Abstract
In the present study, we established a novel murine model of vitiligo by sequential prime/boost immunizations into the hind footpad and tail dermis with tyrosinase-related protein 2 (TRP2)-180 (SVYDFFVWL) peptide, lipopolysaccharides and cytosine–phosphate–guanosine (CpG) oligodeoxynucleotides. Immunized mice developed epidermal depigmentation in the tail skin without hair depigmentation, thereby differentiating this approach from established models of vitiligo. Following intradermal tail immunization, activated CD8+ interferon (IFN)-γ+ T cells were recruited locally to the tail skin. In-vivo cytotoxicity assays demonstrated specific lysis of TRP2-180-presenting cells in immunized mice. Furthermore, the extent of skin depigmentation correlated with the frequency of TRP2-180-specific splenic CD8+ T cells, as determined by IFN-γ and tumour necrosis factor (TNF)-α production, and cytotoxic degranulation evidenced by CD107a staining. These findings suggest a correlation between the presence of TRP2-180-specific CD8+ effector T cells and the development of depigmented skin lesions in our vitiligo model. This new model of vitiligo, characterized by skin depigmentation without hair depigmentation, is more similar to human disease than previous murine models. Therefore, this model is well suited to future studies on the pathogenesis of vitiligo and the development of novel therapeutics for vitiligo.
Keywords: autoreactive CD8+ T cell, epidermal depigmentation, mouse model, vitiligo
Introduction
Vitiligo is a common skin disorder characterized by progressive depigmentation of the skin due to selective loss of melanocytes. It affects 0·1–2% of the worldwide population [1]. In humans, white depigmented patches on the skin are observed commonly in the central face, genitals, hands and feet [2].
Cytotoxic CD8+ T cells play a major role in the pathogenesis of vitiligo. In vitiligo patients, melanocyte-specific cytotoxic CD8+ T cells are detected in the peripheral blood and skin lesions [3,4], and the frequency of melanocyte-specific cytotoxic CD8+ T cells correlates with the severity of disease [5–7]. Moreover, isolated perilesional CD8+ T cells demonstrate cytolytic ability against melanocytes ex vivo [8]. These studies have demonstrated the pathological role of melanocyte-specific cytotoxic CD8+ T cells in mediating vitiligo progression.
To study the pathogenesis of vitiligo in detail, several vitiligo models have been established in mice. In these models, adoptive transfer of melanocyte gp100-specific T cell receptor transgenic CD8+ T cells or genetic immunization of tyrosinase related protein 2 (TRP2) using a gene gun was performed to develop melanocyte-specific cytotoxic CD8+ T cell responses. These methods induced epidermal depigmentation successfully by T cell-mediated destruction of epidermal melanocytes. In addition, hair follicle melanocytes were also destroyed and hairs became depigmented due to vigorous T cell responses against melanocyte antigens.
In vitiligo patients the development of depigmented hair within lesional skin is a rare occurrence, because hair follicles are characterized by immune privilege [9], which protects hair follicle melanocytes from autoimmune responses. Clinically, the presence of depigmented hair in vitiligo lesions represents a more severe form of vitiligo and a poor response to treatment [10].
As previous mouse models of vitiligo induce not only epidermal depigmentation but also hair depigmentation, which is a feature of a rare form of severe vitiligo in humans, a mouse model of vitiligo is needed that resembles more closely the typical human disease course. In the present study, we established a novel mouse model of vitiligo that results in skin depigmentation without hair depigmentation. To accomplish this, we immunized mice with H2-Kb-binding TRP2-180 (SVYDFFVWL) peptide [11] in combination with lipopolysaccharides (LPS) and cytosine–phosphate–guanosine oligodeoxynucleotides (CpG ODN) to destroy self-tolerance to melanocytes. As a result, we primed TRP2-180-specific CD8+ T cells successfully in wild-type C57BL/6 mice, and observed that activated CD8+ T cells were recruited to the tail skin following intradermal boost immunizations at that site. Epidermal depigmentation developed in the tail, but hair follicle melanocytes in the lesional skin remained intact and hair colour was preserved. Furthermore, the extent of depigmented skin correlated with the frequency of TRP2-180-specific CD8+ T cells in the spleen. This new model of vitiligo is more similar to human disease than the previous models of vitiligo. Therefore, we have developed a clinically relevant murine vitiligo model that is well suited to the study of vitiligo pathogenesis and the development of novel therapeutics targeting melanocyte-specific, autoreactive CD8+ T cells.
Materials and methods
Peptides
Anti-mouse OVA257–264 (SIINFEKL) and TRP2-180 (SVYDFFVWL) peptides were purchased from Peptron (Daejeon, Korea). Peptides were dissolved in 5% dimethylsulphoxide (DMSO) phosphate-buffered saline (PBS).
Induction of vitiligo by TRP2-180 immunization
Male C57BL/6 mice were kept in accordance with our institutional guidelines, and used at the age of 4–5 weeks for peptide immunization. Mice were immunized twice subcutaneously at a 1-week interval in the hind footpad with TRP2-180 (50 μg), LPS (5 μg; Invivogen, San Diego, CA, USA) and CpG ODN 1826 (5 μg; Genotech, Daejeon, Korea). One week after the second footpad immunization, TRP2-180 (50 μg), LPS (5 μg) and CpG ODN 1826 (5 μg) were injected twice intradermally at a 1-week interval in the tail dermis. Control mice were either not immunized or were immunized with SIINFEKL (50 μg) instead of TRP2-180.
Histological studies
Haematoxylin and eosin (H&E) staining was performed to observe inflammation in the tail. Tails were excised and fixed in 10% neutral-buffered formalin. Tail tissue was embedded in paraffin, sectioned at a thickness of 5 μm, and stained with H&E according to standard procedures.
TRP2 immunohistochemistry was performed to identify the destruction of melanocytes. Paraffin-embedded tail sections were deparaffinized, rehydrated and treated with Tris-ethylenediamine tetraacetic acid (EDTA) buffer for antigen retrieval. Sections were fixed and permeabilized by acetone at −20°C for 5 min, blocked in 10% normal serum, then stained with anti-TRP2 antibodies (rabbit polyclonal; Abcam, Cambridge, UK) at room temperature for 1 h. Sections were then incubated with 0·3% H2O2 in PBS for 15 min, stained with horseradish peroxidase (HRP)-conjugated anti-rabbit antibodies [ImmPRESS anti-rabbit immunoglobulin (Ig) kit; Vector, Burlingame, CA, USA], and developed by 3′3′-diaminobenzidine (DAB) (ImmPACT DAB; Vector). For evaluation of melanin distribution, excised tails were dehydrated with 30% sucrose in PBS, cryosectioned at a thickness of 30 μm, and observed without further staining. All images were captured by an Olympus BX51 microscope (Olympus, Tokyo, Japan).
Flow cytometric analyses of immune cells in the tail skin during vitiligo induction
One week after the last immunization, tail skin pieces flayed from two mice were pooled, minced into small pieces (1 × 1 mm) and incubated in PBS containing 1 mg/ml of collagenase D (Roche, Indianapolis, IN, USA) plus 40 μg/ml of DNase (Roche) for 1 h at 37°C. Supernatants were filtered using a 70 μm cell strainer, and cells were isolated by centrifugation. The cell pellet was resuspended in 0·65 ml of RPMI-1640 containing 1% fetal bovine serum (FBS). Approximately 8% of the isolated cells were used to identify the subpopulation of immune cells, via staining with anti-CD45-V450, anti-CD3-V500, anti-CD4-allophycocyanin (APC)-cyanin 7 (Cy7) and anti-CD8-phycoerythrin (PE)-Cy7 (all from BD Biosciences, San Jose, CA, USA). After washing, stained cells were analysed on an LSR flow cytometer (BD Biosciences). The absolute cell numbers of each subpopulation in a tail skin were calculated. The remaining cells were used to detect interferon (IFN)-γ-producing CD8+ T cells. Single-cell suspensions (200 μl per well) were stimulated with SIINFEKL (5 μg/ml) or TRP2-180 (5 μg/ml), and brefeldin A (GolgiPlug; BD Biosciences) was added 1 h later. After another 5 h of incubation at 37°C, cells were stained with ethidium monoazide (Sigma-Aldrich, St Louis, MO, USA). After washing, cells were stained with anti-CD3-Pacific blue, anti-CD4-APC-Cy7, anti-CD8-PE-Cy7 and anti-CD44-PE (all from BD Biosciences), permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and stained with anti-IFN-γ-APC (BD Biosciences). Stained cells were analysed on an LSR flow cytometer.
In-vivo cytotoxicity assay
Splenocytes from CD45·1 naive mice were stained with low-dose (0·1 μM) or high-dose (10 μM) CFSE (Molecular Probes, Eugene, OR, USA). CFSElow (0·1 μM) splenocytes were incubated with TRP2-180 (10 μM), and CFSEhigh (10 μM) splenocytes were incubated with SIINFEKL (10 μM) at 37°C for 2 h. These cells (5 × 106 cells of each population) were mixed at a 1:1 ratio and injected retro-orbitally into non-immunized CD45·2 mice or TRP2-180-immunized CD45·2 mice. At 18 h after injection, splenocytes were harvested and analysed by flow cytometry. Target cells were distinguished from recipient cells by CD45·1 gating (anti-CD45·1-V450; BD Biosciences) and dead cells were excluded using 7-amino-actinomycin D (7-AAD; BD Biosciences). The percentage killing was calculated as follows: [100−(percentage of TRP2-180-loaded/percentage of SIINFEKL-loaded) × 100].
Assessment of disease severity
At 5 weeks after the last immunization, the area of depigmented skin per tail of TRP2-180-immunized mice was calculated by using the ImageJ program (National Institutes of Health, Bethesda, MD, USA) and presented as the percentage of depigmented skin/tail.
Assessment of TRP2-180-specific CD8+ T cell response
At 5 weeks after the last immunization, the frequency of TRP2-180-specific CD8+ T cells in the spleen was analysed by intracellular cytokine staining (ICS) for IFN-γ, tumour necrosis factor (TNF)-α and the cytotoxic degranulation marker, CD107a. Splenocytes were resuspended at 107/ml in RPMI-1640 containing 1% FBS in a total volume of 200 μl per well. TRP2-180 was added at a final concentration of 5 μg/ml, anti-CD107a-PE-Cy7 (BD Biosciences) was added at a concentration of 1 μg/ml, and monensin (GolgiStop, BD Biosciences) was added. Brefeldin A was added 1 h later. After another 5 h of incubation at 37°C, splenocytes were stained with ethidium monoazide. After washing, splenocytes were stained with anti-CD3-V500, anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD8-APC-H7 (all from BD Biosciences), then permeabilized using the Cytofix/Cytoperm kit and stained with anti-IFN-γ-APC and anti-TNF-α-PE (all from BD Biosciences). Stained cells were analysed using flow cytometry.
Statistical analysis
Data were presented as the mean ± standard error of the mean. Statistical analyses were performed using GraphPad Prism version 5·0 software (GraphPad Software, La Jolla, CA, USA). A two-tailed Student's t-test was performed to calculate statistical significance for differences between groups. Correlation between the disease severity and the frequency of TRP2-180-specific CD8+ T cells was determined according to Pearson's value correlation test. In all cases, P ≤ 0·05 was considered statistically significant.
Results
Development of epidermal depigmentation without hair depigmentation in the tail skin
To prime melanocyte-specific autoreactive CD8+ T cells, C57BL/6 mice were immunized subcutaneously twice at a 1-week interval into the hind footpad with TRP2-180, LPS and CpG ODN (Fig. 1a). The priming immunization did not result in depigmented skin lesions in any site of the body. For the boost immunizations, 1 week after the second footpad immunization, TRP2-180, LPS and CpG ODN were injected twice intradermally into the tail, again at a 1-week interval (Fig. 1a). Four to 5 weeks after the last immunization, TRP2-180-immunized mice developed a depigmented skin lesion around the tail injection site, whereas neither non-immunized nor SIINFEKL-immunized control mice developed skin lesions (Fig. 1b,c). More than 90% (22 of 24) of the TRP2-180-immunized mice developed depigmented skin lesions and, once visible, depigmented skin lesions were maintained for more than 1 year (data not shown). Histological examination revealed loss of melanin (Fig. 1d) and melanocytes (Fig. 1e) in the affected skin lesions of TRP2-180-immunized mice, but not in the skin of non-immunized and SIINFEKL-immunized control mice. Interestingly, hair colour was preserved in depigmented skin lesions in all mice with vitiligo development (Fig. 2a). Histological examination also demonstrated that hair follicle melanocytes were conserved, despite the disappearance of epidermal melanocytes in the affected skin lesion (Fig. 2b). These results were obtained in all mice developing skin depigmentation.
Fig. 1.
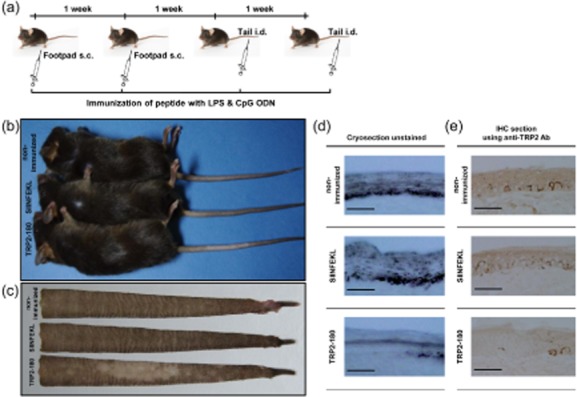
Peptide immunization schedule and development of vitiligo in mice. (a) Peptide immunization schedule. C57BL/6 mice were immunized subcutaneously twice at a 1-week interval in the footpad with tyrosinase-related protein (TRP)2-180 (50 μg), lipopolysacchride (LPS) (5 μg) and cytosine–phosphate–guanosine (CpG) oligodeoxynucleotides (ODN) (5 μg). For boost immunizations, TRP2-180 (50 μg) with the same adjuvants was injected twice intradermally at a 1-week interval into the tail dermis. Control mice were either not immunized or were immunized with SIINFEKL (50 μg) instead of TRP2-180. s.c.: subcutaneous; i.d.: intradermal. (b,c) Gross examination of the tail skin. Five weeks after the last immunization, TRP2-180-immunized mice developed depigmented skin lesions in the tail, but control mice did not (b). Flayed tail skins from these mice are shown (c). (d,e) Histological examination of the tail skin. Depigmented skin lesions of TRP2-180-immunized mice revealed the absence of melanin in unstained cryosections (30 μm) (d) and the absence of melanocytes in immunohistochemistry sections (5 μm) using anti-TRP2 antibodies (e). Scale bars = 200 μm.
Fig. 2.
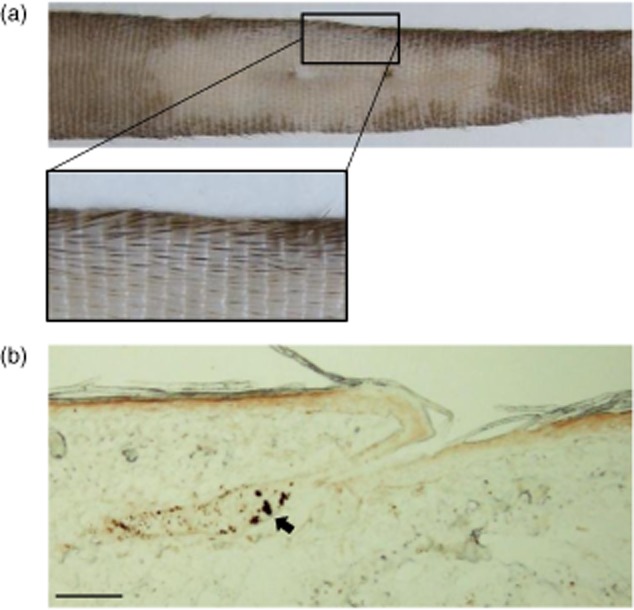
Hair colour and hair follicle melanocytes in depigmented skin lesions. (a) Hair colour in the depigmented skin lesion. In C57BL/6 mice, a black hair stripe is present on the dorsal part of tail. The photograph shows a representative tail with vitiligo 4 months after depigmentation developed. (b) Immunohistochemistry using anti-tyrosinase-related protein (TRP)2 antibodies shows intact hair follicle melanocytes, whereas epidermal melanocytes were not detected. Arrow indicates melanocytes in the hair follicle. Scale bar = 200 μm.
Recruitment of activated CD8+ T cells to tail skin
We examined recruitment of immune cells to the site of immunization in the tail skin 1 week after the last boost immunization, prior to skin depigmentation. Upon histological examination, mononuclear cells were observed at the dermal–epidermal junction in peptide-immunized mice, but not in non-immunized mice (Fig. 3a). We also isolated immune cells from the whole tail skin and performed flow cytometric analysis. The total number of CD45+ leucocytes per tail skin was increased significantly in TRP2-180- or SIINFEKL-immunized mice compared to non-immunized mice (Fig. 3b). The frequency of CD8+ cells within the CD3+ T cell population was also increased in TRP2-180- or SIINFEKL-immunized mice, whereas the frequency of CD4+ cells was not increased (Fig. 3c). In the CD8+ T cell population, antigen-experienced cells were identified by high CD44 expression, and the frequency of CD44high cells was increased significantly in TRP2-180- or SIINFEKL-immunized mice compared to non-immunized mice (Fig. 3d,f). Next, IFN-γ production by CD8+ T cells was evaluated via ICS and flow cytometric analysis using isolated cells from the whole tail skin. IFN-γ-producing CD44high CD8+ T cells were detected even without ex-vivo antigenic peptide restimulation in TRP2-180- or SIINFEKL-immunized mice, and the frequency of IFN-γ-producing T cells increased further with ex-vivo TRP2-180 restimulation when cells were derived from TRP2-180-immunized mice (Fig. 3e,f). Collectively, these results indicate that boost immunizations recruited IFN-γ-producing effector CD8+ T cells to the tail skin. The recruited effector CD8+ T cells could be activated further by cognate antigen which, in the case of TRP2-180, is presented by melanocytes.
Fig. 3.
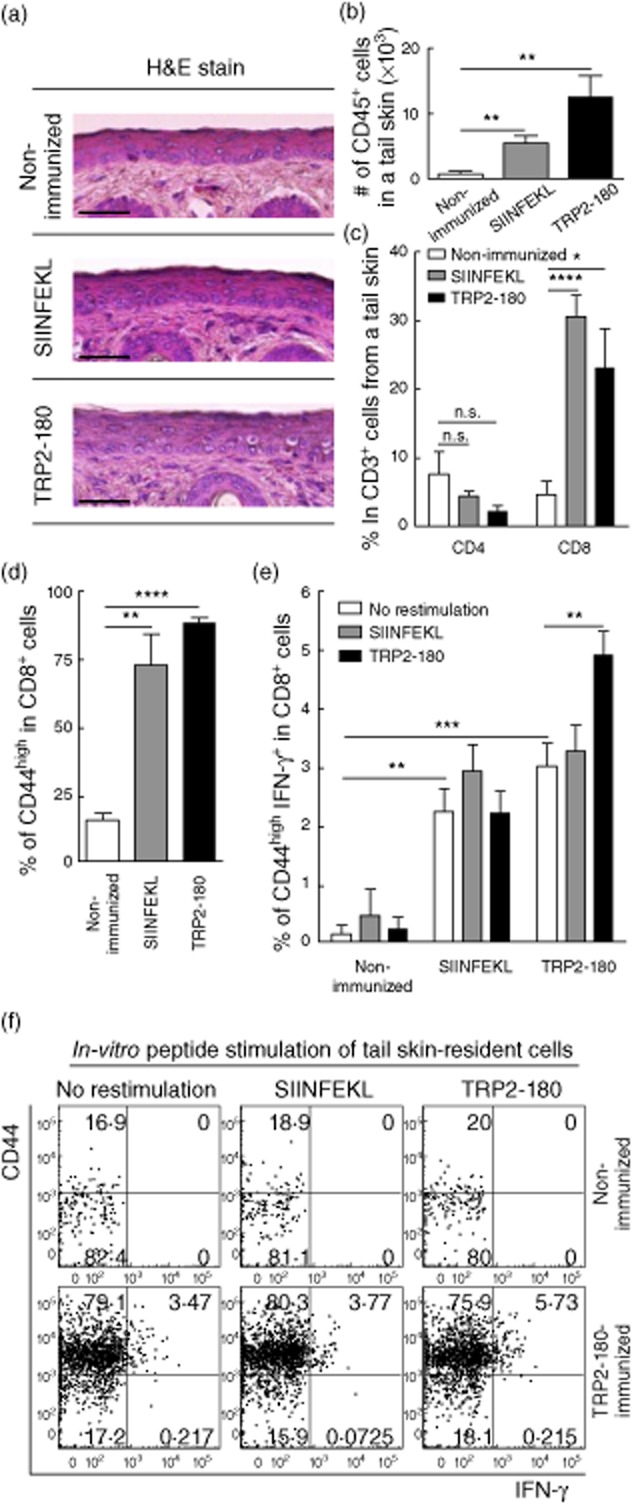
Recruitment and cytokine production of CD8+ T cells in the tail skin. Mice were killed 1 week after the last immunization, prior to skin depigmentation. (a) In haematoxylin and eosin (H&E) staining, mononuclear cells are seen infiltrating to the dermal–epidermal junction in peptide-immunized mice. Scale bars = 200 μm. (b,c) After isolation of immune cells from the tail skin, CD45+ cells were identified by flow cytometry and the calculated total numbers of CD45+ cells are shown (b). The frequency of CD4+ or CD8+ cells within the CD3+ T cell population was analysed by flow cytometry (c). Each bar graph represents the mean ± standard error of the mean (s.e.m.) from five independent experiments with five mice per group (*P < 0·05; **P < 0·01; ****P < 0·0001; n.s.: not significant). (d,e) The frequency of CD44high cells within the CD8+ T cell population was analysed by flow cytometry (d). Interferon (IFN)-γ intracellular cytokine staining (ICS) was performed on cells isolated from the tail skin with or without in-vitro antigenic peptide stimulation, and the frequency of CD44high IFN-γ+ cells within the CD8+ T cell population was analysed by flow cytometry (e). Each bar graph represents the mean + s.e.m. from four independent experiments with four mice per group (**P < 0·01; ***P < 0·001; ****P < 0·0001). (f) Representative plots illustrate CD44high and IFN-γ+ cells within the CD3+ CD8+ T cell gate.
TRP2-180-specific cytotoxicity in the immunized mice
As destruction of melanocytes is the major pathogenic outcome in vitiligo, we determined if cells presenting TRP2-180 peptide were lysed in TRP2-180-immunized mice. We performed in-vivo cytotoxicity assays by pulsing splenocytes from naive mice with either TRP2-180 peptide or the control peptide SIINFEKL, then labelled cells with differential doses of CFSE. Peptide-loaded, CFSE-labelled target cells (CD45·1+) were transferred adoptively into recipient mice (CD45·2+) that were either TRP2-180-immunized or unimmunized controls. Whereas TRP2-180-specific killing was robust in TRP2-180-immunized mice, it was not detectable in non-immunized naive mice (Fig. 4a,b). These data demonstrate that following TRP2-180-immunization, TRP2-180-presenting cells such as melanocytes are lysed readily in vivo.
Fig. 4.
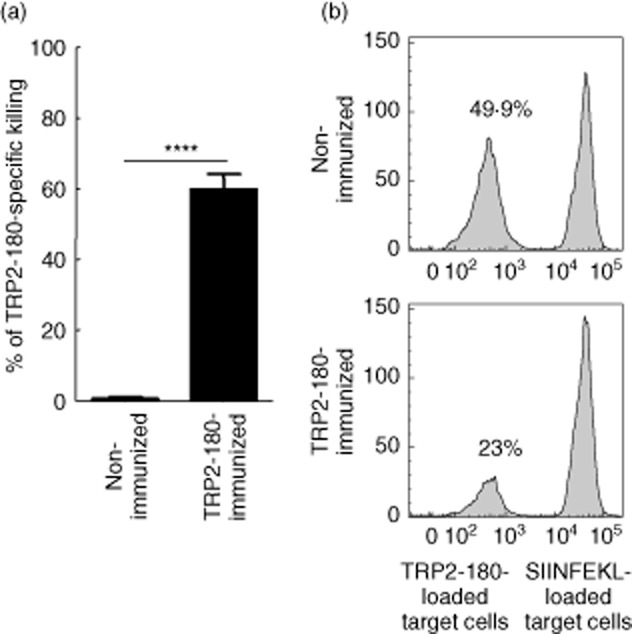
In-vivo cytotoxicity against tyrosinase-related protein (TRP)2-180-loaded target cells. (a) Splenocytes from CD45·1 naive mice were stained with low-dose (0·1 μM) or high-dose (10 μM) carboxyfluorescein succinimidyl ester (CFSE). CFSElow (0·1 μM) splenocytes were loaded with TRP2-180 (10 μM), and CFSEhigh (10 μM) splenocytes were loaded with SIINFEKL (10 μM). These cells were mixed at a 1:1 ratio and injected retro-orbitally into non-immunized CD45·2 mice or TRP2-180-immunized CD45·2 mice. At 18 h after injection, splenocytes were harvested and analysed by flow cytometry. The percentage killing was calculated as follows: [100 – (percentage of TRP2-180-loaded/percentage of SIINFEKL-loaded) × 100]. Each bar graph represents the mean ± standard error of the mean (s.e.m.) from four independent experiments with four mice per group (****P < 0·0001). (b) Histograms represent mice that were not immunized or were immunized with TRP2-180. In each histogram, the left peak represents CFSElow, TRP2-180-loaded target cells and the right peak represents CFSEhigh, SIINFEKL-loaded control target cells. Numbers indicate CFSElow, TRP2-180-loaded target cells as a percentage of total acquired cells.
Correlation of TRP2-180-specific CD8+ T cell response with the disease severity
Finally, we evaluated whether the frequency of TRP2-180-specific effector CD8+ T cells correlated with the severity of vitiligo, which is determined by the relative area of depigmented skin lesions in a tail. TRP2-180-specific effector CD8+ T cell responses were quantified by ICS and flow cytometric analysis for IFN-γ, TNF-α and the cytotoxic degranulation marker, CD107a. All three effector functions of CD8+ T cells correlated significantly and positively with the relative area of depigmented skin lesions (Fig. 5a,b), demonstrating that acquisition of effector function in TRP2-180-specific CD8+ T cells is associated directly with the development of depigmented skin lesion in our vitiligo model.
Fig. 5.
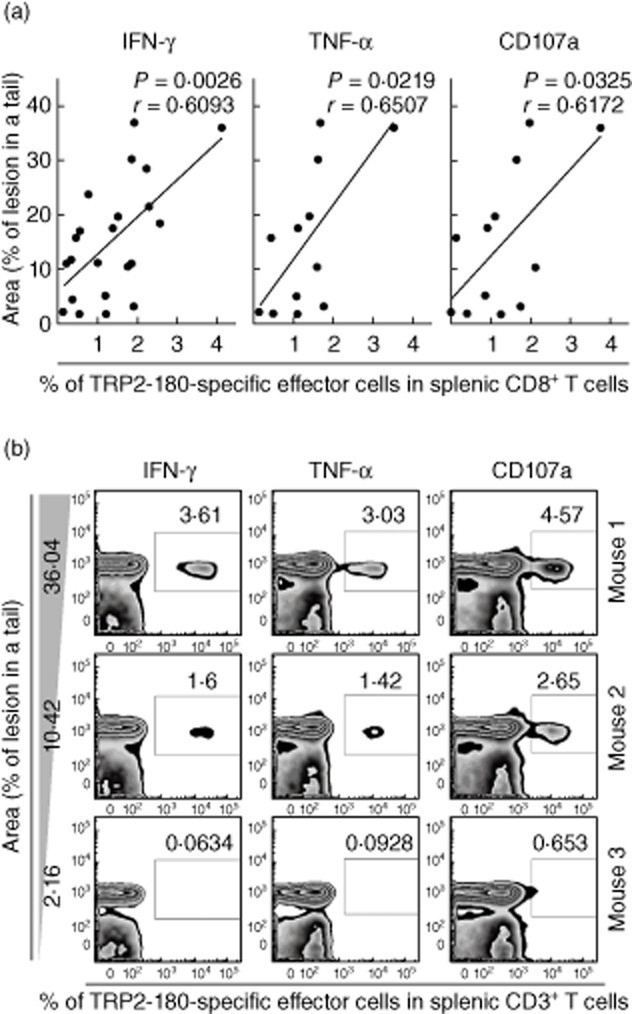
The tyrosinase-related protein (TRP)2-180-specific CD8+ T cell response correlates with disease severity. Five weeks after the last immunization, TRP2-180-specific CD8+ T cells were analysed by intracellular cytokine staining (ICS) for interferon (IFN)-γ, tumour necrosis factor (TNF)-α and the cytotoxic degranulation marker, CD107a in the spleens of mice with evident vitiligo. (a) Correlation analyses were performed between each effector function and disease severity, which was determined by the percentage of depigmented skin lesion/tail using ImageJ. Pearson's value correlation test was used for statistical analysis of correlation. (b) Representative plots from three mice illustrate IFN-γ+ cells, TNF-α+ cells or CD107a+ cells within the CD3+ T cell gate.
Discussion
In the present study, mice were immunized in the footpad and tail dermis with TRP2-180 peptide/LPS/CpG ODN to prime TRP2-180-specific autoreactive CD8+ T cells. Following boost immunizations, TRP2-180-specific CD8+ T cells were recruited to the tail skin, and epidermal depigmentation developed. Importantly, epidermal depigmentation was not accompanied by hair follicle depigmentation, illustrating the similarities between this murine vitiligo model and the common clinical form of human vitiligo.
Our results demonstrate that this immunization strategy can break self-tolerance to melanocyte self-antigens, and prime TRP2-180-specific autoreactive CD8+ T cells even without boost immunizations (data not shown). However, in the absence of boost immunizations, activated autoreactive CD8+ T cells did not destroy melanocytes, and vitiligo did not develop in any site of the body (data not shown). Thus, in our model, epidermal depigmentation developed only after boost immunizations, which recruited autoreactive CD8+ T cells to the immunization site in the tail dermis. Considering a previous report, that the development of vitiligo is regulated by local inflammation in the skin [12], inflammatory signals stimulated by Toll-like receptor (TLR) ligands such as LPS and CpG ODN might be important for triggering melanocyte destruction at the boost immunization site.
Previous studies in mouse models of vitiligo have demonstrated that the development of vitiligo requires IFN-γ production by CD8+ T cells [13], and local IFN-γ release in the lesion promotes further recruitment of melanocyte-specific CD8+ T cells into the site [14]. In our model, IFN-γ-producing CD8+ T cells were detected at the immunization site before epidermal depigmentation (Fig. 3e,f), and IFN-γ production was increased further by in-vitro stimulation of TRP2-180 (Fig. 3e,f). Furthermore, the frequency of TRP2-180-specific, IFN-γ-producing CD8+ T cells in the spleen correlated with the severity of disease determined by the relative area of depigmented lesion (Fig. 5a,b). These data suggest that blocking local recruitment of melanocyte-specific CD8+ T cells or IFN-γ production by them could be promising therapeutic targets to prevent vitiligo progression. Our new murine vitiligo model will be beneficial during the development of immune-modulating therapeutics for vitiligo.
The most interesting feature of our novel vitiligo model is the fact that hair follicle melanocytes were not destroyed and hair colour was conserved. This is an important issue in terms of skin repigmentation. Hair follicle melanocytes are known to provide a resource for regeneration of epidermal melanocytes and eventual repigmentation of skin [15,16]. Therefore, our vitiligo model can be used to study repigmentation mechanisms and drug development for repigmentation of vitiligo lesions.
In summary, we have established successfully a novel mouse model of vitiligo of epidermal depigmentation without hair depigmentation. This was accomplished by immunization with TRP2-180 peptide plus TLR agonists. This new model of vitiligo is more similar to human disease than the previous murine models of vitiligo; therefore, this model is well suited to the study of vitiligo pathogenesis and the development of novel therapeutics that target melanocyte-specific, autoreactive CD8+ T cells.
Acknowledgments
Sooseong You was responsible for design, performance and data interpretation of the study, and wrote the primary writing of the manuscript. Young-Hun Cho performed and analysed experiments. Eui-Cheol Shin conceived, designed and provided funding of the study and reviewed the final manuscript. This work was supported by National Research Foundation grants funded by the Korean government (2012-M3C1A1-048860). This work was also supported by the KAIST (Korea Advanced Institute of Science and Technology) Future Systems Healthcare Project from the Ministry of Education, Science and Technology.
Disclosure
The authors declare no competing financial interests.
References
- 1.Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419–431. doi: 10.1111/j.1346-8138.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 2.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview: part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–332. doi: 10.1046/j.1523-1747.2001.01408.x. [DOI] [PubMed] [Google Scholar]
- 4.Le Gal FA, Avril MF, Bosq J, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–1470. doi: 10.1046/j.0022-202x.2001.01605.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang KS, Caroli CC, Muhm A, et al. HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol. 2001;116:891–897. doi: 10.1046/j.1523-1747.2001.01363.x. [DOI] [PubMed] [Google Scholar]
- 7.Mandelcorn-Monson RL, Shear NH, Yau E, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003;121:550–556. doi: 10.1046/j.1523-1747.2003.12413.x. [DOI] [PubMed] [Google Scholar]
- 8.van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 9.Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26:32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Na GY, Seo SK, Choi SK. Single hair grafting for the treatment of vitiligo. J Am Acad Dermatol. 1998;38:580–584. doi: 10.1016/s0190-9622(98)70121-5. [DOI] [PubMed] [Google Scholar]
- 11.Bloom MB, Perry-Lalley D, Robbins PF, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steitz J, Bruck J, Lenz J, Buchs S, Tuting T. Peripheral CD8+ T cell tolerance against melanocytic self-antigens in the skin is regulated in two steps by CD4+ T cells and local inflammation: implications for the pathophysiology of vitiligo. J Invest Dermatol. 2005;124:144–150. doi: 10.1111/j.0022-202X.2004.23538.x. [DOI] [PubMed] [Google Scholar]
- 13.Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–1917. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97:410–416. doi: 10.1111/1523-1747.ep12480997. [DOI] [PubMed] [Google Scholar]
- 16.Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313–318. doi: 10.4103/0019-5154.57604. [DOI] [PMC free article] [PubMed] [Google Scholar]


