Abstract
Anti-citrullinated peptide antibodies (ACPA) are highly specific for rheumatoid arthritis (RA). However, the predominant B cell epitopes have not yet been defined. The aim of this study was to examine the reactivity of ACPA against different peptides derived from citrullinated proteins and to investigate whether or not these antibodies constitute a homogeneous population. For this purpose, sera from patients with RA (n = 141), systemic lupus erythematosus (SLE) (n = 60), Sjögren's syndrome (SS) (n = 54) and healthy controls (n = 100) were tested for their reactivity against six citrullinated peptides derived from peptidyl arginine deiminase (PAD), vimentin (vim), alpha-enolase (enol), fibrin, type II collagen (col-II) and filaggrin, respectively. A non-citrullinated control peptide derived from PAD was used as control (ctrlPAD621–40). Antibody reactivity against each individual peptide was evaluated by enzyme-linked immunosorbent assay (ELISA). Specificity and cross-reactivity of ACPA were tested by using two prototype sera with homologous and cross-inhibition assays. Specificity of ACPA from two prototype sera was confirmed by purification of anti-peptide antibodies and homologous-inhibition experiments. We found that sera from patients with RA reacted diversely with the six citrullinated peptides. More specifically, PAD211–30 displayed 29·08% sensitivity, vim60–75 29·08%, enol5–21 37·59%, fibrin617–31 31·21%, col-II358–75 29·97% and filaggrin306–24 28·37%, while control ctrlPAD621–40 showed no reactivity. All reactive peptides were found to be highly specific for RA. A notable cross-reaction (>70%) was found mainly between filaggrin and the majority of anti-citrullinated peptide antibodies. We concluded that ACPA in RA constitute a heterogeneous population with limited cross-reactivity and without a predominant epitope.
Keywords: anti-citrullinated peptide antibodies (ACPA), epitopes, pathogenesis, rheumatoid arthritis, synthetic peptides
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease of autoimmune origin with significant morbidity worldwide, accounting for 0·5–1% of the adult population [1]. The aetiology of RA remains unclear, even though immunological, genetic and environmental factors have been reported to be involved in pathogenesis [2–4]. Two important autoantibodies have been described in RA: rheumatoid factor (RF) directed against the Fc fragment of immunoglobulin (Ig)G [5] and autoantibodies against citrullinated peptides/proteins (ACPA) [6]. RF is a well-described autoantibody and is present in approximately 70% of RA patients [7]. RF is also detected in patients with other autoimmune diseases as well as in chronic infections, lymphomas and solid tumours with limited specificity [6]. Thus, interest has shifted towards novel autoantibodies to detect seronegative RA [7] as well as ACPA, which are directed to antigens that contain arginine converted to citrulline residues by the calcium-dependent enzyme family of peptidylarginine deiminases (PAD) [8]. Remarkably, ACPA are detectable in sera of patients with early RA several years before disease onset, and have been associated with a more severe disease outcome [9,10]. Also, autoantibodies can be used as biomarkers to direct clinical judgement towards specific therapeutic approaches, to monitor disease activity or to assess response to treatment. Therefore, the presence of any RF isotype or anti-cyclic citrullinated peptide (CCP) antibodies has been found to predict clinical benefit from anti-CD20 therapy [11].
Previous studies have revealed that ACPA recognize linear peptide epitopes containing citrulline residues, generated by post-translational deimination (citrullination) of arginine residues derived from various proteins [12–14]. However, it has been demonstrated previously that B cell epitopes within the parent proteins vimentin, fibrin, filaggrin [15], PAD [16] and alpha-enolase [12] are recognized consistently by ACPA. Specifically, the vimentin and fibrinogen epitopes are the most frequently recognized by sera from ACPA-positive patients with long-standing RA [17]. Lundberg et al. identified an immunodominant epitope in citrullinated alpha-enolase, to which antibodies are specific for RA [18]. Because RA patients recognize diversely distinct citrullinated epitopes of the same parent protein, an approach based on citrullinated peptides rather than whole proteins has been used in an attempt to obtain optimal discrimination ability [19]. Therefore, ACPA represent a promising tool for the serological diagnosis of RA. However, the predominant B cell epitopes have not yet been defined.
The present study sought to identify the major targets of ACPA that could provide further insights into RA pathogenesis. To this end, we examined in parallel the ACPA reactivity against different peptides derived from citrullinated proteins and investigate whether or not these antibodies constitute a homogeneous population. Previous studies have shown that ACPA recognize a wide spectrum of different citrullinated epitopes, implying that this specific autoantibody population is characterized by heterogeneity [20–22]. Mechanisms such as epitope spreading and isotype expansion might be involved in disease pathogenesis contributing to ACPA diversity.
Materials and methods
Serum samples
Serum samples were obtained, after informed consent and ethical approval, from 141 consecutive patients with RA who were attending the outpatient Rheumatology Clinic at Laiko University General Hospital in Athens, Greece. All patients with RA fulfilled the 1987 American College of Rheumatology (ACR) classification criteria [23]. The disease control group consisted of 104 patients with other autoimmune diseases, including systemic lupus erythematosus (SLE) (n = 60) and Sjögren's syndrome (SS) (n = 54). The SLE and SS patients were classified according to the 1997 ACR revised criteria and the 2002 American–European Consensus Group revised criteria, respectively [24,25]. The control group included 100 sera obtained from healthy volunteers (blood donors and healthy laboratory personnel, age- and sex-matched with the RA patients). Patients with hepatitis B or C virus were excluded. Serum samples were stored at −20°C until tested.
Synthetic peptides
Linear citrullinated (Cit) peptides derived from the sequence of human proteins were as follows: PAD211–30 (VCitVFQATCitGKLSSKCSVVLG) derived from PAD, vim60–75 (VYATCitSSAVCitLCitSSVP) derived from vimentin, enol5–21 (KIHACitEIFDSCitGNPTVE) derived from alpha-enolase, fibrin617–31 (HSTKCitGHAKSCitPVCitG) derived from the alpha chain of fibrinogen, col-II358–75 (GACitGLTGCitPGDAGPPGPP) from type II collagen and fillaggrin306–24 [SHQEST(Cit)G(Cit)SRGRSGRSG] derived from filaggrin. A non-citrullinated peptide, ctrlPAD621–40 (EPLGLQCTFINDFFTYHIRH) derived from PAD was used as control. The peptide sequences are listed in Table 1. All synthetic peptides were synthesized as multiple antigenic peptides (MAP) and purchased from Biosynthesis (Lewisville, TX, USA). The peptides were purified by high performance liquid chromatography (HPLC) and subjected to amino acid mass analysis and mass spectroscopy (MS) to confirm their purity and identity.
Table 1.
The peptide sequences used in the direct binding and inhibition experiments
| Citrullinated peptide | Parent protein | Sequence |
|---|---|---|
| CtrlPAD621–40 (non-citrullinated control) | PAD4 | EPLGLQFINDFFTYHIRH |
| PAD211–30 | PAD4 | VCitVFQATCitGKLSSKCSVVLG |
| Vim60–75 | Vimentin | VYATCitSSAVCitLCitSSVP |
| Enol5–21 | Alpha-enolase | KIHACitEIFDSCitGNPTVE |
| Fibrin617–31 | Fibrin | HSTKCitGHAKSCitPVCitG |
| Col-II358–75 | Collagen | GACitGLTGCitPGDAGPPGPP |
| Filaggrin306–24 | Filaggrin | SHQESTCitGCitSRGRSGRSG |
Cit: citrulline; PAD: peptidyl arginine deiminase.
Enzyme-linked immunosorbent assay (ELISA) protocol
Sensitivity and specificity were determined by evaluating antibody reactivity against the synthetic peptides in serum of patients with RA (n = 141), SLE (n = 60), SS (n = 54) and healthy donors (n = 100) using ELISA assays. Briefly, 96-well high-binding polystyrene microplates (Costar™ Corning, NY, USA) were coated with each peptide diluted in 0·05 M carbonate–bicarbonate buffer pH = 9·6, at a concentration of 1 or 5 μg/ml; the optimum concentration was determined after preliminary experiments for each peptide individually. After a 2-h incubation at room temperature (RT), wells were washed with phosphate-buffered saline–0·1% Tween 20 (PBST) and blocked with blocking buffer (BB) (BB: 5% bovine serum albumin, 0·1% Tween 20 in PBS). Sera diluted in BB were incubated in duplicate on the plates for 2 h at RT; optimal dilutions were selected by preliminary titration for each peptide, ranging from 1:50 to 1:800 in BB. Afterwards, antigen-specific antibodies were detected by alkaline phosphatase-conjugated, affinity purified, anti-human IgG (Jackson Immunoresearch, West Grove, PA, USA) diluted 1:1100 in BB and developed in p-nitrophenyl phosphatase disodium substrate solution (pNPP; Sigma, St Louis, MO, USA). The absorbance at 405 nm was recorded by an ELISA reader (Molecular Devices, Sunnyvale, CA, USA). The cut-off values for each peptide assay were determined using the mean optical density (OD) plus 2 standard deviations of the sera from the 100 healthy controls. RF activity has not been found to interfere with the ELISA assay after using purified IgMk RFs from SS patients.
To increase the sensitivity of anti-citrullinated antibodies, an equamolar mixture, containing all six citrullinated peptides, was used for coating Costar plates. After 2 h incubation at RT, microplates were washed three times with PBST and blocked with BB for 1 h at RT. Human sera were diluted at 1:100 in BB. ELISA was performed with the same protocol as described above. The in-house method was compared with the QUANTA Lite CCP3 IgG ELISA kit (Inova Diagnostics, San Diego, CA, USA) as a gold standard method. The assay was performed according to the manufacturer's instructions.
Homologous and cross-inhibition experiments
To investigate specificity and cross-reactivity of ACPA, homologous and cross-inhibition experiments were performed using two highly reactive sera against the QUANTA Lite CCP3 IgG ELISA kit from RA patients (prototype sera). The specificity of purified anti-citrullinated peptide antibodies was evaluated by homologous inhibition, using various concentrations (0–200 μg/ml) of peptides or control peptide as inhibitor. CtrlPAD621–40 was chosen as a control, as it exhibited low reactivity during the first series of experiments. Each of the two prototype sera was pretreated in optimum dilution – determined in preliminary experiments – with increasing concentrations (from 0 to 250 μg/ml) of peptides and control peptide. The mixture was then incubated for 1 h at 37°C and refrigerated overnight at 4°C. Subsequently, all dilutions were tested by ELISA for reactivity against the peptides. Inhibition percentages were calculated as [(OD 0 mg/ml)–(OD 200 mg/ml)]/(OD 0 mg/ml) × 100% for each serum. ELISA assays were performed as described above.
In order to investigate whether the six peptides could cross-inhibit one another, a second series of inhibition assays was performed. The two prototype sera were pretreated with each one of the six peptides – in optimum dilution for each peptide – overnight at 4°C with increasing concentrations of each peptide separately, as well as with control peptide (0–200 μg/ml). Subsequently, the ELISA protocol was applied.
Purification of anti-peptide antibodies of the prototype sera
IgG purification from total human serum
Purification of anti-peptide antibodies from the two prototype sera was also performed to confirm ACPA specificity. Total IgG of each prototype serum was purified using a Protein A-Sepharose affinity chromatography column (Sigma-Aldrich, St Louis, MO, USA). Sepharose beads were swelled according to the manufacturer's instructions. Serum samples were diluted in PBS pH 7·4 and applied to the column. The latter was washed with PBS and bound immunoglobulins were eluted with 0·1 M HCl-glycine pH 2·7. The pH was fixed immediately to 7·4 using 0·5 M NaOH in PBS. Immunoglobulins were dialyzed versus PBS O/N at 4°C. The amount of total IgG in each sample was measured by ultraviolet (UV) absorption at 280 nm on a UV-visible photometer (Helios Gamma, Spectronic Unicam, Cambridge, UK) and evaluated by ELISA. To investigate further the presence and specificity of anti-peptide antibodies of the two prototype sera used for the homologous and cross-inhibition experiments, purification techniques were performed.
Isolation of anti-citrullinated peptide antibodies
To isolate anti-citrullinated peptide antibodies from the total IgG antibodies of the two prototype sera, each peptide was coupled covalently to a 96-well plate (Nunc Covalink F8; Nunc, Roskilde, Denmark) at a concentration of 1 or 5 μg/ml for 1 h at RT. After washing three times with PBST the wells were blocked with 200 μl of BB and incubated for 1 h at RT. Sera diluted in BB were incubated for 2 h at RT; optimal dilutions were selected by preliminary titration for each peptide, ranging from 1:50 to 1:800. Plates were washed three times with PBST. Finally, antibodies bound to their specific antigen were eluted using 100 μl/well 5 M NaSCN (sodium isothiocyanate) for 15 min at RT and dialyzed with 10 KMWCO dialysis cassettes (Slide-A-Lyzer, Thermo Fisher Scientific, Waltham, MA, USA) against PBS at 4°C overnight under gentle shaking. Concentration of purified antibodies in the eluates was determined by UV absorption at 280 on a UV-visible photometer (Helios Gamma) and then concentrated to the desired concentration using a Visking dialysis tube 25/16 mm (regenerated cellulose, 12–14 kDa MWCO; Serva Electrophoresis GmbH, Heidelberg, Germany); antibody reactivity was tested by ELISA.
Testing of anti-citrullinated peptide binding
The presence of anti-citrullinated peptide antibodies detected in the eluates was confirmed by ELISA, using synthetic peptides as coating antigens. The concentration of anti-peptide antibodies used for the confirmation ELISA protocol was determined after a series of preliminary experiments. The specificity of purified anti-citrullinated peptide antibodies was then evaluated by homologous inhibitions using various concentrations (0–200 μg/ml) of each synthetic peptide or control peptide (ctrlPAD621–40) as inhibitor.
Results
Prevalence of anti-peptide antibodies in RA and other control diseases
The reactivity against each linear peptide was tested by ELISA in sera of 141 RA patients, 100 healthy subjects and 114 disease controls. Sera from patients with RA reacted diversely with the six citrullinated peptides. More specifically, we showed that 29·08% of sera from patients with RA reacted with PAD211–30, 29·08% recognized vim60–75, 37·59% enol5–21, 31·21% fibrin617–31, 29·97% col-II358–75 and 28·37% filaggrin306–24. The control peptide, ctrlPAD621–40, showed no reactivity in any of the tested sera. Additionally, all citrullinated peptides (PAD211–30, vim60–75, enol5–21, fibrin617–31, col-II358–75 and filaggrin306–24) have been found to be highly specific for RA (specificity: 91·59, 93·93, 95·79, 91·59, 98·13 and 97·66%, respectively). The percentage of RA patients with reactivity against 1, 2, 3, 4, 5 or 6 citrullinated peptides was 61, 45, 31, 24, 17 and 5%, respectively (Fig. 1a). Patients with RA and various reactivities against one or more citrullinated peptides did not present any difference in their clinical picture or in disease severity. The percentage of SLE and SS patients with reactivity against one to six citrullinated peptides ranged ranged from 23 to 0% and 22 to 0%, respectively (Fig. 1b and c). No clinical differences were recorded among SLE and SS patients with and without reactivity against the citrullinated peptides.
Fig. 1.
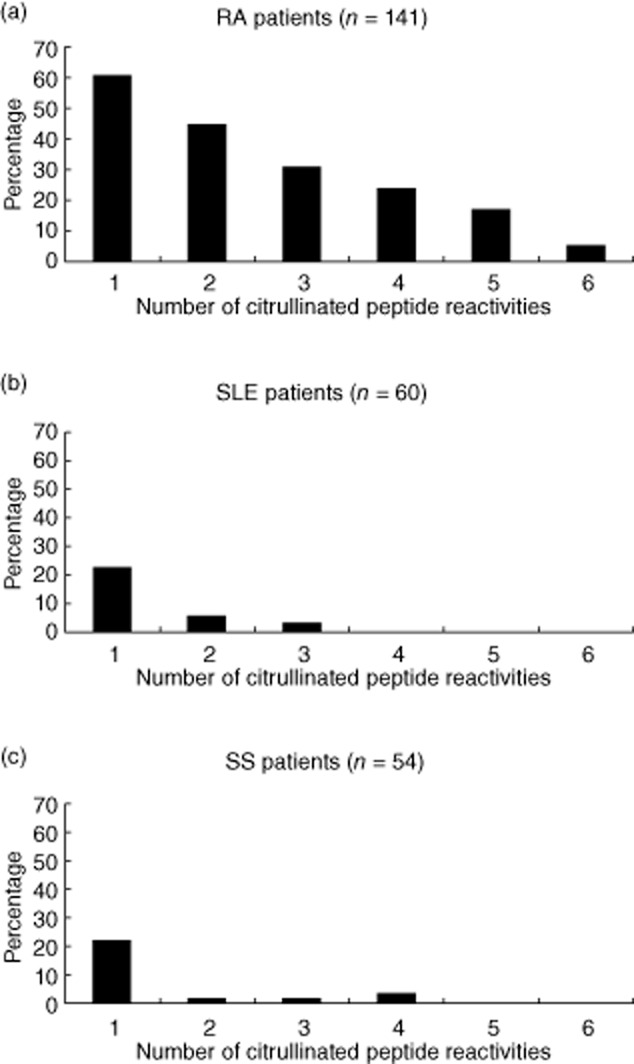
Histograms showing the percentage of (a) rheumatoid arthritis (RA), (b) systemic lupus erythematosus (SLE) and (c) Sjögren's syndrome (SS) patients with the number of peptide reactivities against the six citrullinated peptides.
SLE patients were found positive for antibodies against PAD211–30, vim60–75, enol5–21, fibrin617–31, col-II358–75 and filaggrin306–24 in 13·33, 6·67, 1·67, 21·67 and 1·67%, respectively and SS patients in 14·81, 12·96, 7·41, 3·70, 5·56 and 1·85%, respectively. Specificity of antibodies against PAD211–30, vim60–75, enol5–21, fibrin617–31, col-II358–75 and filaggrin306–24 among SLE patients ranged from 14·58 to 20·68%, respectively, and for SS patients from 6·67 to 22·75%, respectively.
To detect antibodies against an equamolar peptide mixture (containing all six citrullinated peptides) or anti-CCP3 antibodies, we tested sera from RA, SLE and SS patients as well as from healthy subjects. More specifically, sera from 141 RA, 60 SLE, 54 SS patients and 100 healthy donors were tested for antibodies against the equamolar peptide mixture. For the anti-CCP3 commercial kit reactivity, we used sera from 102 RA, 30 SLE, 30 SS patients and 42 healthy donors. The estimated sensitivity for anti-CCP3 and the equamolar mixture was 60·78 and 46·08%, respectively, while specificity was found to be 94·12 and 82·22%, respectively.
Fine specificity of ACPA antibodies
The percentage of homologous inhibition after incubation with all the synthetic peptides ranged between 85·67 and 95·29% for prototype serum 1, with the exception of vim60–75, whereas homologous inhibition dropped to 63·53%. For prototype serum 2, homologous inhibition ranged between 86·41 and 95·15%. Cross-inhibition for anti-PAD211–30 antibodies ranged from 11·96 to 79·91% for serum 1 and 11·05 to 85·29% for serum 2. For anti-vim60–75 antibodies, cross-inhibition ranged from 28·47 to 81·94% for serum 1 and 9·45 to 74·30% for serum 2. For anti-enol5–21 antibodies, cross-inhibition ranged from 4·36 to 65·01% for serum 1 and 3·03 to 65·19% for serum 2. For anti-fibrin617–31 antibodies, cross-inhibition ranged from 7·13 to 82·15% for serum 1 and 10·43 to 78·99% for serum 2. For anti-col-II358–75 antibodies, cross-inhibition ranged from 29·11 to 89·73% for serum 1 and 20·01 to 75·94% for serum 2. For anti-filaggrin306–24 antibodies, cross-inhibition ranged from 4·97 to 49·03% serum 1 and 3·47 to 28·04% for serum 2. The percentage of inhibition and cross-inhibition for prototype serum 1 and 2 are illustrated in Figs 2 and 3, respectively. A significant cross-reaction (>70%) was recorded between filaggrin and antibodies against peptides derived from PAD, vimentin, fibrin and type II collagen, between fibrin and antibodies against peptides from vimentin and anti-type II collagen and between PAD and antibodies against peptide from type II collagen for prototype serum 1. For prototype serum 2, notable cross-inhibition (>70%) was found between filaggrin and antibodies for peptides from PAD, vimentin, fibrin and between PAD and antibodies for type II collagen.
Fig. 2.
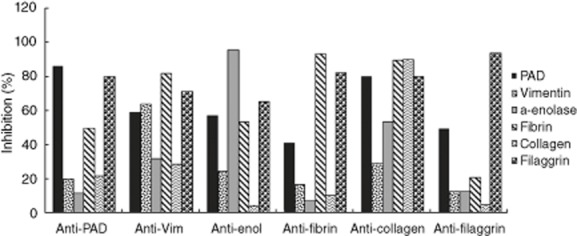
Percentages of inhibition and cross-inhibition for prototype serum 1.
Fig. 3.
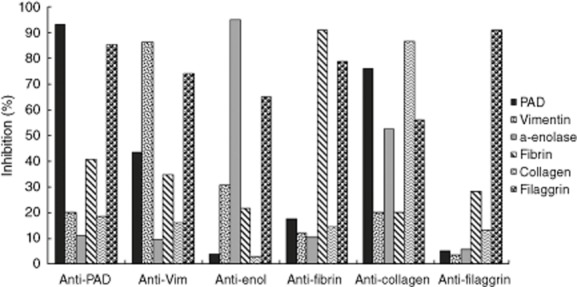
Percentages of inhibition and cross-inhibition for prototype serum 2.
Inhibition assays with purified anti-peptide antibodies
The percentages of inhibition of purified anti-peptide antibodies of the two prototype sera after incubation with the homologous peptides were 83·09% for PAD211–30, 62·60% for vim60–75, 87·15% for enol5–21, 87·96% for fibrin617–31, 84·53% for col-II358–75, 92·64% for filaggrin306–24 for serum 1 and 84·09% for PAD211–30, 78·67% for vim60–75, 55·02% for enol5–21, 92·80% for fibrin617–31, 83·65% for col-II358–75 and 90·12% for fillaggrin306–24, respectively, for serum 2.
Discussion
Given that no predominant B cell epitope has been identified as yet, in the present study we aimed to evaluate simultaneously the reactivity of ACPA against different peptides derived from citrullinated proteins in RA and to investigate whether or not these antibodies constitute a homogeneous population [26,27]. Anti-citrullinated protein-antibodies and anti-CCPs are considered a hallmark of RA, and they are both detected in the serum of RA patients with high specificity [20,22]. Despite this association, it is not expected that the antibodies recognizing citrullinated peptides would be the same with those recognizing CCP peptides. However, previous studies have shown that anti-CCPs are a collection of ACPA also directed against citrullinated peptides, with overlapping and non-overlapping reactivities [20,22]. Thus, anti-CCP positivity reflects ACPA positivity, although the CCP sequence remains unknown, underlying the use of anti-CCPs as a biomarker of ACPA reactivity [28]. To extend the spectrum of ACPA reactivity, we used six specific synthetic peptides representing citrullinated epitopes derived from parent proteins that have been used in other studies [12–16]. Among the most frequently recognized epitopes are those derived from vimentin and fibrinogen [29,30], while all these parent proteins have been recognized as citrullinated antigens in serum and/or joint fluid of patients with RA. Recently, Van Beers et al. have shown limited serum reactivity against specific citrullinated epitopes compared to the extended repertoire of citrullinated polypeptides contained in synovial fluid of RA patients, including vimentin and fibrinogen, implying that the formation of in-situ immune complexes might contribute to disease onset [31]. Interestingly, we also included citrullinated PAD-4 peptide, as anti-PAD4 antibodies have been detected in the serum of RA patients before disease onset and have been implicated in disease pathogenesis [16]. An approach based on citrullinated epitopes rather than whole proteins has been selected to achieve optimal reactivity.
Specificity for all citrullinated peptide was more than 90% while sensitivity was approximately 30% in the RA population, with the exception of alpha-enolase, which reached almost 38%. These sensitivity values, although not very different, are lower compared to other studies. These differences could be attributed to genetic and environmental factors characterizing the different RA populations [32] as well as to various experimental and methodological parameters [8,16–18,33]. In a recent study, almost the same citrullinated epitopes have been used to develop a multiplex immunofluorescence-based assay that proved to be comparable with the ELISA assays, and the recorded serum reactivities were found similar to those observed in our study. However, the citrullinated collagen epitope was slightly different and reactivity reached only 19% [34]. Conversely, both specificity and sensitivity for all six citrullinated peptides were low among SLE and SS patients and among healthy controls. The above data indicate that reactivity against these citrullinated peptides is highly specific for RA patients, suggesting that citrullination of various autoantigens might be an important – if not unique – and unifying mechanism of peripheral breakdown of immune tolerance in RA.
The fact that patients with established RA exhibit an expansion of the isotype repertoire, including IgM and IgA anti-CCPs, should be taken into consideration [21,35]. However, IgM and IgA isotypes of anti-CCPs can be detected in RA and patients with undifferentiated arthritis but in lower frequencies compared to IgG [35]. In addition, it has been shown that IgG ACPA in RA patients might also recognize citrullinated peptides [21]. After performing the assay by using the equamolar mixture, we found that both sensitivity and specificity were lower compared to anti-CCP3 reactivity for this particular population of RA patients. This finding supports the hypothesis that CCP3 peptides contain more specific epitopes with broader diversity not necessarily derived from different parent proteins, compared to the aforementioned equamolar mixture.
By choosing two prototype sera highly positive for anti-CCP3 antibodies from RA patients, we investigated the specificity of ACPA by performing homologous and cross-inhibition experiments. For both sera, preincubation with increasing concentrations of the homologous citrullinated peptide resulted in inhibition of more than 85% for almost all the peptides. In addition, cross-reactivity was found to be minimal in both sera, while a significant (>70%) cross-reaction was observed mainly between filaggrin and the majority of anti-citrullinated peptide antibodies. Conversely, anti-filaggrin and anti-alpha-enolase antibodies showed the least cross-reactivity, as they have been found to react only with the citrullinated peptide derived from filaggrin and alpha-enolase, respectively (homologous derivative peptides), and therefore they might be considered the most specific for these two prototype sera. Consistent with the above data, other groups have also supported that serum from anti-CCP-positive RA patients might react against one or more citrullinated epitopes with limited or even no cross-reactivity [20,22]. However, it is noteworthy that the repertoire of citrullinated peptides recognized by the ACPA seems to have been already developed before disease onset, while recognition of citrullinated peptides does not change as disease evolves [36]. Furthermore, this expansion of ACPA response has been correlated closely with an elevation of specific inflammatory cytokines such as tumour necrosis factor (TNF)-α and interferon (IFN)-γ that precedes the appearance of arthritis [37]. Thus, the breakdown of peripheral immune tolerance is centred probably in the preclinical phase of the disease, when citrullination is taking place and citrullinated autoantigens are being presented and recognized by the immune system, eliciting an autoimmune response [38]. Various contributors could interfere with these processes and therefore affect the diversity of ACPA specificity, including genetic features [39,40] and environmental factors [41]. Epitope spreading is another mechanism that could expand the diversity of ACPA responses against different citrullinated epitopes of the same parent protein among individuals or RA populations with different ethnicities and genetic background. The importance of intra/interparticle epitope spreading has been well defined in other systemic autoimmune diseases such as SLE and SS, affecting the diversification of autoimmune responses against Ro/SSA, La/SSB and U1-RNP autoantigens [42,43]. As a result, distinct ACPA-antibody-producing B cells could arise in the blood of RA, contributing to disease pathogenesis. Finally, immunological parameters such as T cell immunity [44] and affinity maturation affecting the detection of ACPA antibodies by ELISA, should be taken into account when interpreting data concerning ACPA specificity. From a technical viewpoint, the idiotype/anti-idiotype network is another issue in autoimmunity [45]. Healthy children of anti-Ro-/anti-La-positive women might exhibit no apparent reactivity because of the blockade of anti-idiotype antibodies which, at the same time, offer protection to the fetus [46]. Similarly, it is possible that ACPA reactivity might be masked because of the presence of anti-idiotype antibodies.
In summary, ACPA is a population of antibodies that exhibit diversity and heterogeneity to a variety of citrullinated epitopes and peptides. Extended studies from different cohorts are required to investigate the relevant ACPA spectrum of B cell immunity in RA and identify, if possible, the most frequent or dominant epitopes. To this end, it is necessary to examine new citrullinated epitope mixtures derived from the same and/or other parent proteins. T cell immunity against citrullinated peptides should also be evaluated as the cellular component of the immune system, in combination with the underlying genetic background, might affect ACPA responses.
Disclosure
The authors have no financial or personal conflicts of interest to declare.
References
- 1.Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35:10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac'h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–228. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Worthington J. Investigating the genetic basis of susceptibility to rheumatoid arthritis. J Autoimmun. 2005;25:16–20. doi: 10.1016/j.jaut.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011;36:65–75. doi: 10.1016/j.jaut.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh U, Vishwanath A, Verma PK, et al. Is rheumatoid factor still a superior test for the diagnosis of rheumatoid arthritis? Rheumatol Int. 2010;30:1115–1119. doi: 10.1007/s00296-009-1338-0. [DOI] [PubMed] [Google Scholar]
- 6.Willemze A, Toes RE, Huizinga TW, Trouw LA. New biomarkers in rheumatoid arthritis. Neth J Med. 2012;70:392–399. [PubMed] [Google Scholar]
- 7.Somers K, Geusens P, Elewaut D, et al. Novel autoantibody markers for early and seronegative rheumatoid arthritis. J Autoimmun. 2011;36:33–46. doi: 10.1016/j.jaut.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 10.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 11.Lal P, Su Z, Holweg CT, et al. Inflammation and autoantibody markers identify rheumatoid arthritis patients with enhanced clinical benefit following rituximab treatment. Arthritis Rheum. 2011;63:3681–3691. doi: 10.1002/art.30596. [DOI] [PubMed] [Google Scholar]
- 12.Kinloch A, Tatzer V, Wait R, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebbag M, Moinard N, Auger I, et al. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur J Immunol. 2006;36:2250–2263. doi: 10.1002/eji.200535790. [DOI] [PubMed] [Google Scholar]
- 14.Vossenaar ER, Despres N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanmarti R, Graell E, Perez ML, et al. Diagnostic and prognostic value of antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R135. doi: 10.1186/ar2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auger I, Martin M, Balandraud N, Roudier J. Rheumatoid arthritis-specific autoantibodies to peptidyl arginine deiminase type 4 inhibit citrullination of fibrinogen. Arthritis Rheum. 2010;62:126–131. doi: 10.1002/art.27230. [DOI] [PubMed] [Google Scholar]
- 17.Snir O, Widhe M, Hermansson M, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62:44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg K, Kinloch A, Fisher BA, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–3019. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 19.Raats JM, Wijnen EM, Pruijn GJ, van den Hoogen FH, van Venrooij WJ. Recombinant human monoclonal autoantibodies specific for citrulline-containing peptides from phage display libraries derived from patients with rheumatoid arthritis. J Rheumatol. 2003;30:1696–1711. [PubMed] [Google Scholar]
- 20.Ioan-Facsinay A, el-Bannoudi H, Scherer HU, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–193. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 21.Ioan-Facsinay A, Willemze A, Robinson DB, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008;58:3000–3008. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- 22.Snir O, Widhe M, von Spee C, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009;68:736–743. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 25.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routsias JG, Tzioufas AG, Moutsopoulos HM. The clinical value of intracellular autoantigens B-cell epitopes in systemic rheumatic diseases. Clin Chim Acta. 2004;340:1–25. doi: 10.1016/j.cccn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Yiannaki EE, Tzioufas AG, Bachmann M, et al. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin Exp Immunol. 1998;112:152–158. doi: 10.1046/j.1365-2249.1998.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Stadt LA, van der Horst AR, de Koning MH, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis. 2010;70:128–133. doi: 10.1136/ard.2010.132662. [DOI] [PubMed] [Google Scholar]
- 29.Hida S, Miura NN, Adachi Y, Ohno N. Influence of arginine deimination on antigenicity of fibrinogen. J Autoimmun. 2004;23:141–150. doi: 10.1016/j.jaut.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Iobagiu C, Magyar A, Nogueira L, et al. The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J Autoimmun. 2011;37:263–272. doi: 10.1016/j.jaut.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 31.van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, Pruijn GJ. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 32.Drosos AA, Lanchbury JS, Panayi GS, Moutsopoulos HM. Rheumatoid arthritis in Greek and British patients. A comparative clinical, radiologic, and serologic study. Arthritis Rheum. 1992;35:745–748. doi: 10.1002/art.1780350705. [DOI] [PubMed] [Google Scholar]
- 33.Perez ML, Gomara MJ, Kasi D, et al. Synthesis of overlapping fibrin citrullinated peptides and their use for diagnosing rheumatoid arthritis. Chem Biol Drug Des. 2006;68:194–200. doi: 10.1111/j.1747-0285.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 34.Hansson M, Mathsson L, Schlederer T, et al. Validation of a multiplex chip-based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res Ther. 2012;14:R201. doi: 10.1186/ar4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006;54:3799–3808. doi: 10.1002/art.22279. [DOI] [PubMed] [Google Scholar]
- 36.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–1561. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 37.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 39.Behrens M, Trejo T, Luthra H, Griffiths M, David CS, Taneja V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. J Autoimmun. 2010;35:1–9. doi: 10.1016/j.jaut.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas-Villarraga A, Diaz FJ, Calvo-Paramo E, et al. Familial disease, the HLA-DRB1 shared epitope and anti-CCP antibodies influence time at appearance of substantial joint damage in rheumatoid arthritis. J Autoimmun. 2009;32:64–69. doi: 10.1016/j.jaut.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Svendsen AJ, Hjelmborg JV, Kyvik KO, et al. The impact of genes on the occurrence of autoantibodies in rheumatoid arthritis. A study on disease discordant twin pairs. J Autoimmun. 2013;41:120–125. doi: 10.1016/j.jaut.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Routsias JG, Kyriakidis N, Latreille M, Tzioufas AG. RNA recognition motif (RRM) of La/SSB: the bridge for interparticle spreading of autoimmune response to U1-RNP. Mol Med. 2010;16:19–26. doi: 10.2119/molmed.2009.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Routsias JG, Tzioufas AG. B-cell epitopes of the intracellular autoantigens Ro/SSA and La/SSB: tools to study the regulation of the autoimmune response. J Autoimmun. 2010;35:256–264. doi: 10.1016/j.jaut.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Penzotti JE, Doherty D, Lybrand TP, Nepom GT. A structural model for TCR recognition of the HLA class II shared epitope sequence implicated in susceptibility to rheumatoid arthritis. J Autoimmun. 1996;9:287–293. doi: 10.1006/jaut.1996.0037. [DOI] [PubMed] [Google Scholar]
- 45.Tzioufas AG, Routsias JG. Idiotype, anti-idiotype network of autoantibodies: pathogenetic considerations and clinical application. Autoimmun Rev. 2010;9:631–633. doi: 10.1016/j.autrev.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Stea EA, Routsias JG, Clancy RM, Buyon JP, Moutsopoulos HM, Tzioufas AG. Anti-La/SSB antiidiotypic antibodies in maternal serum: a marker of low risk for neonatal lupus in an offspring. Arthritis Rheum. 2006;54:2228–2234. doi: 10.1002/art.21954. [DOI] [PubMed] [Google Scholar]


