Abstract
Exposure of human bronchial epithelial (HBE) cells from normal and asthmatic subjects to extracts from Alternaria alternata evoked a rapid and sustained release of ATP with greater efficacy observed in epithelial cells from asthmatic patients. Previously, Alternaria allergens were shown to produce a sustained increase in intracellular Ca2+ concentration ([Ca2+]i) that was dependent on the coordinated activation of specific purinergic receptor (P2Y2 and P2X7) subtypes. In the present study, pretreatment with a cell-permeable Ca2+-chelating compound (BAPTA-AM) significantly inhibited ATP release, indicating dependency on [Ca2+]i. Alternaria-evoked ATP release exhibited a greater peak response and a slightly lower EC50 value in cells obtained from asthmatic donors compared to normal control cells. Furthermore, the maximum increase in [Ca2+]i resulting from Alternaria treatment was greater in cells from asthmatic patients compared to normal subjects. The vesicle transport inhibitor brefeldin A and BAPTA-AM significantly blocked Alternaria-stimulated incorporation of fluorescent lipid (FM1-43)-labelled vesicles into the plasma membrane and ATP release. In addition, inhibiting uptake of ATP into exocytotic vesicles with bafilomycin also reduced ATP release comparable to the effects of brefeldin A and BAPTA-AM. These results indicate that an important mechanism for Alternaria-induced ATP release is Ca2+ dependent and involves exocytosis of ATP. Serine and cysteine protease inhibitors also reduced Alternaria-induced ATP release; however, the sustained increase in [Ca2+]i typically observed following Alternaria exposure appeared to be independent of protease-activated receptor (PAR2) stimulation.
Key points
Exposure of human bronchial epithelial (HBE) cells to fungal aeroallergens derived from Alternaria alternata stimulates Ca2+-dependent and Ca2+-independent ATP release across the apical membrane.
The Ca2+-dependent component was blocked by inhibitors of both ATP uptake and transport of exocytotic vesicles to the plasma membrane.
Treatment with inhibitors that target cysteine proteases significantly blocked Ca2+-dependent ATP release evoked by Alternaria in normal HBE cells, but not in cells derived from asthmatic patients.
The magnitude of ATP release and associated intracellular Ca2+ mobilization was significantly greater in bronchial epithelial cells obtained from patients with asthma.
These findings establish a novel role for ATP release as a mechanism underlying Alternaria aeroallergen activation of airway mucosal immunity and that cells derived from patients with asthma exhibit greater responsiveness to these allergens.
Introduction
Alternaria alternata is a common fungus found in the soil and on decaying vegetation (Sanchez & Bush, 2001; Weber, 2001; Seminario-Vidal et al. 2009). Inhaled Alternaria spores evoke airway hyper-responsiveness in individuals with asthma and in patients with severe asthma; life-threatening respiratory distress has been associated with increased exposure to Alternaria aeroallergens (Black et al. 2000; Downs et al. 2001; Weber, 2001; Kauffman & van der Heide, 2003; Bush & Prochnau, 2004; Shi et al. 2008; Sipos et al. 2009; Smith, 2010; Hayes et al. 2013). In regions where Alternaria spore counts are high, sensitization is more strongly correlated with asthma and children sensitized to Alternaria were found to be more likely to develop airway hyper-responsiveness (Downs et al. 2001; Salo et al. 2006; Yagami et al. 2010). Although the molecular mechanisms responsible for the association between Alternaria and asthma are poorly understood, previous studies have shown that exposure to Alternaria extract induced expression and secretion of thymic stromal lymphopoietin (TSLP; Kouzaki et al. 2009). TSLP secretion was dependent on Alternaria-derived protease activity and was significantly inhibited by silencing protease-activated receptor (PAR2) expression. TSLP contributes to the adaptive immune response and promotes allergic airway inflammation by increasing the synthesis and secretion of Th2 cytokines (Zhong et al. 2003; Ahmad et al. 2006; Shi et al. 2008; Ying et al. 2008; Halim et al. 2012). More recently, interleukin 33 (IL-33) secretion was shown to be stimulated by airway exposure to Alternaria (Kouzaki et al. 2011). IL-33 is a member of the IL-1 family of cytokines that specifically activates ST2 (IL-33Rα) receptors on a variety of immune cells resulting in release of Th2 cytokines that are important in the development of allergic inflammation (Cherry et al. 2008; Smith, 2010; Yagami et al. 2010; Fujita et al. 2012).
When airway epithelial cells are exposed to Alternaria, IL-33 translocates from the nucleus into the cytoplasm and is subsequently secreted into the extracellular fluid (Kouzaki et al. 2011). Results from immunohistochemistry experiments using native human bronchial epithelium showed that IL-33 is predominantly localized within the nucleus of basal cells (Kouzaki et al. 2011). A signalling mechanism that participates in regulation of IL-33 secretion involves autocrine release of ATP from the epithelium followed by purinergic receptor activation and a sustained increase in [Ca2+]i. Inhibition of Ca2+ mobilization with cell-permeable Ca2+-chelating compounds completely blocks IL-33 secretion. Moreover, silencing the expression of P2Y2 or P2X7 receptors in cultured human bronchial epithelial cells or treating P2Y2-deficient mice with Alternaria extract significantly abrogates IL-33 and Th2 cytokine secretion by the airway epithelium (Kouzaki et al. 2011).
Earlier studies of ATP release from airway epithelial cells have shown that increases in [Ca2+]i either by application of the calcium ionophore ionomycin or by activation of G-protein-coupled receptors that regulate release of Ca2+ from internal stores evoke ATP release (Lazarowski et al. 2003; Praetorius & Leipziger, 2009; Ransford et al. 2009; Seminario-Vidal et al. 2009; Kreda et al. 2010; Hackett et al. 2011; Lazarowski et al. 2011; Okada et al. 2011). The mechanisms of release involve calcium-dependent exocytosis of nucleotide phosphates and Mucin-5, subtype AC-containing vesicles (Kreda et al. 2007, 2010) as well as activation of ATP-conductive pathways that include pannexin 1 channels (Cherry et al. 2008; Ransford et al. 2009; Lazarowski et al. 2011; Seminario-Vidal et al. 2011; Halim et al. 2012) and/or connexin hemichannels (Seminario-Vidal et al. 2009; Sipos et al. 2009; Baroja-Mazo et al. 2013). Previous studies have demonstrated that exposure to specific serine proteases such as thrombin can evoke ATP release by stimulating protease-activated receptors to elicit Ca2+ mobilization, RhoA activation and subsequent opening of pannexin/connexin channels (Sipos et al. 2009). The specific mechanism whereby Rho/ROCK (a member of the family of Rho GTPases and its effector, Rho associated kinase) activation leads to ATP release is presently unclear, but it appears likely that the resulting cytoskeletal rearrangements that occur in response to ROCK phosphorylation may facilitate membrane vesicle insertion or directly activate ATP-conductive pathways in the plasma membrane (Lazarowski et al. 2011).
At this time the mechanism underlying the effects of Alternaria on ATP release from airway epithelial cells is unknown. Furthermore, it is unclear whether differences exist between normal and asthmatic bronchial epithelial cells with respect to ATP release and Ca2+ mobilization following exposure to Alternaria. In the present study we compared the effects of Alternaria on human bronchial epithelial cells derived from normal (HBE) and asthmatic (HBE-As) subjects. These cells were found to express marker proteins characteristic of human basal cells and to release IL-33 in response to Alternaria stimulation (Hackett et al. 2011; Kouzaki et al. 2011). The results provide evidence to support at least two distinct mechanisms responsible for Alternaria-evoked ATP release. Moreover, experiments revealed enhanced ATP release and Ca2+ mobilization by HBE-As cells compared to HBE cells following Alternaria exposure and increases in [Ca2+]i that were independent of PAR2 activation.
Methods
Materials
The compounds used for imaging experiments (FM1-43 and the acetoxymethyl ester form of Fura-2, Fura-2-AM) were purchased from Invitrogen/Life Technologies (Carlsbad, CA, USA). Trypsin, E64, leupeptin, probenicid, carbenoxolone, 1,2-bis(2-aminophenoxy)ethane-N,N, N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM), brefeldin A, bafilomycin A1 and phorbol 12-myristate 13-acetate were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Alternaria extract was purchased from Greer Laboratories (Lenoir, NC, USA).
Cell culture
Bronchial epithelial (HBE and HBE-As) cells were purchased from Lonza (Walkersville, MD, USA). 16HBE14o− cells were obtained through a Material Transfer Agreement with the California Pacific Medical Center Research Institute (San Francisco, CA, USA). Bronchial epithelial growth media (BEGM) supplemented with defined growth factors and retinoic acid from bullet kits were also obtained from Lonza and Eagle's minimum essential medium with Earle's salts (MEM), fetal bovine serum (FBS), penicillin and streptomycin were purchased from Life Technologies. HBE and HBE-As cells were grown in BEGM supplemented with growth factors and retinoic acid at 37°C in a humidified CO2 atmosphere. 16HBE14o− cells were grown under the same conditions in MEM supplemented with 10% FBS, penicillin and streptomycin. Cells used for ATP measurements were cultured on 35 mm-diameter dishes until confluent. Imaging experiments were performed using cells grown on chamber slides (Fisher Scientific, Pittsburgh, PA, USA).
Alternaria extract
Alternaria alternata extract was prepared by Greer Laboratories. The cellular antigens were obtained from Alternaria plated from a master-spore and allowed to germinate. Antigens were then harvested using acetone and extracted into buffer solution, then dialysed and lyophilized. To ensure that differences in ATP release between normal HBE and HBE-As cells were due to responsiveness of the epithelial cells, all comparisons were conducted in parallel, using the same lot number of freshly prepared solutions (reconstituted in Hanks’ balanced salt solution (HBSS) with 1 mm CaCl2 and 1 mm MgCl2) of Alternaria extract.
Quantitative RT-PCR
Total RNA was isolated from epithelial cells using TRizol (Sigma-Aldrich Co.) according to the manufacturer's protocol. Complimentary DNA was then prepared from total RNA (750 ng) using the Quantitech Reverse Transcription two-step qRT-PCR kit (Qiagen, Valencia, CA, USA). Quantitative PCR (qPCR) was performed using an Applied Biosystems 7300 Real time PCR System (Life Technologies). PCR conditions: 50°C for 2 min, 95°C for 10 min, 45 cycles of 95°C for 15 s and 60°C for 1 min. The list of primers used for PCR experiments is presented in Table 1. Each reaction was carried out in triplicate in 96-well plates. Relative expression of the target gene was calculated using the 2ΔCT method. Messenger RNA levels were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and specificity for the different mRNA transcripts was assessed by analysis of melting point dissociation curves.
Table 1.
Primer sequences and PCR product characteristics
| Primer | Sequences (F and R; 5′ to 3′) | Accession no. or publication | mRNA size (bp) | Position | Product size (bp) | Tm (°C) |
|---|---|---|---|---|---|---|
| KRT6A | CCAAGGCAGACACTCTCACA | NM_005554.3 | 2450 | 857–1090 | 234 | 60.02 |
| GCAGCTCCTCGTACTTGGTC | 60.02 | |||||
| CK14 | TTCTGAACGAGATGCGTGAC | NM_000526.4 | 1653 | 848–1036 | 189 | 59.99 |
| GCAGCTCAATCTCCAGGTTC | 59.96 | |||||
| KRT16 | AGGGCCAGAGCTCCTAGAAC | NM_005557.3 | 1720 | 1545–1697 | 153 | 59.98 |
| GCTTTATTAGCCCACCACCA | 59.96 | |||||
| MUC5AC | CACCTCCTTCAACACCACCT | NM_017511.1 | 18750 | 3294–3454 | 161 | 60.00 |
| GAACGTAGCTGCAGTCACCA | 60.01 | |||||
| GAPDH | ATGACATCAAGAAGGTGGTG | NM_002046 | 1401 | 941–960 | 177 | 52.2 |
| CATACCAGGAAATGAGCTTG | 51.1 |
Tm is the primer melting temperature.
Western immunoblotting
Protein samples were prepared from cell pellet homogenates in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), then incubated on ice for 30 min and centrifuged (12,000 g for 10 min) to remove insoluble debris. Total protein was measured using the bicinchoninic acid (BCA) protein assay reagent kit (Pierce, Rockford, IL, USA). Proteins (100 μg) were separated on 8% NuPAGE Bis-Tris precast gels (Life Technologies) and electro-blotted onto nitrocellulose membranes. Membranes were then probed with primary antibody (cytokeratin 6A (KR-6A), 1:1000 dilution or cytokeratin 16 (KR-16), 1:1000 dilution; Novus Biologicals, Littleton, CO, USA), followed by peroxidase-conjugated secondary antibody (goat anti-rabbit IgG-HRP SC-2004, 1:5000 dilution, Santa Cruz Biotechnologies) and visualized using an enhanced Novex ECL HRP chemiluminescent substrate reagent kit (Life Technologies).
Immunohistochemistry
Cells were cultured on chamber slides until reaching 75% confluence before fixation. The cells were rinsed with phosphate-buffered saline solution (PBS) and fixed in 4% paraformaldehyde for 20 min at room temperature (RT), then washed three times in PBS solution. Washed cells were treated with 1 ml Triton X for 20 min, then washed with PBS. After fixation the cells were blocked in 5% bovine serum albumin (BSA) for 2 h at RT. Later, cells were incubated in primary antibody KR-6A or KR-16 (Novus Biologicals) diluted 1:200 in 5% BSA and washed in PBS. After a 1 h incubation with secondary antibody (Alexa Fluor 488 goat anti-rabbit IgG; Life Technologies), cells were again washed three times in PBS. For immunofluorescence, cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen/Life Technologies) for 20 min at RT. All the steps were performed under low-light conditions.
ATP measurements
ATP release into the medium was monitored using a luciferase/luciferin bioluminescence ATP determination kit (Invitrogen/Life Technologies). Standard curves were generated using 35 mm dishes containing 1 ml of standard reaction solution (SRS) placed within the sample chamber of a Glomax 20/20 luminometer (Promega, Madison, WI, USA). The background was measured and averaged for each ATP standard solution. Reactions were initiated by adding ATP standard solution (range: 1–1000 nm) to the SRS. The ATP concentration measurements were averaged, followed by subtraction of the mean background luminescence. ATP release from cultured epithelial cells was measured following the protocol used for the standard curve, substituting cell monolayers grown on 35 mm dishes for the ATP standard solutions. Apical solution ATP concentrations were calculated from the standard curve and expressed as nanomolar per square centimetre.
FM1-43 imaging experiments
Cells were grown on chamber slides before removing growth media and incubation in FM1-43 labelling solution (5 μg ml−1 FM1-43 dissolved in Hanks’ balanced salt solution (HBSS) with 1 mm CaCl2 and 1 mm MgCl2) for 1 h to facilitate endocytosis. Cells were then washed three times with HBSS to remove FM1-43 from the extracellular solution prior to mounting slides onto the stage of a Nikon Diaphot inverted microscope equipped for fluorescence imaging. FM1-43 was excited at 490 nm and emission was measured at 560 nm. Images were acquired using Image-1 MetaMorph software (Universal Imaging Corporation, West Chester, PA, USA). Movement of vesicles labelled with FM1-43 to the plasma membrane could be detected in serial pseudocolour images indicating changes in fluorescence intensity acquired during the experiment and by measuring increases in fluorescence intensity associated with the plasma membrane following Alternaria exposure.
Intracellular [Ca2+] measurements
Epithelial cells were cultured on chamber slides for 48–72 h in either BEGM with growth factor supplements or MEM containing 10% FBS depending on the cell type. Initially, growth media was replaced with HBSS containing 10 mm Hepes buffer, pH 7.4. The cells were loaded with Fura-2-AM for 60 min according to manufacturer's instructions, washed in HBSS and mounted onto the stage of a Nikon Diaphot inverted microscope equipped for fluorescence imaging. Single cell fluorescence was visualized using a Nikon UV-fluor 20× or 40× oil-immersion objective. Excitation (340 nm/380 nm), image acquisition and data analyses were performed using Image-1 MetaMorph software running on a Dell Optiplex 760 microcomputer. [Ca2+]i was measured as the ratio of fluorescence emitted at 510 nm when the cells were alternately excited at 340 nm and 380 nm (F340/F380). [Ca2+]i was calculated following calibration using the Fura-2-AM calcium imaging calibration kit available from Invitrogen/Life Technologies.
Statistics
Data are reported as the mean ± standard error of the mean (SEM). Statistical comparisons between control and a single treatment condition were performed using an unpaired, two-tailed t test. Comparisons between control and multiple treatment conditions were carried out by ANOVA followed by Tukey's post test. A value of P < 0.05 was considered to be significant. Analysis of concentration–response curves was performed using PRISM 5 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Basal cell mRNA and protein expression by normal HBE, HBE-As and 16HBE14o− cells
The expression of cytokeratin 14 (KR-14), cytokeratin 16 (KR-16) and cytokeratin 6A (KR-6A), previously shown to be highly expressed in airway basal cells along with MUC5AC, a marker for differentiated human airway epithelial cells, were measured in HBE, HBE-As and 16HBE14o− cells and the results presented in Fig. 1. Analysis of mRNA expression (Fig. 1A and B) revealed that KR-14, KR-16 and KR-6A were significantly higher in HBE and HBE-As cells compared to 16HBE14o− cells. In contrast, 16HBE14o− cells showed greater MUC5AC expression compared to HBE and HBE-As cells. Figure 1C and D shows the results of immunocytochemistry comparing KR-16 and KR-6A expression in 16HBE14o− and HBE cells and corresponding western blots using the same antibodies. Both KR-16 and KR-6A proteins were expressed more abundantly in HBE cells as compared to 16HBE14o− cells. No fluorescence was detected in control cells treated with only secondary antibody (data not shown). Molecular weight estimates for KR-16 (∼50 kDa) and KR-6A (∼55 kDa) were consistent with predictions based on amino acid sequence data (NP_005545.1, NP_005548.2). These results indicate that HBE and HBE-As cells show characteristics of basal cells and that 16HBE16o− cells exhibit a more differentiated phenotype.
Figure 1. Cell type characterization of cultured human airway epithelial cells.
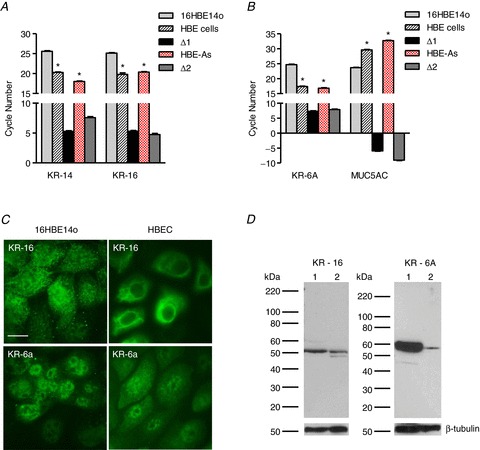
A, comparison of threshold cycle (CT) values for cytokeratin 14 (KR-14) and cytokeratin 16 (KR-16) expressed by 16HBE14o−, HBE and HBE-As cells. ΔCT values (16HBE14o−– HBE and 16HBE14o−– HBE-As) are represented as Δ1 and Δ2, respectively (n= 6; * significantly different from 16HBE14o− cells). B, threshold cycle values for cytokeratin 6A (KR-6A) and mucin (MUC5AC) obtained from 16HBE14o−, HBE and HBE-As cells. ΔCT values (16HBE14o−– HBE and 16HBE14o−– HBE-As) are represented as Δ1 and Δ2, respectively (n= 6; * significantly different from 16HBE14o− cells). C, immunocytochemistry showing KR-16 and KR-6A protein expression in 16HBE14o− and HBE cells (scale bar = 5 μm). No fluorescence was observed with secondary antibody alone (data not shown). D, western blots comparing KR-6A and KR-16 protein expression between 16HBE14o− and HBE cells. Blots were re-probed with anti-β-tubulin antibody to document protein loading in each lane. Lane 1, HBE cells; lane 2, 16HBE14o− cells.
Alternaria evokes a sustained release of ATP
Treatment with Alternaria extract produced a sustained release of ATP beginning ∼2 min after addition to the apical surface of HBE cells. Removal of Alternaria extract by washing the cells with HBSS resulted in a rapid return to baseline levels, indicating that the cells did not exhibit significant ATP leakage after 30 min of exposure to Alternaria (Fig. 2A). If Alternaria extract was pre-exposed to a temperature of 80°C for 5 min prior to HBE cell stimulation, no increase in ATP release could be detected (Fig. 2B). However, if the same monolayer of cells was subsequently treated with apical Alternaria that was not exposed to high temperature, ATP release was measureable. The concentration-dependent effects of Alternaria on HBE, HBE-As and 16HBE14o− cells are reported in Fig. 2C. The threshold Alternaria concentration for stimulation of ATP release was ∼50 μg ml−1 and the onset time for release was dependent on Alternaria concentration. At concentrations >200 μg ml−1, maximum levels of ATP release were significantly higher in HBE-As cells compared to normal HBE cells (Fig. 2C). EC50 values obtained from fitting the concentration–response data with a four-parameter logistic function were 149 ± 10 (n= 6) and 201 ± 15 μg ml−1 (n= 6) for HBE-As and HBE cells, respectively, and 116 ± 12 μg ml−1 (n= 3) for 16HBE14o− cells. These results also indicated that the efficacy of ATP release at concentrations >200 μg ml−1 was greater for HBE-As cells compared to normal HBE cells and exhibited 1.35-fold greater potency relative to HBE cells.
Figure 2. Time course of ATP release and Alternaria concentration–response relationships.
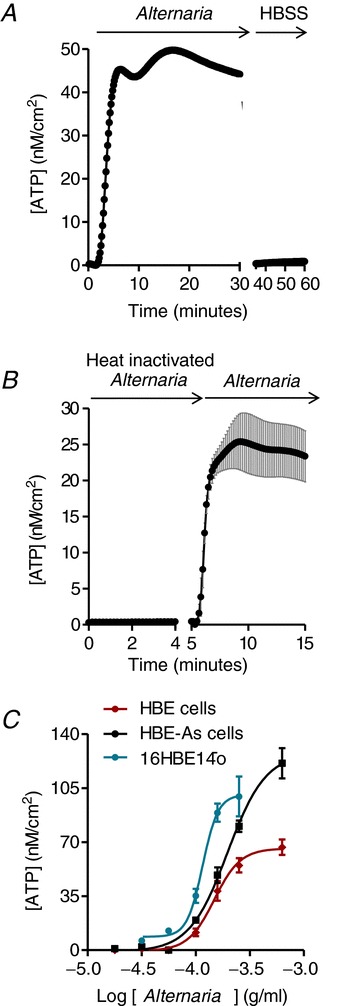
A, Alternaria extract (200 μg ml−1) evokes a sustained release of ATP over a period of 30 min. Washout of the extract with HBSS reduces ATP release to pre-stimulus levels (n= 3). B, preheating Alternaria extract (200 μg ml−1) to 80°C for 5 min abolished ATP release; however, subsequent addition of the same concentration of non-inactivated extract produced a rapid increase in ATP concentration (n= 3). C, Alternaria concentration–response relationships for 16HBE14o−, HBE and HBE-As cells. The EC50 values are reported in Results (n= 6).
Lowering intracellular [Ca2+] reduces Alternaria-induced ATP release
Figure 3 shows the effects of lowering [Ca2+]i using the cell-permeable calcium-chelating agent BAPTA-AM on Alternaria-evoked ATP release from HBE-As and HBE cells. Pretreatment with BAPTA-AM reduced maximum ATP release by 72% in HBE cells and by approximately 50% in HBE-As cells. The initial rate of ATP release was also significantly faster in HBE-As cells following Alternaria exposure (384 ± 52 nm min−1) compared to HBE cells (198 ± 29 nm min−1) and BAPTA-AM pretreatment significantly reduced the rate of release by 48% and 69%, respectively.
Figure 3. Chelating intracellular Ca2+ with BAPTA-AM significantly inhibited Alternaria-evoked ATP release.
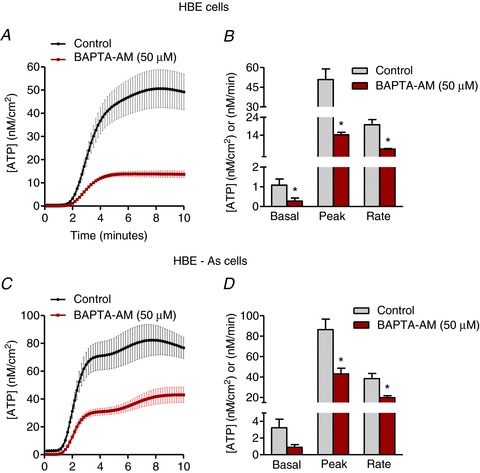
A, kinetics of ATP release from HBE cells elicited by 200 μg ml−1 Alternaria extract with and without 100 μm BAPTA-AM pretreatment for 30 min (n= 6). B, comparison of basal, peak and rate of ATP release between untreated and BAPTA-AM-treated HBE cell monolayers following Alternaria exposure (n= 6; * significantly different from control). C, ATP release from HBE-As cells stimulated with 200 μg ml−1 Alternaria extract with and without 100 μm BAPTA-AM pretreatment for 30 min (n= 6). D, basal, peak and rate of ATP release from untreated and BAPTA-AM-treated HBE-As cells after stimulation with Alternaria extract (n= 6; * significantly different from control).
Alternaria-induced Ca2+ mobilization
A comparison of the effects of Alternaria on [Ca2+]i between HBE and HBE-As cells is shown in Fig. 4. Images in panels A and B are HBE-As cells before (A) and 100 s after (B) Alternaria treatment. Nearly all of the HBE-As cells reached their peak (410 nm) at 100 s, then [Ca2+]i decreased exponentially with a time constant of 66 s to a new plateau concentration equal to 213 nm. In contrast, the increase in [Ca2+]i in normal HBE cells was delayed relative to HBE-As cells, reaching a sustained maximum of 260 nm for >10 min following Alternaria stimulation. These results indicated that HBE-As cells responded more rapidly and with a greater initial increase in [Ca2+]i, followed by a decrease in [Ca2+]i below the sustained steady-state concentration observed in normal HBE cells.
Figure 4. Effects of Alternaria exposure on [Ca2+]i in HBE and HBE-As cells.
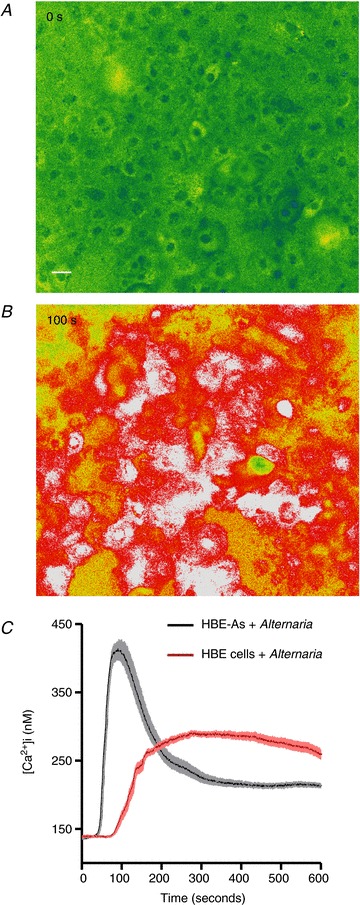
A, background levels of [Ca2+]i in HBE cells prior to Alternaria stimulation (Scale bar = 10 μm). B, Alternaria (200 μg ml−1) evoked a sustained increase in [Ca2+]i in nearly all HBE cells within the field. C, mean ± SEM values for [Ca2+]i from HBE and HBE-As cells following Alternaria exposure (n= 75 cells from 3 experiments).
Alternaria stimulates exocytotic release of ATP
The effects of Alternaria (200 μg ml−1) on intracellular vesicle movement was determined by measuring redistribution and incorporation of fluorescent lipid (FM1-43) into the plasma membrane. Images in Fig. 5A and B along with movies comparing vehicle and Alternaria-treated HBE cells (see Supplemental material Fig. 1S, available online only) illustrates the redistribution of FM1-43 after Alternaria treatment. Measurements of relative fluorescence intensity at the plasma membrane under control and after pretreatment with 5 μg ml−1 brefeldin A and BAPTA-AM are shown in Fig. 5C. Both brefeldin A and BAPTA-AM inhibited the Alternaria-evoked redistribution of FM1-43 to the plasma membrane. Similarly, brefeldin A also inhibited ATP release from HBE cells by 54% and significantly reduced the initial rate of ATP release by 57% (Fig. 6A and B). Bafilomycin (100 nm), which inhibits vesicular uptake of ATP, also blocked both the magnitude and rate of Alternaria-evoked ATP release from HBE cells by 64% (Fig. 6C and D). Moreover, experiments with HBE-As cells following treatment with brefeldin A or bafilomycin showed comparable reductions in the amount (56% and 67%, respectively) and rate (54% and 70%) of ATP release as observed with HBE cells (Fig. 7A and B). These findings indicated that more than 50% of the Alternaria-evoked ATP release depended on transport and fusion of ATP-containing vesicles with the plasma membrane.
Figure 5. Effects of Alternaria on FM1-43 movement in HBE cells.
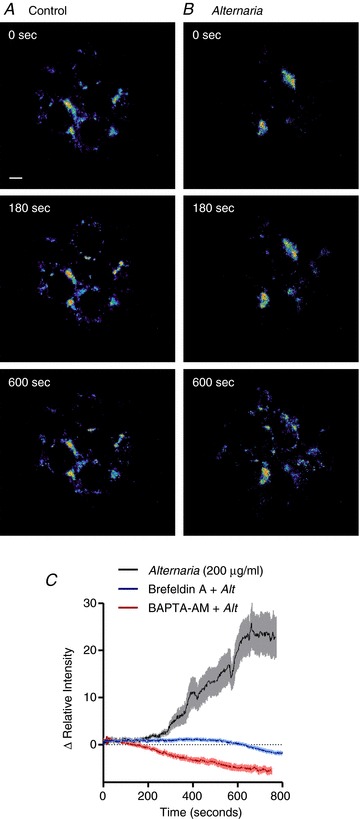
A, time course of FM1-43-labelled vesicle movement in untreated control cells (representative of 5 experiments) (Scale bar = 5 μm). B, redistribution of the FM1-43 fluorescence pattern following stimulation with 200 μg ml−1 Alternaria (representative of 5 experiments). C, measurements of fluorescence intensity changes associated with the plasma membrane following stimulation with Alternaria, or after pretreatment with 100 μm BAPTA-AM or 5 μg ml−1 brefeldin A (n= 5).
Figure 6. Inhibition of vesicle transport or ATP uptake into exocytotic vesicles significantly reduced ATP release in HBE cells.
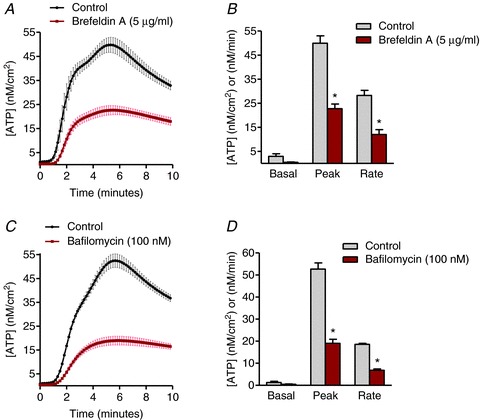
A, kinetics of ATP release evoked by 200 μg ml−1 Alternaria with and without brefeldin A (5 μg ml−1) pretreatment (n= 6). B, measurements of basal, peak and rate of ATP release stimulated by Alternaria in the presence and absence of 5 μg ml−1 brefeldin A (n= 6; * significantly different from control). C, time course of ATP release produced by 200 μg ml−1 Alternaria with and without bafilomycin A (100 nm) pretreatment (n= 6). D, basal, peak and rate of ATP release produced by Alternaria in the presence and absence of 100 nm bafilomycin A (n= 6; * significantly different from control).
Figure 7. Effects of ATP exocytosis inhibitors on ATP release from HBE-As cells.
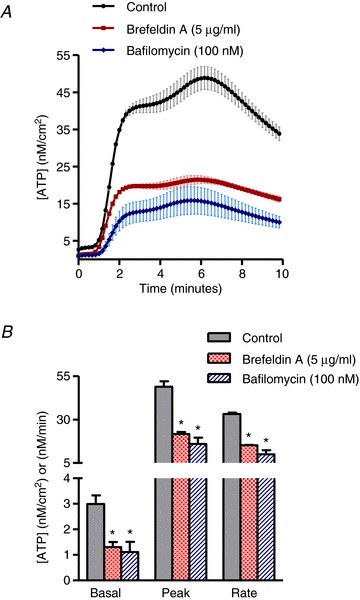
A, time course of ATP release induced by 200 μg ml−1 Alternaria with and without brefeldin A (5 μg ml−1) or bafilomycin A (100 nm) pretreatment (n= 6). B, measurements of basal, peak and rate of ATP release evoked by Alternaria in the presence and absence of 5 μg ml−1 brefeldin A and 100 nm bafilomycin A (n= 6; * significantly different from control).
Transporter-mediated ATP release
To investigate the possible role of membrane transport pathways on Alternaria-induced ATP release we investigated the effects of known inhibitors of pannexin 1-mediated ATP release (Table 2). Treatment with probenecid (500 μm) had no effect on Alternaria-evoked ATP release. Similarly, exposure to an inhibitor of Rho kinase (ROCK) Y27632 (200 μm) also had no inhibitory effect on ATP release. Partial inhibition was observed following pretreatment with carbenoxolone (100 μm), but the responses to these inhibitors was not consistent with previously reported effects on ATP release involving pannexin 1 channels (Ransford et al. 2009; Seminario-Vidal et al. 2011).
Table 2.
Effects of carbenoxolone (CBX; 100 μm), Rho/ROCK inhibitor Y27632 (200 μm) and probenecid (PBC; 500 μm) on ATP release from HBE cells (n= 4)
| Control | CBX | Y27632 | PBC |
|---|---|---|---|
| 55 ± 2.8 | *25 ± 2.8 | 58 ± 5.5 | 53 ± 2.0 |
Values are mean ± SEM (nm cm−2). *Significantly different from control.
Effects of serine and cysteine protease inhibitors on Alternaria-evoked ATP release
Serine and cysteine protease inhibitors were also shown to reduce the maximum levels of ATP release from HBE cells by 58 and 47%, respectively (Fig. 8A and C). In addition, pretreatment of HBE cells with BAPTA-AM (100 μm) completely blocked the inhibitory effects of protease inhibition on Alterneria-induced ATP release suggesting that the increase in vesicular ATP secretion was dependent on protease activity (Fig. 8D). Similarly, the pan-specific protease inhibitor leupeptin also reduced ATP release from HBE-As cells by 52%, whereas no significant decrease was observed following treatment with the cysteine protease inhibitor E64 (Fig. 8B and C).
Figure 8. Protease inhibitors differentially reduce Alternaria-evoked, Ca2+-dependent ATP release from HBE and HBE-As cells.
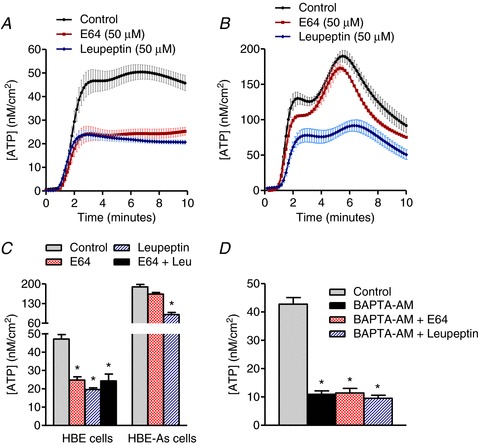
A, time course showing the effects of E64 (50 μm) and leupeptin (50 μm) pretreatment on Alternaria (200 μg ml−1)-evoked ATP release from normal HBE cells (n= 6). B, ATP release from HBE-As cells pretreated with 50 μm E64 or 50 μm leupeptin, followed by exposure to 200 μg ml−1 of Alternaria extract (n= 6). C, comparison of E64 and leupeptin effects on the steady-state levels of ATP release evoked by Alternaria. Note that no additive effects of E64 and leupeptin were detected in HBE cells (n= 6; * significantly different from control). D, protease inhibitors have no significant effect on the Ca2+-independent ATP release mechanism (n= 6; * significantly different from control).
Trypsin or PAR2 agonist peptide pretreatment does not block the effects of Alternaria
To determine whether the Alternaria-evoked increase in [Ca2+]i was blocked by serine protease pretreatment, HBE and HBE-As cells were initially exposed to 5 μm trypsin, then stimulated again with the same trypsin concentration to test for trypsin desensitization. Figure 9A shows the effects of the initial treatment (2), recovery (3) and subsequent stimulation with 100 μg ml−1 Alternaria (4) on [Ca2+]i in HBE cells. All of the cells rapidly responded to trypsin before [Ca2+]i quickly decreased to a new steady-state concentration. Treatment with an additional 5 μm trypsin produced no further increase in [Ca2+]i. Subsequent addition of Alternaria resulted in an increase in [Ca2+]i (Fig. 9B and C). Similar data were obtained with 16HBE14o− cells (Fig. 9D). In a separate experiment, treatment of HBE cells with 5 μm trypsin alone did not evoke ATP release (data not shown). Pretreatment with PAR2 agonist peptide (50 μm SLIGKV-NH2) also produced an increase in [Ca2+]i in both HBE (Fig. 9E) and HBE-As cells (Fig. 9F), but similar to the earlier results with trypsin, subsequent addition of Alternaria in the continuous presence of SLIGKV-NH2 did not block the increase in [Ca2+]i typically produced by Alternaria.
Figure 9. Inhibition of PAR2 receptor activation by pretreatment with trypsin does not block the effects of Alternaria extract on [Ca2+]i.
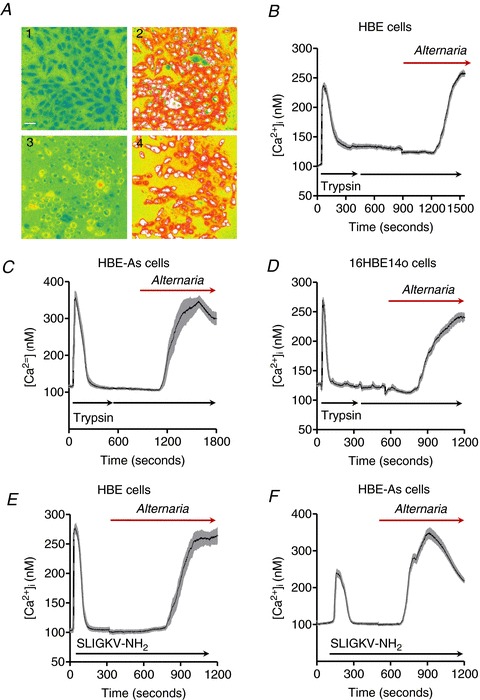
A, images of HBE cells before (1) and after (2) an initial 5 μm trypsin treatment, followed by a second addition of 5 μm trypsin (3), and finally, exposure to 100 μg ml−1 Alternaria extract (4) (representative images from a total of 5 separate experiments). B, mean ± SEM of [Ca2+]i measurements from HBE cells that initially responded to the addition of 5 μm trypsin (n= 25 cells). C, a separate series of experiments showing [Ca2+]i measurements (mean ± SEM) from HBE-As cells that also responded to the addition of 5 μm trypsin prior to treatment with Alternaria (100 μg ml−1; n= 25 cells). D, the experiment described in B and C was repeated under the same conditions using 16HBE14o− cells except that the Alternaria concentration used for these experiments was 50 μg ml−1 (n= 25 cells). E and F, effects of maximal stimulation of HBE cells (E; n= 25 cells) and HBE-As cells (F; n= 25 cells) with the PAR2 agonist peptide SLIGKV-NH2 (50 μm) prior to apical addition of 100 μg ml−1 Alternaria extract.
Discussion
The pseudostratified epithelium of the airways consists of multiple cell types that interact with the basement membrane. Within this epithelium, basal cells represent a population of relatively undifferentiated cells abundantly expressed in both large and small human airways (Zhong et al. 2003; Kreda et al. 2010; Hackett et al. 2011; Kouzaki et al. 2011). In addition to their putative role as progenitor cells, basal cells secrete various signalling and defence molecules including cytokines, arachidonic acid metabolites and proteases that play an important role in the innate immune function of the airways. Recent studies have revealed that basal cells within the human bronchial epithelium serve as a primary site for IL-33 expression and release in response to Alternaria exposure (Kouzaki et al. 2011). Initial experiments with HBE and HBE-As cells in the present study demonstrated that both cell lines express high levels of protein markers that are characteristic of basal cells (Hackett et al. 2011). In contrast, 16HBE14o− cells express much higher levels of MUC5AC compared to HBE or HBE-As cells and lower amounts of the basal cell cytokeratin markers, consistent with a more differentiated phenotype. The basal cell characteristics of HBE and HBE-As cells along with the previous finding (Kouzaki et al. 2011) that Alternaria exposure stimulates secretion of IL-33 from cultured HBE cells, makes them useful models for understanding the mechanisms of ATP release that contribute to Alternaria-evoked IL-33 secretion.
Exposure of HBE and HBE-As cells to Alternaria produced a sustained release of ATP that was completely abolished when Alternaria extract was removed from the apical bathing solution. This result indicated that exposure to the extract did not produce damage to the plasma membrane that would permit leakage of ATP from the cells. Moreover, exposure of Alternaria extract to high temperature blocked ATP release, suggesting that heat labile components of the extract were responsible for stimulating ATP secretion. Analysis of varying Alternaria concentrations on ATP release revealed that 16HBE14o− cells were less than 1.5-fold more sensitive to the extract compared to normal and asthmatic HBE cells; however, it is not known whether this difference could be related to the level of differentiation associated with 16HBE14o− cells. An interesting distinction between the effects of Alternaria on HBE cells and HBE-As cells was the significantly greater efficacy in stimulating ATP release associated with HBE-As cells coupled with a minimal change in sensitivity as indicated by the slightly lower EC50 value relative to HBE cells. Although the mechanism responsible for this difference is not clear, the greater amount of ATP release appears to correlate with results of Ca2+-imaging experiments showing a higher initial increase in Ca2+ mobilization in HBE-As cells compared to HBE cells when exposed to the same concentration of Alternaria extract. The enhanced [Ca2+]i response could result from more rapid and robust stimulation of multiple purinergic receptor subtypes, causing greater mobilization of Ca2+ in HBE-As cells compared to normal HBE cells. It is also possible that HBE-As cells maintain higher levels of releasable Ca2+ within various internal stores so that upon stimulation, a greater increase in [Ca2+]i occurs. At this time, the level of Alternaria aeroallergen exposure necessary to induce allergic inflammation or exacerbation of an asthmatic episode is not known. However, previous in vivo experiments in mice showed that Alternaria extract could evoke an inflammatory response involving eosinophil recruitment, release of IL-33 and Th2 cytokine secretion into the airways (Kouzaki et al. 2011).
A substantial portion of Alternaria-evoked ATP release was dependent on [Ca2+]i. Pretreatment of both HBE and HBE-As cells with the cell-permeable Ca2+-chelating compound BAPTA-AM inhibited more than 50% of the response and also slowed the rate of release. This result demonstrated that Alternaria induces Ca2+-dependent and Ca2+-independent ATP secretion. Previous studies with airway epithelial cells have shown that Ca2+-dependent ATP release can involve exocytosis of vesicles containing nucleotide phosphates as well as mucin (Kauffman & van der Heide, 2003; Kreda et al. 2007, 2010). Experiments with polarized monolayers of Calu-3 cells (an adenocarcinoma cell line exhibiting a phenotype characteristic of airway submucosal gland epithelium) showed Ca2+-dependent apical release of ATP, UDP-glucose and MUC5AC following PAR and P2Y2 receptor stimulation. More recently, experiments with primary human bronchial epithelial cells induced to develop goblet cell hyperplasia exhibited increased mucin secretion and ATP release that was blocked by disruption of exocytosis with brefeldin A and enhanced by increases in [Ca2+]i compared to control monolayers (Okada et al. 2011). Experiments designed to measure ATP and adenosine concentrations within the thin (∼7 mm) liquid layer lining the surface of primary human bronchial epithelial cells showed that hypotonic challenge stimulated sufficient amounts of ATP release to activate apical P2Y2 receptors (Okada et al. 2006). However, ATP release was not Ca2+ dependent, suggesting a non-exocytotic release mechanism from the ciliated cells constituting most of the cell types within the monolayer. The subsequent regulatory volume decrease was reduced by apyrase and augmented by ATP or UTP, indicating that ATP was critical for regulating cell volume (Okada et al. 2006). In the present study, Alternaria stimulated fluorescent lipid (FM1-43)-containing vesicle transport to the plasma membrane. This effect was blocked by pretreatment of HBE cells with the exocytosis inhibitor brefeldin A and by chelating intracellular Ca2+ with BAPTA-AM. Furthermore, Ca2+-dependent ATP release following HBE and HBE-As cell exposure to Alternaria was also shown to be blocked by pretreatment with brefeldin A and by the vacuolar H+-ATPase inhibitor bafilomycin, which inhibits uptake of ATP into exocytotic vesicles (Zhong et al. 2003). These findings indicated that >50% of ATP release evoked by Alternaria was the result of exocytosis. ATP release was not blocked by probenecid or by the Rho/ROCK inhibitor Y27632. Moreover, treatment of HBE cells with phorbol 12-myristate 13-acetate in an attempt to mimic increased diacylglycerol (DAG) levels within the cells also failed to stimulate ATP release as was previously reported for DAG in astrocytes (Mungenast, 2011). Interestingly, pretreatment with carbenoxolone (100 μm) inhibited ∼50% of the ATP release following Alternaria exposure; however, the molecular identity of the efflux pathway could not be determined. Thus the mechanism responsible for Ca2+-independent ATP release following exposure to Alternaria does not appear to be the same pathway activated in response to cell swelling or protease exposure as previously described (Lazarowski et al. 2011) and at this time remains unknown.
Previous studies have shown that Alternaria extracts possess protease activity capable of activating PAR receptors (Kouzaki et al. 2009; Boitano et al. 2011) and that PAR receptor activation can elicit ATP release from airway epithelial cells (Praetorius & Leipziger, 2009; Seminario-Vidal et al. 2009, 2011; Kreda et al. 2010). To determine whether Alternaria-evoked ATP release was dependent on endogenous protease activity, two different protease inhibitors, E64, which blocks a variety of cysteine proteases, and leupeptin, a pan-specific protease inhibitor, capable of blocking serine, threonine and cysteine proteases, were examined. The results showed that both inhibitors were effective in blocking ATP release from HBE cells and that no additive effects were observed when E64 and leupeptin were applied together prior to Alternaria exposure. In addition, if HBE cells were pretreated with BAPTA-AM to block Ca2+-dependent ATP release, then the protease inhibitors had no effect. These findings support the idea that cysteine protease activity associated with Alternaria extract was responsible for stimulating exocytotic ATP release from HBE cells. Similar effects of leupeptin on ATP release from HBE-As were also observed; however, E64 did not inhibit release. This result suggests that HBE cells and HBE-As appear to respond to distinct proteases present in Alternaria extract.
In an earlier study, the effects of Alternaria filtrate on 16HBE14o− cells suggested that sustained increases in [Ca2+]i were dependent on PAR2 receptors and that inactivating them with trypsin or producing desensitization by pretreating with activating peptides abolished [Ca2+]i mobilization (Boitano et al. 2011). In contrast to these findings, results from the present study showed that pretreatment of HBE cells and HBE-As cells with 5 μm trypsin resulted in a rapid and transient increase in [Ca2+]i that returned to near baseline levels within 5 min following trypsin exposure. A second treatment with the same concentration of trypsin produced no further increase in [Ca2+]i. However, unlike the results previously reported for 16HBE14o− cells, subsequent exposure to Alternaria extract did evoke a sustained increase in [Ca2+]i. To determine whether this response was due to differences between HBE and 16HBE14o− cells, we repeated the experiment under identical conditions with 16HBE14o− cells, but observed a similar sustained increase in [Ca2+]i evoked by Alternaria extract. Thus differences between our results and the study by Biotano et al. (2011) were not due to differences in the cell types used for these experiments. One possible explanation might involve differences in the Alternaria preparations. In the present study Alternaria extract was obtained from a commercial source (Greer) whereas experiments performed in the previous study used an Alternaria filtrate that was prepared by the authors and used for in vitro experiments (Boitano et al. 2011). We suggest that the Greer extract used in the present study contained components that were either inactivated or not present in the filtrate used by Boitano et al. (2011). This difference could also explain why higher concentrations of the Greer extract were required to increase [Ca2+]i compared to concentrations reported by Boitano et al. (2011) and why treatment with protease inhibitors such as E64 did not block responses to Alternaria filtrate that were reported in the previous study.
The results of the present study indicate that Alternaria-evoked ATP release from airway epithelial cells involves multiple pathways with distinct kinetics. Although a significant fraction of ATP release was blocked by protease inhibitors, the effect was not directly linked to activation of epithelial cell PAR2 receptors. How Alternaria-associated protease activity stimulates ATP release and Ca2+ mobilization is not clear. Previous studies using human bronchial epithelial cells have shown that protease exposure produced increases in reactive oxygen species (ROS) and oxidant injury (Aoshiba et al. 2001). Interestingly, oxidative stress has been shown to stimulate ATP release from primary pulmonary endothelial and epithelial cells (Ahmad et al. 2006). Extracellular ATP binding to specific purinergic receptors leads to activation of ERK and phosphoinositide-3 kinase signalling pathways that increase resistance and survival of cells experiencing oxidative stress. These earlier findings along with the results of this study suggest the hypothesis that Alternaria-derived proteases are capable of inducing oxidative stress resulting in stimulation of ATP release, purinoceptor activation and sustained mobilization of intracellular [Ca2+]. Differences in the response to E64 and leupeptin pretreatment between normal HBE and HBE-As cells could mean that the transduction mechanism leading to ROS generation in asthmatic cells is not sensitive to cysteine protease activity associated with Alternaria extract, a distinction that could be helpful in identifying protease-sensitive pathways that couple to ROS production. At this time the underlying molecular mechanisms that link protease activity with oxidative stress have not been defined in any cell system, so further experiments will be required to address this hypothesis.
In closing, the results of this investigation support four major conclusions. First, exposure of HBE and HBE-As cells to Alternaria stimulates ATP release by Ca2+-dependent exocytosis and by undefined Ca2+-independent mechanisms that do not appear to involve pannexin 1 channels. Secondly, HBE-As cells exhibited significantly greater ATP release in response to Alternaria exposure compared to normal HBE cells with a relatively modest increase in sensitivity and both Ca2+-dependent and independent pathways contributed to the increased level of ATP release. Third, pretreatment with a protease inhibitor that is known to block cysteine protease activity was effective in blocking exocytotic ATP release from normal HBE cells, but only the pan-specific protease inhibitor leupeptin was capable of inhibiting release from normal HBE and HBE-As cells. This result suggests that HBE-As cells respond to a different protease or group of proteases within Alternaria extract that stimulate exocytosis. Finally, sustained Ca2+ mobilization evoked by Alternaria and required for IL-33 release does not appear to be exclusively dependent on the activation of PAR2 as previously reported for the more differentiated and immortalized 16HBE14o− cell line. Additional studies will be necessary to identify the mechanism of Ca2+-independent ATP release and the underlying signalling pathways responsible for protease-dependent, exocytotic ATP release.
Acknowledgments
None.
Glossary
- BEGM
bronchial epithelial growth media
- HBE cells
human bronchial epithelial cells derived from normal subjects
- HBE-As cells
HBE cells from asthmatic subjects
- IL-33
interleukin 33
- PAR2
protease-activated receptor
Additional information
Competing interests
The authors have no conflicts to disclose.
Author contributions
N.P., P.J.M. and C.L. were involved in the ATP release measurements, qRT-PCR experiments and immunohistochemistry studies. P.J.M. and S.M.O’G. conducted the Ca2+ and FM1-43 imaging experiments. N.P. and T.M. performed the western blot experiments. S.M.O’G, N.P. and H.K. collaborated on the experimental design and wrote the manuscript. Experiments were performed in the laboratory of S.M.O'G at the University of Minnesota. All authors have approved the final version of the manuscript.
Funding
This study was supported by a grant from the National Institutes of Health (HL110539) to H.K. and S.M.O’G.
Supplementary material
Supplemental material Fig. 1S
References
- Ahmad S, Ahmad A, White CW. Purinergic signalling and kinase activation for survival in pulmonary oxidative stress and disease. Free Radic Biol Med. 2006;41:29–40. doi: 10.1016/j.freeradbiomed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L556–L564. doi: 10.1152/ajplung.2001.281.3.L556. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A, Barberà-Cremades M, Pelegrín P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–504. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, Xuan W, Downie SR, Tobias A, Peat JK. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–459. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- Fujita J, Kawaguchi M, Kokubu F, Ohara G, Ota K, Huang SK, Morishima Y, Ishii Y, Satoh H, Sakamoto T, Hizawa N. Interleukin-33 induces interleukin-17F in bronchial epithelial cells. Allergy. 2012;67:744–750. doi: 10.1111/j.1398-9995.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Hayes D, Jr, Jhaveri MA, Mannino DM, Strawbridge H, Temprano J. The effect of mold sensitization and humidity upon allergic asthma. Clin Respir J. 2013;2:135–144. doi: 10.1111/j.1752-699X.2012.00294.x. [DOI] [PubMed] [Google Scholar]
- Kauffman HF, van der Heide S. Exposure, sensitization, and mechanisms of fungus-induced asthma. Curr Allergy Asthma Rep. 2003;3:430–437. doi: 10.1007/s11882-003-0080-z. [DOI] [PubMed] [Google Scholar]
- Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, van Heusden CA, O’Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol. 2010;588:2255–2267. doi: 10.1113/jphysiol.2009.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Sesma JI, Seminario L, Esther CR, Jr, Kreda SM. Nucleotide release by airway epithelia. Subcell Biochem. 2011;55:1–15. doi: 10.1007/978-94-007-1217-1_1. [DOI] [PubMed] [Google Scholar]
- Mungenast AE. Diacylglycerol signalling underlies astrocytic ATP release. Neural Plast. 2011;2011:537659. doi: 10.1155/2011/537659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol. 2011;45:253–260. doi: 10.1165/rcmb.2010-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H, Bush RK. A review of Alternaria alternata sensitivity. Rev Iberoam Micol. 2001;18:56–59. [PubMed] [Google Scholar]
- Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signalling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Leu SW, Xu F, Zhou X, Yin H, Cai L, Zhang L. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129:202–210. doi: 10.1016/j.clim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- Weber RW. Alternaria alternata. Ann Allergy Asthma Immunol. 2001;87:A4. doi: 10.1016/s1081-1206(10)62219-3. [DOI] [PubMed] [Google Scholar]
- Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–5750. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- Zhong X, Malhotra R, Guidotti G. ATP uptake in the Golgi and extracellular release require Mcd4 protein and the vacuolar H+-ATPase. J Biol Chem. 2003;278:33436–33444. doi: 10.1074/jbc.M305785200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


