAP (100 μm). This dose of SNAP resulted in a statistically significant level of TD relaxation similar to what was observed during increases of imposed flow in the duct (Gashev et al. 2004). Figure 1 demonstrates a relaxation (decrease in lymphatic tone) and the inhibition of parameters of the active lymph pump, after treatment of the TD segments with the NO donor SNAP (100 μm). After a subsequent 15 min wash out of the SNAP with APSS, we repeated recordings of the TD active lymph pump to confirm the reversibility of SNAP. All vessels restored their activity back to control levels after elimination of SNAP (data not shown). Following a second control period, we pre-treated TD segments with the sGC inhibitor ODQ (30 μm), which induced changes in TD contractility similar to that seen with NO synthase blockade (Gasheva et al. 2006). ODQ also prevented the NO-induced relaxation during the second application of the NO donor SNAP (100 μm) in the continuous presence of ODQ (30 μm).
Figure 1. Administration of the soluble guanylate cyclase inhibitor ODQ (30 μm) prevents the effects of the nitric oxide donor SNAP (100 μm) on the responses of the active lymph pump in rat thoracic duct at different transmural pressures (inlet and outlet pressures set equally).
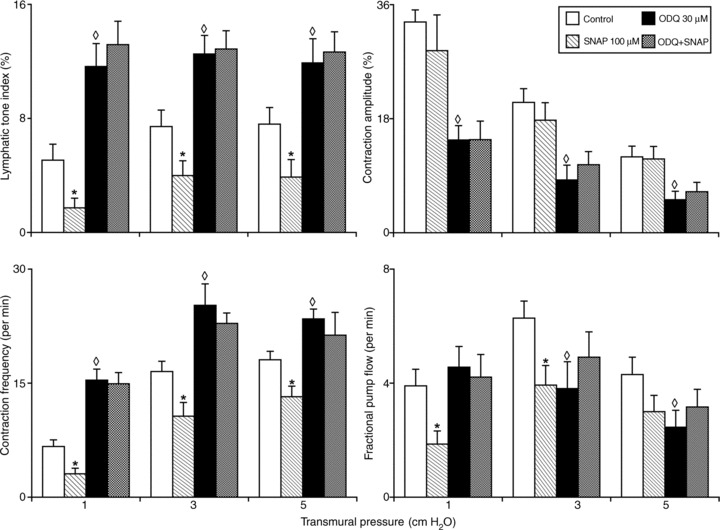
*Significant differences (P < 0.05) between control and SNAP treatment within each level of transmural pressure; ◊ indicates significant differences (P < 0.05) between active lymph parameters at control and after administration of ODQ within each level of transmural pressure. ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; SNAP, nitric oxide donor S-nitroso-N-acetylpenicillamine.
The lymphatic tone index under control conditions was ∼5–7% at all levels of transmural pressure tested, whereas treatment by SNAP induced a further dose-dependent relaxation in the duct: the lymphatic tone index was 67, 45 and 49% less (statistically significant) than that at control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. SNAP (100 μm) did not significantly alter the contraction amplitude. However, SNAP produced statistically significant negative chronotropy, the reduction of contraction frequency compared with control conditions. As shown in Fig. 1, TD treated with SNAP exhibited 54, 35 and 27% lower contraction frequencies when compared with that in control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. Because of this negative chronotropy in the TD, its pumping ability was greatly reduced after SNAP treatment. The fractional pump flow in thoracic segments treated by SNAP was reduced by 51, 32 and 30% at 1, 3 (statistically significant) and 5 cm H2O transmural pressures respectively. We observed a similar tendency of changes in the lymphatic pump flow after SNAPM (Table 1).
Table 1.
Influence of transmural pressure on parameters of the active lymph pump in rat thoracic duct (control and after administration of ODQ and SNAP)
| Transmural pressure (cm H2O) | Treatment | Diastolic diameter (±μ) | Systolic diameter (±μ) | LPF (nl min−1) |
|---|---|---|---|---|
| 1 | Control | 761 ± 45 | 494 ± 34 | 1866 ± 379 |
| SNAP 100 μm | 785 ± 39 | 548 ± 45 | 1021 ± 297 | |
| ODQ 30 μm | 714 ± 46 | 583 ± 39 | 2039 ± 503 | |
| ODQ + SNAP | 696 ± 43 | 578 ± 41 | 1771 ± 506 | |
| 3 | Control | 801 ± 43 | 626 ± 48 | 3202 ± 431 |
| SNAP 100 μm | 829 ± 41 | 672 ± 37 | 2301 ± 438 | |
| ODQ 30 μm | 764 ± 40 | 695 ± 51 | 1646 ± 421 | |
| ODQ + SNAP | 753 ± 39 | 664 ± 45 | 2185 ± 495 | |
| 5 | Control | 808 ± 40 | 706 ± 41 | 2151 ± 276 |
| SNAP 100 μm | 841 ± 43 | 741 ± 46 | 1680 ± 312 | |
| ODQ 30 μm | 779 ± 41 | 736 ± 47 | 1087 ± 235 | |
| ODQ + SNAP | 764 ± 39 | 711 ± 47 | 1334 ± 269 |
LPF, lymphatic pump flow; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; SNAP, nitric oxide donor S-nitroso-N-acetyl-penicillamine. Values are means ± SEM; n= 9.
The sGC inhibitor ODQ (30 μm) induced changes in TD contractility similar to NO synthase blockade, but opposite to the effects of SNAP treatment. As shown in Table 1 and Fig. 1, treatment by ODQ induced constriction in TD: the lymphatic tone index was 127, 69 and 57% statistically significantly higher than that at control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. At the same time, the contraction amplitude was reduced statistically significantly during sGC inhibition, demonstrating an ODQ-induced negative inotropy in TD: we observed the contraction amplitude decrease 56, 60 and 57% below contraction amplitude in control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. Furthermore, ODQ caused a statistically significant positive chronotropy in TD – an increase of contraction frequency compared with control conditions. As shown in Fig. 1, the TD segments treated by ODQ exhibited 130, 53 and 30% higher contraction frequencies when compared with that in control conditions at 1, 3 and 5 cm H2O transmural pressures. Because of these ODQ-induced changes in the contractile activity of the TD, its pumping ability was differentially altered after ODQ treatment dependent upon the pressure. Fractional pump flow in thoracic segments treated by 30 μm of ODQ was not significantly changed at 1 cm H2O, but was statistically significantly decreased 40 and 42% at 3 and 5 cm H2O transmural pressures respectively. A similar tendency of changes was observed in lymphatic pump flow.
Lastly, the subsequent application of the NO donor SNAP (100 μm) combined with continuous application of ODQ (30 μm) did not significantly change any of parameters of the contractility of the TD segments at any levels of transmural pressure compared to ODQ alone. Thus, ODQ successfully blocked all effects of the NO donor. The experimental data, presented in Fig. 1 and Table 1, indicate that essentially all the contractile effects of NO on TD involve the subsequent activation of sGC. The raw data of the active lymph pump parameters in the TD are presented in Table 1.
Effects of the cyclic guanosine monophosphate analogue on pressure-dependent regulation of contractility in thoracic duct
In separate sets of experiments we treated isolated segments of the rat TD with abluminal administration of the cGMP analogue 8pCPTcGMP (1–100 μm, n= 11) or the cGMP/PKG inhibitor Rp-8-Br-PET-cGMPS (10–50 μm, n= 9). Figure 2 demonstrates the relaxation and inhibition of all active lymph pump parameters of the TD treated with cGMP analogue 8pCPTcGMP. The lymphatic tone index under control conditions was 3–6% at all levels of transmural pressure, whereas treatment by 8pCPTcGMP (10 and 100 μm) induced relaxation in the duct. At higher doses of the cGMP analogue (100 μm), the lymphatic tone index was significantly decreased; 73, 76 and 71% less than at control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. Furthermore, the contraction amplitude was progressively reduced during the cGMP analogue application, indicating a cGMP-induced negative inotropy in TD. At higher doses of the cGMP analogue (100 μm), the phasic contractions of TD were essentially abolished at 1 cm H2O of transmural pressure and at 3 and 5 cm H2O transmural pressures we observed statistically significant decreases in the contraction amplitude of 83 and 80% below control conditions respectively. In addition, 8pCPTcGMP caused the reduction of contraction frequency compared with control conditions; statistically significant at 10 and 100 μm of the cGMP analogue. As shown in Fig. 2, the TD segments treated by 100 μm of cGMP analogue exhibited 99% decrease in contraction frequencies when compared with that in control conditions at 3 and 5 cm H2O transmural pressures. Because of such negative inotropy and chronotropy of the TD, its pumping ability was greatly reduced after 8pCPTcGMP treatment. For instance, fractional pump flow in treated thoracic segments was 54, 57 and 58% decreased statistically significantly after 10 μm of the cGMP analogue at 1, 3 and 5 cm H2O transmural pressures respectively; and FPF was essentially zero at 100 μm (Fig. 2). Similar tendency of changes was observed in the lymphatic pump flow. Importantly, we observed a statistically significant dose-dependency (1 μm versus 100 μm) of the 8pCPTcGMP-induced inhibition of all parameters of the TD pumping (Fig. 2). The raw data of the active lymph pump parameters in TD obtained in these experiments are presented in Table 2.
Figure 2. Effects of the cyclic guanosine monophosphate analogue 8pCPTcGMP (1–100) on the active lymph pump in rat thoracic duct at different transmural pressures (inlet and outlet pressures set equally).
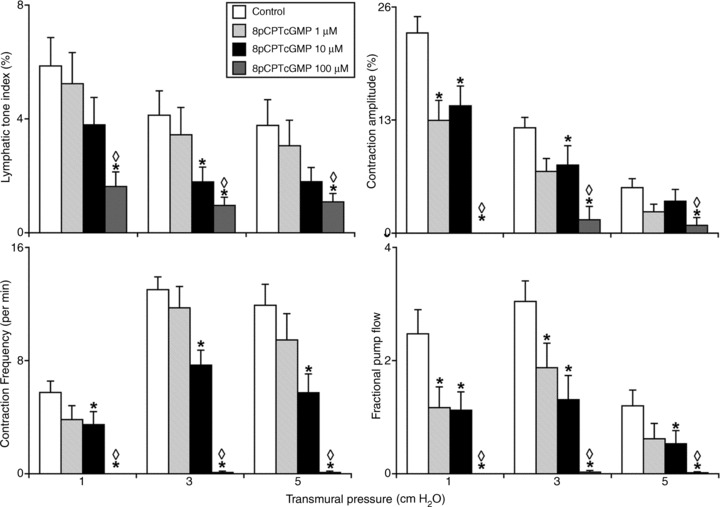
*Significant differences (P < 0.05) between active lymph parameters at control and after 8pCPTcGMP within each level of transmural pressure; ◊ indicates significant differences (P < 0.05) between control and ODQ treatment at 1 μm and 100 μm within each level of transmural pressure. 8pCPTcGMP, 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate sodium salt; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Table 2.
Influence of transmural pressure on parameters of the active lymph pump in rat thoracic duct (control and after administration of 8pCPTcGMP)
| Transmural pressure (cm H2O) | Treatment | Diastolic diameter (±μ) | Systolic diameter (±μ) | LPF (nl min−1) |
|---|---|---|---|---|
| 1 | Control | 734 ± 36 | 557 ± 34 | 1107 ± 255 |
| 8pCPTcGMP 1 μm | 740 ± 35 | 641 ± 41 | 455 ± 143 | |
| 8pCPTcGMP 10 μm | 751 ± 36 | 640 ± 42 | 506 ± 175 | |
| 8pCPTcGMP 100 μm | 768 ± 37 | 768 ± 37 | 0 ± 0 | |
| 3 | Control | 766 ± 38 | 670 ± 38 | 1401 ± 188 |
| 8pCPTcGMP 1 μm | 772 ± 40 | 715 ± 39 | 911 ± 260 | |
| 8pCPTcGMP 10 μm | 786 ± 41 | 725 ± 47 | 596 ± 206 | |
| 8pCPTcGMP 100 μm | 792 ± 42 | 778 ± 39 | 19 ± 19 | |
| 5 | Control | 778 ± 41 | 737 ± 43 | 563 ± 155 |
| 8pCPTcGMP 1 μm | 783 ± 41 | 763 ± 41 | 315 ±161 | |
| 8pCPTcGMP 10 μm | 794 ± 43 | 764 ± 44 | 261 ± 132 | |
| 8pCPTcGMP 100 μm | 800 ± 43 | 791 ± 41 | 12 ± 12 |
8pCPTcGMP, 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate sodium salt LPF, lymphatic pump flow. Values are means ± SEM; n= 11.
Effects of cyclic guanosine monophosphate/cyclic guanosine monophosphate-dependent protein kinase inhibition on the pressure-dependent regulation of contractility in thoracic duct
Figure 3 demonstrates the effects of administration of the cGMP/PKG inhibitor Rp-8-Br-PET-cGMPS (10–50 μm) on the active lymph pump in TD. The lymphatic tone index in control conditions was 3–6% at all levels of transmural pressure, whereas treatment by Rp-8-Br-PET-cGMPS-induced constriction. At the higher dose of the cGMP/PKG inhibitor (50 μm), the lymphatic tone index was statistically significantly 154, 137 and 154% greater than in control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. In addition, the contraction amplitude was reduced by the cGMP/PKG inhibitor, demonstrating its negative inotropy on the TD. At 50 μm Rp-8-Br-PET-cGMPS we observed contraction amplitude decreases of 38, 38 (statistically significant) and 25% (statistically non-significant) during 1, 3 and 5 cm H2O transmural pressures compared to control conditions respectively. At the same time, Rp-8-Br-PET-cGMPS induced slight positive chronotropy in TD compared with control conditions. As shown in Fig. 3, treatment by the cGMP/PKG inhibitor (50 μm) produced an 88% increased contraction frequency (statistically significant when compared to control conditions at 1 cm H2O of transmural pressure). At 3 and 5 cm H2O transmural pressures, the changes were non-significant (28% and 17% higher contraction frequencies respectively) after application of Rp-8-Br-PET-cGMPS at 10 μm. Because of the balance between the negative inotropy and positive chronotropy TD pumping ability (fractional pump flow) was not changed significantly after Rp-8-Br-PET-cGMPS treatment (Fig. 3). A similar tendency was observed in the lymphatic pump flow. The raw data of the parameters of the active lymph pump in TD obtained in these experiments are presented in Table 3.
Figure 3. Influence of the cyclic guanosine monophosphate-dependent protein kinase inhibitor Rp-8-Br-PET-cGMPS (10–50 μm) on the active lymph pump in rat thoracic duct at different transmural pressures (inlet and outlet pressures set equally).
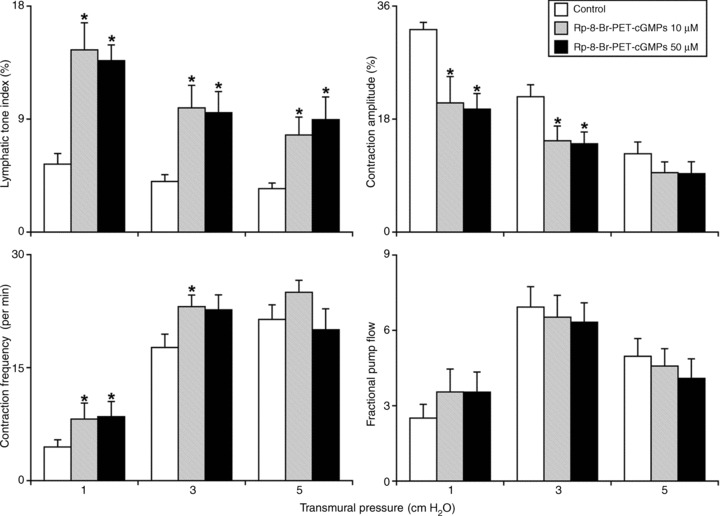
*Significant differences (P < 0.05) between control and Rp-8-Br-PET-cGMPS treatment within each level of transmural pressure. Rp-8-Br-PET-cGMPS, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-isomer, triethylammonium salt.
Table 3.
Influence of transmural pressure on parameters of active lymph pump in rat thoracic duct (control and after administration of Rp-8-Br-PET-cGMPS)
| Transmural pressure (cm H2O) | Treatment | Diastolic diameter (±μ) | Systolic diameter (±μ) | LPF (nl min−1) |
|---|---|---|---|---|
| 1 | Control | 641 ± 35 | 420 ± 17 | 854 ± 244 |
| Rp-8-Br-PET-cGMPS 10 μm | 578 ± 37 | 436 ± 26 | 1091 ± 383 | |
| Rp-8-Br-PET-cGMPS 50 μm | 584 ± 36 | 451 ± 30 | 1080 ± 335 | |
| 3 | Control | 659 ± 38 | 510 ± 31 | 2430 ± 422 |
| Rp-8-Br-PET-cGMPS 10 μm | 620 ± 37 | 520 ± 32 | 2084 ± 359 | |
| Rp-8-Br-PET-cGMPS 50 μm | 621 ± 34 | 526 ± 32 | 1996 ± 291 | |
| 5 | Control | 665 ± 35 | 582 ± 39 | 1714 ± 259 |
| Rp-8-Br-PET-cGMPS 10 μm | 637 ± 35 | 574 ± 37 | 1458 ± 217 | |
| Rp-8-Br-PET-cGMPS 50 μm | 627 ± 31 | 567 ± 34 | 1234 ± 230 |
LPF, lymphatic pump flow; Rp-8-Br-PET-cGMPS, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-isomer, triethylammonium salt. Values are means ± SEM; n= 9.
Effects of cyclic guanosine monophosphate/cyclic guanosine monophosphate-dependent protein kinase inhibition on the imposed flow sensitivity of the thoracic duct
We then compared the changes in contractile responses in TD due to imposed flow before and after abluminal administration of cGMP/PKG inhibitor Rp-8-Br-PET-cGMPS (10–50 μm). The detailed results of these experiments are shown in Fig. 4. In TD, in control conditions, we observed the typical imposed flow-induced inhibition of lymphatic contractility we described in previous studies (Gashev, 2002, 2008, 2010; Gashev et al. 2002, 2004, 2006; Gashev & Zawieja, 2010): in control, increases in the imposed flow gradient up to 5 cm H2O statistically significantly decreased the lymphatic tone index to 53%. In addition, the imposed flow pressure gradient (up to 5 cm H2O) significantly inhibited pumping, by both inotropic (35% decrease in contraction amplitude) and chronotropic (56% decrease in contraction frequency) influences in controls. Because of the imposed flow, the fractional pump flow was diminished statistically significantly, particularly at high levels of imposed flow. At an imposed flow gradient of 5 cm H2O, the fractional pump flow was 67% lower than control with no imposed flow (Fig. 4), demonstrating a strong imposed flow-induced inhibition of the active lymph pump in control conditions. While the cGMP/PKG inhibitor (50 μm) significantly increased tone at all imposed flow levels, cGMP/PKG inhibition had insignificant effects on the influence of imposed flow on the lymphatic tone index (30% after cGMP/PKG inhibition compared to a 53% decrease in control). However, cGMP/PKG inhibition prevented some of the imposed flow-induced effects on the active pump. The cGMP/PKG inhibitor completely eliminated the negative inotropic effect of imposed flow: contraction amplitude was unchanged with imposed flow gradients up to 5 cm H2O in the presence of the cGMP/PKG inhibitor (Fig. 4) compared to a 35% flow-dependent decrease in the contraction amplitude without the cGMP/PKG inhibitor. The negative chronotropic effect of imposed flow was also greatly diminished in the presence of Rp-8-Br-PET-cGMPS: while the imposed flow statistically reduced the contraction frequency in control conditions, cGMP/PKG blockade prevented the decrease in contraction frequency during the imposed flow (no statistically significant change in contraction frequency after cGMP/PKG blockade). With cGMP/PKG inhibition by Rp-8-Br-PET-cGMPS (50 μm), the fractional pump flow in TD was not significantly diminished during an imposed flow gradient of 5 cm H2O (versus a significant 67% decrease under control). A similar tendency was seen in the lymphatic pump flow. The raw data of the parameters of the active lymph pump in TD obtained from these experiments are presented in Table 4.
Table 4.
Influence of imposed flow pressure gradients on parameters of active lymph pump in rat thoracic duct (control and after administration of the cyclic guanosine monophosphate-dependent protein kinase inhibitor, Rp-8-Br-PET-cGMPS)
| Imposed flow gradient (cm H2O) | Treatment | Diastolic diameter (±μ) | Systolic diameter (±μ) | LPF (nl min−1) |
|---|---|---|---|---|
| 0 | Control | 638 ± 43 | 485 ± 38 | 2336 ± 314 |
| Rp-8-Br-PET-cGMPS 10 μm | 579 ± 37 | 496 ± 33 | 1538 ± 291 | |
| Rp-8-Br-PET-cGMPS 50 μm | 593 ± 39 | 511 ± 34 | 1461 ± 256 | |
| Control | 647 ± 43 | 538 ± 44 | 1537 ± 302 | |
| Rp-8-Br-PET-cGMPS 10 μm | 586 ± 38 | 513 ± 34 | 1367 ± 277 | |
| Rp-8-Br-PET-cGMPS 50 μm | 598 ± 40 | 525 ± 40 | 1277 ± 216 | |
| 3 | Control | 652 ± 44 | 559 ± 43 | 1151 ± 323 |
| Rp-8-Br-PET-cGMPS 10 μm | 606 ± 39 | 524 ± 36 | 1480 ± 270 | |
| Rp-8-Br-PET-cGMPS 50 μm | 608 ± 40 | 529 ± 40 | 1320 ± 198 | |
| 5 | Control | 654 ± 45 | 552 ± 43 | 823 ± 265 |
| Rp-8-Br-PET-cGMPS 10 μm | 616 ± 43 | 513 ± 39 | 1592 ± 323 | |
| Rp-8-Br-PET-cGMPS 50 μm | 616 ± 41 | 526 ± 41 | 1295 ± 223 |
LPF, lymphatic pump flow; Rp-8-Br-PET-cGMPS, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-isomer, triethylammonium salt. Values are means ± SEM; n= 7.
Western blot analyses of expression of cyclic guanosine monophosphate-dependent protein kinase isoforms in thoracic duct, vena cavae and aorta
To determine which isoforms of PKG are expressed in TD and their relative abundance as well as compare their expression to that seen in large blood vessels from the thorax, we performed Western blot analyses for the PKG-Iα and PKG-Iβ isoforms in TD, vena cavae and aorta. Figure 5 shows that PKG-Iα protein is expressed ∼10 fold higher in TD compared to the vena cavae or aorta. The ratio of PKG-Iα/GAPDH were 1.03 ± 0.38, 0.09 ± 0.02 and 0.14 ± 0.03 respectively for TD, vena cavae and aorta. PKG-Iβ protein was expressed about equally in TD and vena cava, both being ∼2 times higher than that in the aorta. The ratio of PKG-Iβ/GAPDH were 0.76 ± 0.21, 0.81 ± 0.17 and 0.34 ± 0.06 respectively for TD, vena cavae and aorta.
Figure 5. Western blot analyses of PKG-Iα and PKG-Iβ in TD (n= 9 and 8 for the corresponding isoforms), VC (n= 6 and 6) and A (n= 6 and 6). n depicts number of samples of each tissue type used for analyses.
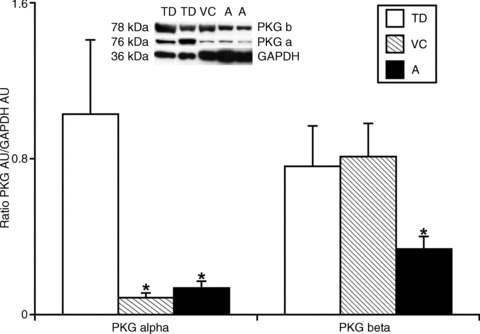
*Significant differences (P < 0.05) between the relative levels of PKG-Iα and PKG-Iβ expression in the different types of vessels compare to TD. Representative blot on the top demonstrates samples of TD, VC and A tested for corresponding proteins. A, aorta; PKG, cyclic guanosine monophosphate-dependent protein kinase; TD, thoracic duct; VC, vena cavae.
Immunohistochemical labelling of cyclic guanosine monophosphate-dependent protein kinase in thoracic duct
We also performed immunohistochemical labelling of frozen sections of rat TD (n= 4) to confirm and localize the PKG-Iα protein in the cells of the TD wall. Figure 6 demonstrates the representative images of these findings. Figure 6A shows PKG-Iα and eNOS signals confirming that the PKG-Iα protein was localized outside of the endothelial cells, while Fig. 6B demonstrates co-localization of PKG-Iα and α-smooth muscle actin signals within the muscle cells in rat TD.
Figure 6. Immunohistochemical labelling of PKG-Iα protein in the wall of the rat thoracic duct.
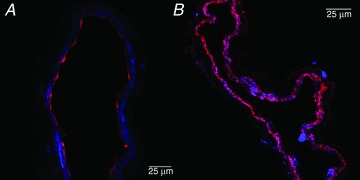
Representative average projections of the confocal image stacks through frozen fixed thoracic duct sections. A, PKG-α (blue) and endothelial nitric oxide synthase (red) signals confirm subendothelial localization of PKG-Iα protein. B, predominant co-localization of PKG-Iα (blue) and α-actin (red) signals confirm the presence of PKGIα protein inside muscle cells in rat thoracic duct. PKG, cyclic guanosine monophosphate-dependent protein kinase.
Discussion
The TD is the major lymphatic trunk responsible for the transport of lymph accumulated from the majority of the lymphatic networks of the body into the great veins of the neck. This largest lymphatic vessel is tightly involved in the maintenance of fluid and macromolecular homeostasis and carries immune cells and biologically active substances vitally important to support proper immune function. The status of lymph flow and pressure in the TD is also crucial for the effective collection of lymph formed in the internal organs of thoracic cavity, including heart (where the extracardiac lymphatic vessels finally transport cardiac lymph to the TD) and lungs. Therefore, any disturbances of the lymph flow in the TD could alter lymph transport in thoracic organs and thus influence the function of the heart and/or lungs. We believe that a better understanding of the functional role of the TD, as well as other pertinent lymphatics opens up possibilities for the development of novel therapeutic approaches to improve major body functions through the maintenance of an effective lymph flow.
Studies during the last decade have demonstrated unique physiological characteristics of the TD. The TD, as well as lymph vessels from other regions of the body, possesses typical bell curve-shaped sensitivity of the lymph pump to wall stretch. However, maximum pumping in the TD occurs at comparatively low levels of transmural pressure (∼2 cm H2O), with a modest effectiveness of the active TD pumping (FPF of ∼2 volumes min−1) (Gashev et al. 2004; Gashev, 2008). These observations correlate with recently published results of investigations of biomechanical properties of the TD wall, where the TD exhibited the flattest length–active tension curve, i.e. its weakest ability to create active tension compared to other regional lymphatics. This is particularly true for peak active tension, in which there was less than a 5% change in peak active tension from 0.75 to 1.3 lo (Gashev et al. 2012). These findings support earlier conclusions of a relatively greater conductive function of the TD as compared to its pumping function under basal conditions. This allows the active pumping of the TD to remain relatively less sensitive to changes in pressure and more likely to be regulated primarily by changes in flow/shear (Gashev et al. 2004; Gasheva et al. 2006). Thus, changes in the tone of the TD are important for its ability to regulate resistance and provide a low local resistance during periods of high lymph flow. We have previously demonstrated that the TD is extremely sensitive to flow (Gashev et al. 2004; Gasheva et al. 2006). Another unique characteristic of the TD relates to its wall shear stress-dependent regulation, which it realizes solely through NO-dependent mechanisms (Gasheva et al. 2006, 2007). At the same time, TD is very sensitive to the changes in intrinsic flow produced by the spontaneous phasic contractions, which are linked to corresponding phasic fluctuations in wall shear stress and the presumed endothelial release of NO. This supports an intrinsic flow-dependent diastolic relaxation of TD (Gasheva et al. 2006). Such a decrease in tone is an important regulatory mechanism that maintains pumping in the TD in an efficient mode: it improves its diastolic filling (enhanced lusitropy), makes lymphatic contractions stronger (enhanced inotropy) and propels more lymph forward during each contraction (elevated EF) while decreasing contraction frequency (reduced chronotropy) (Gasheva et al. 2006). This mechanism is important to maintaining an effective transport of lymph in the TD during periods of comparatively low or moderate levels of lymph flow.
The unique features of the stretch-related sensitivity of the TD can be reasonably explained by the existence of the TD-specific combinations of the contractile protein isoforms reported earlier – predominantly cardiac α-actin and vascular α-actin were found (Muthuchamy et al. 2003) and recent findings indicate that myosin light chain 20 (MLC(20)) diphosphorylation, but not monophosphorylation, in TD was significantly decreased during comparatively low increases in transmural pressure (Nepiyushchikh et al. 2011). However, at the same time, the molecular regulatory mechanisms underlying the unique features of the wall shear stress-related sensitivity of the TD remain primarily undiscovered, therefore limiting further development of effective strategies to influence TD lymph flow during various pathologies.
In this study, we investigated involvement of the sGC/cGMP/PKG pathway in the flow/shear stress-dependent modulation of TD contractility. In our first set of experiments, we found that the sGC inhibitor, ODQ, was able to completely prevent the NO donor-induced inhibition of TD contractility. This finding, at least, with respect to the lymphatic pump frequency is similar to those found previously (von der Weid et al. 2001). Such findings confirm a crucial role of sGC in NO-dependent modulation of the TD active lymph pump, which as we mentioned above appears solely responsible for the flow/ shear stress-dependent adaptive contractile reactions of the TD. Next, we treated isolated rat TD segments by abluminal administration of the cGMP analogue 8pCPTcGMP, thus mimicking the influence of NO-dependent/wall shear stress changes in TD contractility. We found that 8pCPTcGMP-induced dose-dependent relaxation (1 μm versus 100 μm) and inhibition of all parameters of the active lymph pump similar to results of previous studies (Gashev et al. 2004) in which an imposed flow-induced relaxation (decrease in lymphatic tone) of TD occurs, together with negative influences on contraction amplitude, contraction frequency and fractional pump flow. Our present findings using the cGMP analogue are similar to effects of increased wall shear stress evaluated in earlier isolated TD studies (Gasheva et al. 2006, 2007), i.e. 8pCPTcGMP decreased lymphatic tone, contraction amplitude, contraction frequency and fractional pump flow in TD to the same degree observed in the isolated TD segments. Therefore, we conclude that the analogue of cGMP, 8pCPTcGMP (1–100 μm), can mimic the extrinsic flow-induced changes in the TD. Taking into account the fact that all of such imposed flow-induced changes of TD contractility depend on NO release (Gasheva et al. 2006, 2007), these results indicate that flow-mediated relaxation in rat TD occurs through involvement of a NO-activated cGMP-involved regulatory pathway.
In this study, we found that this cGMP/PKG inhibitor eliminated the intrinsic flow-dependent/NO-dependent relaxation. These findings correlate with previous data (Gasheva et al. 2006) where we have shown that l-NAME administration (NO synthase blockade) to TD segments increased their lymphatic tone indices 160, 140 and 120% above control at 1, 3 and 5 cmH2O of transmural pressure respectively (Gasheva et al. 2006). In the current study, we found that cGMP/PKG inhibition increased the lymphatic tone indices similarly to 154, 137 and 154% greater than control conditions at 1, 3 and 5 cm H2O transmural pressures respectively. Moreover, the blockade of NO-synthase (l-NAME administration) in previous studies caused a positive chronotropic effect (increase in contraction frequency) and negative inotropic effect (decrease contraction amplitude) and because of these effects, the fractional pump flow did not significantly change (Gasheva et al. 2006). We found the same patterns in our current experiments with the cGMP/PKG inhibitor administration. In addition, Rp-8-Br-PET-cGMPS completely blocked the NO-induced intrinsic contraction-generated flow relaxation in rat TD in a manner similar to l-NAME treatments seen in a previous study (Gasheva et al. 2006). Thus, we confirmed that wall shear stress/NO-dependent regulatory mechanisms of the TD contractility are predominantly cGMP/PKG-dependent. We confirmed this further by analysing the influence of the cGMP/PKG inhibitor on the contractility of rat TD during imposed flow. In the presence of the cGMP/PKG inhibitor, while the overall tone was elevated (as one would expect if you block any intrinsic NO-dependent relaxations) the effect of an imposed flow on the lymphatic tone index was greatly diminished (only 30% of the effect of imposed flow observed in control conditions). In addition, the cGMP/PKG inhibitor eliminated the negative inotropic effects of imposed flow and greatly diminished its negative chronotropic effects. These results demonstrate the predominance of the cGMP/PKG pathway in the extrinsic flow-induced relaxation in rat TD. It does not completely preclude the potential existence of other PKG-independent mechanisms that could be involved under certain conditions. In particular, it has been demonstrated that elevation of cGMP can produce changes in levels of cAMP through stimulation or inhibition of cAMP–PDEs. Such cross-talk of cGMP with the cAMP system might present a physiologically important mechanism of cGMP signalling that is independent of PKG (Beavo & Brunton, 2002; Rybalkin et al. 2003); its existence in TD should be verified in future studies. We conclude that the contraction-generated intrinsic flow relaxation of TD is predominantly a NO/PKG-dependent process. However, under pathological or physiological conditions (when extrinsic flow inside TD is high) we speculate that additional PKG-independent mechanisms could be at play.
The results of our molecular analyses demonstrate a unique profile of PKG isoforms in TD compared with large thoracic blood vessels. Specifically the 10-fold increased expression of the PKG-Iα isoform in the TD could prove to be particularly important. This provides further evidence of the importance of PKG in the flow-dependent regulatory adaptation of TD contractility. Additionally this may help explain the unusually low basal tone typically observed in the TD, as well as its unique high sensitivity to low levels of flow/shear. We have previously shown that this very low tone is an important factor in the refilling of the lymphatic pump (Gasheva et al. 2006) and that it is dependent on the lymphatic endothelial production of NO. The current data from our Western blot analyses demonstrate an extremely large expression of the PKG-Iα isoform in the TD compared to regional blood vessels. Earlier it has been demonstrated that the PKG-Iα isoform has the highest sensitivity to cGMP of all the known PKG isoforms (Ruth et al. 1991). One established in vivo target for PKG-Iβ is IRAG [inositol 1,4,5-trisphosphate (IP3) receptor-associated PKG (cGK1b) substrate], which has been identified in a complex with the smooth muscle IP3 receptor type 1 and PKG. Phosphorylation of IRAG by PKG inhibits IP3-induced Ca2+ release from intracellular stores, therefore supporting muscle relaxation (Schlossmann et al. 2000; Ammendola et al. 2001; Geiselhoringer et al. 2004). This pathway may also be involved in the NO/cGMP/PKG-dependent decrease in lymphatic pacemaker activity and thus lymph pump frequency (von der Weid et al. 2001).
Thus, the findings described above together with high expression of PKG-Iα isoform in the TD may be a critical feature of it, which helps explain its uniquely low basal tone. This is supportive for enhancements of the TD lymph pumping activity in conditions of low or moderate diastolic filling (Gasheva et al. 2006). Results of our immunohistochemical analyses, which confirmed the localization of PKG-Iα predominantly in rat TD muscle cells, provide additional strong molecular foundations of our functional observations. However, we believe that further studies, potentially using gene-targeted approaches, could help to confirm the functional importance of the PKG-Iα isoform dominance in the TD. Furthermore, future comparisons of the expression of these isoforms in lymph vessels from other tissue beds that do not exhibit as strong a flow/shear-dependent effect would clarify the molecular basis for the regional variability of lymphatic function. Nevertheless, our current results already provide conclusive novel evidence that PKG-Iα protein may be an important target for the development of therapeutic interventions during lymph transport dysfunctions.
In conclusion, in this study, for the first time we tested the hypothesis that the cGMP/PKG-mediated pathway is important to the intrinsic and extrinsic flow-dependent modulation of lymphatic contractility in rat TD. We found that the sGC inhibition induced changes in TD contractility similar to NO synthase blockade and prevented the NO donor-induced relaxation. A cGMP analogue mimicked the changes in TD contractility seen with extrinsic flow-induced relaxation, while the cGMP/PKG inhibitor eliminated the intrinsic flow-dependent relaxation, and largely prevented the extrinsic flow-dependent relaxation in TD. We found that TD expressed ∼10-fold more PKG-Iα compared to the aorta or vena cava, while the PKG-Iβ isoform was expressed equally in the TD and vena cava, both being ∼2 times higher than that in the aorta. PKG is predominantly found in TD muscle cells in close proximity to the endothelium. We conclude that the high sensitivity of rat TD to flow/shear is highly dependent upon the sGC/cGMP/PKG regulatory pathway and may in part be due to the high expression of the PKG-Iα isoform, which is highly sensitive to cGMP (Ruth et al. 1991). This important information extends our understanding of the intrinsic processes that regulate lymph flow and provides potential targets for the therapeutic manipulation of lymphatic function.
Acknowledgments
Dr Michael Davis is greatly acknowledged for the use of the diameter analysis program. Dr H. Glen Bohlen is acknowledged for his help in the measurements of NO in studies that conceptually helped to lead this work. The authors thank Aleksey Gashev for his help in primary data analysis.
Glossary
- 8pCPTcGMP
cGMP analogue 8-(4-Chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate sodium salt
- AMP
contraction amplitude
- APSS
albumin-physiological salt solution
- AU
adsorption units
- cGMP
cyclic guanosine monophosphate
- EDD
end-diastolic diameter
- eNOS
endothelial NO synthase
- ESD
end-systolic diameter
- LTI
lymphatic tone index
- FPF
fractional pump flow
- FREQ
contraction frequency
- LPF
lymphatic pump flow
- NO
nitric oxide
- ODQ
sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PKA
(cAMP)-dependent protein kinase
- PKG
cGMP-dependent protein kinase
- Rp-8-Br-PET-cGMPS
cGMP/PKG inhibitor guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-isomer, triethylammonium salt
- sGC
soluble guanylate cyclase
- SNAP
NO donor S-nitroso-N-acetylpenicillamine
- TD
thoracic duct
- TTBS
tween-supplemented Tris-buffered saline
Additional information
Competing interests
None.
Author contributions
Conception and design of the experiments – O.Y.G., D.C.Z. Collection, analysis and interpretation of data – O.Y.G., A.A.G., D.C.Z. Drafting the article or revising it critically for important intellectual content – O.Y.G., A.A.G., D.C.Z. All authors approved the final version of the manuscript.
Funding
This work was supported by National Institutes of Health RO1 grants HL-070308 and AG-030578.
References
- Ammendola A, Geiselhoringer A, Hofmann F, Schlossmann J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. J Biol Chem. 2001;276:24153–24159. doi: 10.1074/jbc.M101530200. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nature Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation. 2005;12:361–372. doi: 10.1080/10739680590934772. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Elmedal Laursen B, Mulvany MJ, Simonsen U. Involvement of guanylyl cyclase, protein kinase A and Na+ K+ ATPase in relaxations of bovine isolated bronchioles induced by GEA 3175, an NO donor. Pulm Pharmacol Ther. 2006;19:179–188. doi: 10.1016/j.pupt.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Gashev AA. Physiologic aspects of lymphatic contractile function: current perspectives. Ann N Y Acad Sci. 2002;979:178–187. doi: 10.1111/j.1749-6632.2002.tb04878.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann N Y Acad Sci. 2008;1131:100–109. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- Gashev AA. Basic mechanisms controlling lymph transport in the mesenteric lymphatic net. Ann N Y Acad Sci. 2010;1207(Suppl 1):E16–20. doi: 10.1111/j.1749-6632.2010.05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology. 2010;17:277–287. doi: 10.1016/j.pathophys.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am J Physiol Heart Circ Physiol. 2006;290:H2295–2308. doi: 10.1152/ajpheart.00260.2005. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Zhang RZ, Muthuchamy M, Zawieja DC, Davis MJ. Regional heterogeneity of length-tension relationships in rat lymph vessels. Lymphat Res Biol. 2012;10:14–19. doi: 10.1089/lrb.2011.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation. 2007;14:827–839. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- Geiselhoringer A, Werner M, Sigl K, Smital P, Worner R, Acheo L, Stieber J, Weinmeister P, Feil R, Feil S, Wegener J, Hofmann F, Schlossmann J. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 2004;23:4222–4231. doi: 10.1038/sj.emboj.7600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey EW, Schwarte RC. The role of nitric oxide signalling in the formation of the neuromuscular junction. J Neurocytol. 2003;32:591–602. doi: 10.1023/B:NEUR.0000020612.87729.98. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Schwarte RC. Nitric oxide and cyclic GMP regulate early events in agrin signalling in skeletal muscle cells. Exp Cell Res. 2010;316:1935–1945. doi: 10.1016/j.yexcr.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Longacher M, Neiswender H, Schwarte RC, Browning DD. Guanylate cyclase and cyclic GMP-dependent protein kinase regulate agrin signalling at the developing neuromuscular junction. Dev Biol. 2007;307:195–201. doi: 10.1016/j.ydbio.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger HJ, Kovalcheck S, Zweifach BW, Barnes GE. 1977. Quantitative analysis of active lymphatic pumping. In Proceedings of the VII Summer Computer Simulation Conference, pp. 562–565. Simulation Councils, Inc., La Jolla, CA.
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Sipkema P, Goto M, Hiramatsu O, Nakamoto H, Toyota E, Kajita T, Shigeto F, Yada T, Ogasawara Y, Kajiya F. Exogenous NO suppresses flow-induced endothelium-derived NO production because of depletion of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2005;288:H553–558. doi: 10.1152/ajpheart.00408.2004. [DOI] [PubMed] [Google Scholar]
- Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signalling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol. 2011;589:5415–5429. doi: 10.1113/jphysiol.2011.218446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmegeers S, Sips P, Buys E, Decaluwe K, Brouckaert P, Van de Voorde J. Role of the soluble guanylyl cyclase alpha1-subunit in mice corpus cavernosum smooth muscle relaxation. Int J Impot Res. 2008;20:278–284. doi: 10.1038/sj.ijir.3901627. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Takahashi N. Acetylcholine-induced release of endothelium-derived relaxing factor from lymphatic endothelial cells. Am J Physiol Heart Circ Physiol. 1991;260:H1172–1178. doi: 10.1152/ajpheart.1991.260.4.H1172. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Yokoyama S. Nitric oxide and the lymphatic system. Jpn J Physiol. 1994;44:327–342. doi: 10.2170/jjphysiol.44.327. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Mizuno R, Ikomi F, Kawai Y. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol Ther. 2005;105:165–188. doi: 10.1016/j.pharmthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- PHS Policy. 1996. http://grants.nih.gov/grants/olaw/references/phspol.htm.
- Qin X, Zheng X, Qi H, Dou D, Raj JU, Gao Y. cGMP-dependent protein kinase in regulation of basal tone and in nitroglycerin- and nitric-oxide-induced relaxation in porcine coronary artery. Pflugers Arch. 2007;454:913–923. doi: 10.1007/s00424-007-0249-8. [DOI] [PubMed] [Google Scholar]
- Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Russo I, Doronzo G, Mattiello L, De Salve A, Trovati M, Anfossi G. The activity of constitutive nitric oxide synthase is increased by the pathway cAMP/cAMP-activated protein kinase in human platelets. New insights into the antiaggregating effects of cAMP-elevating agents. Thromb Res. 2004;114:265–273. doi: 10.1016/j.thromres.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F. The activation of expressed cGMP-dependent protein kinase isozymes I alpha and I beta is determined by the different amino-termini. Eur J Biochem. 1991;202:1339–1344. doi: 10.1111/j.1432-1033.1991.tb16509.x. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- Shukla P, Sun C, O’Rourke ST. Melatonin inhibits nitric oxide signalling by increasing PDE5 phosphorylation in coronary arteries. Am J Physiol Heart Circ Physiol. 2012;303:H1418–1425. doi: 10.1152/ajpheart.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terluk M, Cunha Da Silva E, Antunes T, Assreuy J. The presence of the endothelial layer reduces nitric oxide-induced hyporesponsiveness to phenylephrine in rat aorta. Endothelium. 2004;11:181–187. doi: 10.1080/10623320490512228. [DOI] [PubMed] [Google Scholar]
- Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol. 2003;53:157–163. doi: 10.2170/jjphysiol.53.157. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol. 2001;280:H2707–2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Williams JM, White CR, Chang MM, Injeti ER, Zhang L, Pearce WJ. Chronic hypoxic decreases in soluble guanylate cyclase protein and enzyme activity are age dependent in fetal and adult ovine carotid arteries. J Appl Physiol. 2006;100:1857–1866. doi: 10.1152/japplphysiol.00662.2005. [DOI] [PubMed] [Google Scholar]
- Ying L, Xu X, Liu J, Dou D, Yu X, Ye L, He Q, Gao Y. Heterogeneity in relaxation of different sized porcine coronary arteries to nitrovasodilators: role of PKG and MYPT1. Pflugers Arch. 2012;463:257–268. doi: 10.1007/s00424-011-1040-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol. 1993;264:H1460–1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol Heart Circ Physiol. 1991;260:H1935–1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]


