Abstract
B cells originate from precursors in the bone marrow, and the first cells which migrate to the peripheral blood have been classified as ‘transitional B cells’. Transitional B cells have been characterized in human blood with stage 1 (T1) and stage 2 (T2) subsets being proposed. In the present study, 27 normal human bone marrow samples were analysed for transitional B cell markers by eight-colour flow cytometry. T1 transitional B cells (CD45+CD19+CD10+IgM+IgDlo) and T2 transitional B cells (CD45+CD19+CD10+IgM+IgD+) were identified in normal bone marrow samples at a mean frequency of 3·2 and 3·1% of total B lineage cells, respectively. A majority of the bone marrow transitional B cells were CD24hiCD38hi, the phenotype of blood transitional B cells. Consistent with recent peripheral blood data, T2 B cells had a significantly higher CD21 expression compared with T1 B cells (72·4 versus 40·9%) in the bone marrow. These data raise the possibility that transitional B cells are capable of differentiating from T1 to T2 B cells within the bone marrow. Furthermore, transitional cells at either stages 1 or 2 might be capable of migrating out of the bone marrow.
Keywords: bone marrow, CD21, flow cytometry, human, transitional B cells
Introduction
B cells originate from haematopoietic stem cells present in bone marrow (BM) [1]. Developing B cells undergo several stages of differentiation in the BM before they enter the peripheral compartment [2]. Cells that are developmentally intermediate between immature BM B lineage cells and fully mature naive B cells in the peripheral blood (PB) and secondary lymphoid tissues are termed ‘transitional B cells’. This term was first used in the mouse in 1995 [3] and three stages of murine transitional B cells, namely T1, T2 and T3, have been identified [4]. More recently, transitional B cells have been identified in humans, defined as new emigrant B cells, which have been selected for self-tolerance in the BM and have been exported to the periphery [5]. It is postulated that the transitional cells, after leaving the BM, are subjected to peripheral checks to prevent the production of autoantibodies [6]. Transitional B cells that survive selection against autoreactivity develop eventually into naive B cells [7–9]. Earlier reports described a CD19+immunoglobulin (Ig)M+IgD+CD24hiCD38hiCD27– phenotype of transitional B cells, which is distinct from mature naive B cells that are CD38lo, and both germinal centre and memory B cells that are CD27+ [10–13].
Bright CD24 and CD38 co-expression have been proposed to be transitional B cell markers [5,10,12]. Studies on the PB [9,14] B cell compartment identified two transitional B cell subsets, T1 and T2 on the basis of CD24 and CD38 staining, with the former being brighter for both markers than the latter. A more recent publication used CD21 and IgD expression to divide peripheral blood transitional B cells into T1 and T2 subsets. T2 cells were reported to have a high expression of CD21 and IgD, whereas expression of these markers was low on T1 cells. Both these subsets displayed a CD5+CD10+CD20+CD27– phenotype [7]. This report provided evidence that T2 (CD21hi) B cells are more mature than T1 (CD21lo) B cells, because the T2 subset had greater proliferation and Ig secretion in vitro than the T1 subset. Furthermore, following haematopoietic stem cell transplantation (HSCT), T1 B cells populated the PB before T2 B cells, suggesting that T1 B cells represent primary BM B cell emigrants [7].
Obtaining normal human BM is difficult, and thus few studies have searched for transitional B cells in this location. Early BM studies identified transitional B cells using the CD19+CD24hiCD38hi classification [5,10], and a more recent study has identified T1 and T2 cells in four samples of human BM using a CD19+CD10+CD24hiCD38hiIgD+ classification [9]. In the present study, we used eight-colour flow cytometry to provide a comprehensive immunophenotype of B lineage cells in 27 samples of normal human BM. We used this strategy to assess BM samples for the presence of T1 and T2 transitional B cell subsets.
Materials and methods
Patient samples
Bone marrow (BM) aspirate samples were obtained from patients as indicated by routine clinical care. Samples from patients who were receiving chemotherapy or those who had received a haematopoietic stem cell transplant were excluded. This study was approved by the St Vincent's Hospital Human Research Ethics Committee (file number 11/095) and signed informed consent was obtained from all patients, in accordance with the Declaration of Helsinki.
BM aspirate samples were assessed by microscopy and flow cytometry, and BM trephine samples taken at the same time were assessed by morphology and immunohistochemistry. Samples displaying no detectable abnormalities via this multi-disciplinary approach were classified as normal, and were included into the study. Twenty-seven normal adult BM samples (median age = 51 years; interquartile range = 43–63 years; 14/27 males) were collected.
Monoclonal antibodies
BM samples were collected in heparinized tubes. Immunofluorescence staining was performed with the following monoclonal antibodies (mAbs) and fluorochromes from BD Biosciences (San Jose, CA, USA): CD45 (V500), CD19 (allophycocyanin; APC), CD10 (phycoerythrin-cyanine 7; PE-Cy7), IgM (PE), IgD (peridinin chlorophyll protein-cyanine 5·5; PerCP-Cy5·5), CD5 (fluorescein isothiocyanate; FITC), CD20 (APC coupled with haemocyanine dye; APC-H7), CD27 (APC-H7) and CD21 (PE). The following mAbs from Biolegend (San Diego, CA, USA) were utilized: IgM (Pacific Blue), CD24 (FITC) and CD38 (PE).
Immunofluorescence staining
Eight antibodies (total volume of 44 μl) were added to each test tube in the combinations described in Table 1. The BM was washed twice in phosphate-buffered saline (PBS) and resuspended in 1% (v/v) PBS/fetal calf serum (FCS); 100 μl was added to each test tube to ensure that a minimum of 10 000 B cell events were recorded in all samples. The sample was vortexed and then incubated for 10 min at room temperature; 2 ml of FACS Lyse (BD Biosciences) was added to the sample and incubated further for 10 min at room temperature. Subsequently, 2 ml of PBS was added, and the sample was centrifuged (800 g at room temperature) for 5 min. The supernatant was discarded, and the cells were resuspended by vortex in 200 μl of stabilizing fixative (BD Biosciences). The sample was then acquired on a FACSCanto II flow cytometer (BD Biosciences) using 405, 488 and 633 nm lasers, and analysed using FACSDiva software (BD Biosciences). Positive staining was determined by a comparison with negatively stained cells in the same specimen.
Table 1.
Antibody panels for bone marrow samples
| Tube | Pacific blue | V500 | FITC | PE | PerCP-Cy5·5 | PE-Cy7 | APC | APC-H7 |
|---|---|---|---|---|---|---|---|---|
| 1 | IgM | CD45 | CD5 | CD21 | IgD | CD10 | CD19 | CD27 |
| 2 | IgM | CD45 | CD24 | CD38 | IgD | CD10 | CD19 | CD27 |
FITC: fluorescein isothiocyanate; PE: phycoerythrin; PerCP-Cy.5: peridinin chlorophyll protein-cyanin 5.5; APC: allophycocyanin; Ig: immunoglobulin.
Statistical analysis
Data analysis was performed with the assistance of GraphPad Prism version 5 software. Descriptive statistics were generated and t-tests were performed to compare means between two groups. Significant differences were classified as P < 0·05. Data are expressed as mean ± standard error of the mean (s.e.m.).
Results
Strategy for identification of transitional B cell subsets in BM
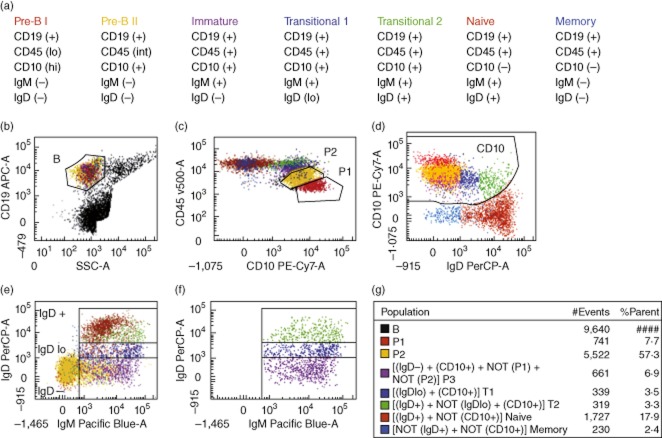
Bone marrow aspirate cells were analysed by eight-colour flow cytometry to identify transitional B cells. The phenotypic criteria that were utilized are listed in Fig. 1a, and the flow cytometry strategy to identify these populations is presented in the remainder of Fig. 1. First, CD19 was used to identify cells of the B cell lineage. CD19 is present on all B cells except for the most immature cells of this lineage [15]. CD19-positive cells displaying low side-scatter (Fig. 1b) were selected for further analysis, excluding CD19-positive plasma cells with higher side-scatter. Next, the CD10 and CD45 staining properties of these B lineage cells were used to identify the two most immature B lineage populations (Fig. 1c). The earliest CD19-positive cells in the B cell lineage are CD45dim and CD10bright, and have been termed pre-B-I cells [15]. At the next stage in maturation CD45 expression rises to intermediate and CD10, while still clearly positive, is less bright than on the pre-B-I stage. These cells have been termed pre-B-II cells [15]. These two populations are identified readily in Fig. 1c. Pre-B-I cells have considerable similarities to stage 1 haematogones and pre-B-II cells resemble stage 2 haematogones [16,17]. These two populations were negative for IgM and IgD (Fig. 1d,e), consistent with their classification as early B lineage cells.
Fig. 1.
Identification of transitional B cell subsets in normal human bone marrow (BM). The dot-plots presented display the flow cytometric approach used to identify various B cell subsets in a representative sample. Each B cell subset was assigned a particular colour. (a) Phenotypic criteria for the characterization of B lineage subsets (b) CD19+ cells with low side-scatter (SSC) were gated as B cells. (b) All BM cells except those with high SSC. In (c–f), only B cells (gated in b) are shown. (c) Pre-B I cells (P1, red) were identified as CD45loCD10hi, and pre-B II cells (P2, yellow) were identified as CD45intCD10+. (d) CD10+ B cells were gated as shown. The CD19– T and natural killer (NK) cells (not shown) were used as a negative control to appropriately draw the CD10+ gate. (e) Identification of B cell subsets. The most immature B cells [CD19+CD10+ immunoglobulin (Ig)D–IgM–] were used as a negative control to define the IgD+ and IgM+ cell populations. Gates were placed to identify the IgD–, IgDlo and IgD+ cell populations; B cells with IgD intensities higher than pre-B cells (CD45lo) and lower than mature B cells (CD10–) were classified as low IgD intensity. (f) This is a duplicate of (e); however, only the CD10+IgM+ cells are shown. (g) The gating algorithms employed to derive B cell subsets are shown. Pre-B I cells (P1, red) were identified as CD45loCD10hi and pre-B II cells (P2, yellow) were identified as CD45intCD10+. All other subsets were CD45bright. Immature (P3, purple) B cells were CD10+ and IgD–; T1 (dark blue) B cells were IgDlo and CD10+; T2 (light green) B cells were IgD+ and CD10+. Mature CD10– B cells were divided into IgD+ cells (maroon; naive and natural effector B cells), and IgD– cells (light blue; IgM only, and switched memory B cells).
The IgM+ B cells were then divided into IgD–, IgDlo and IgD+ subsets (Fig. 1e). B cells with IgD intensities higher than pre-B cells and lower than IgD+ mature B cells were classified as IgDlo. Figure 1f shows only the IgM+ CD10+ B cells. The three subsets in this figure are: IgD– (immature B cells), IgDlo (T1 cells) and IgD+ (T2 cells). CD10+sIg+ cells have been described as stage 3 haematogones [16], but this term does not distinguish between the three CD10+sIg+ subsets displayed in Fig. 1f. The gating algorithms employed to assign a particular colour to each developmental B cell stage are shown in Fig. 1g.
Mature CD10-negative B cells were identified in Fig. 1d, and were divided further into subsets based on the intensity of IgD expression. IgDlo/+ mature B cells include naive and natural effector memory B cells, while CD10–IgD– B cells represent IgM-only, and switched memory cells [18–20]. Plasma cells in bone marrow typically display higher side-scatter than lymphocytes [21], and such cells were excluded by the side-scatter gating shown in Fig. 1b. However, plasma cells with low side-scatter might be present in bone marrow samples [15]. Any such cells are CD10- and IgD-negative [15,17] and would have been excluded from the transitional B cell populations.
Enumeration of transitional B cells in BM
B cell subsets were characterized based on the phenotypic criteria presented in Fig. 1a. The mean frequencies of all the identified BM B cell subsets are presented in Fig. 2. A key finding of this study was that T1 and T2 populations were detected in all individuals. The mean cell frequency as a percentage of total BM B cells was 3·16 ± 0·34% (± s.e.m.) for T1 B cells, and 3·07 ± 0·45% for T2 B cells. Naive B cells and pre-B II cells were the most abundant B cell populations in the normal BM.
Fig. 2.
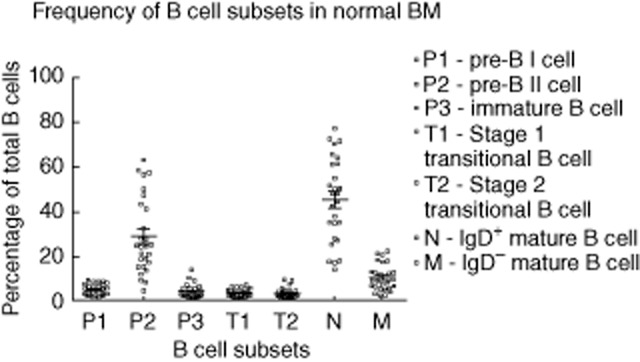
Enumeration of B cell subsets in normal bone marrow (BM). The mean [± standard error of the mean (s.e.m.)] frequencies of various B cell subsets in the normal BM are presented in this chart (n = 27). The means (± s.e.m.) of each subset are as follows: pre-B I (4·7 ± 0·5%), pre-B II (28·7 ± 3·3%), immature (4 ± 0·6%), T1 (3·2 ± 0·3%), T2 (3·1 ± 0·4%), IgD+ mature B cells (naive and natural effector) (45·2 ± 3·6%) and IgD– mature B cells (IgM only, and switched memory) (10·2 ± 1·1%).
Further phenotypic features of transitional B cells in BM
The T1 and T2 subsets were assessed for bright CD24 and CD38 co-expression, features that have been used previously to define transitional B cells [5,12]. A majority of the T1 and T2 B cells identified by the strategy in Fig. 1 had the CD24hiCD38hi phenotype, which provides strong evidence that these are indeed bona fide transitional B cell populations. The results showed that the expression of these two markers was significantly higher on the T1 B cells when compared to the T2 B cells (Fig. 3). Moreover, the differences remained significant when CD24 and CD38 were analysed individually (data not shown).
Fig. 3.
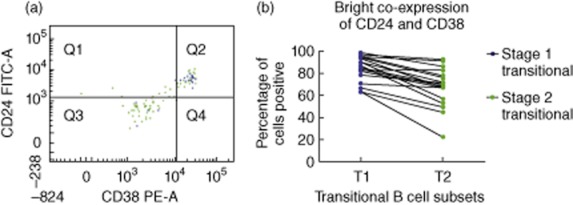
Bright CD24 and CD38 co-expression on bone marrow (BM) transitional B cell subsets. (a) A representative normal BM sample is presented here, and only transitional B cell subsets are displayed [T1 (blue; CD10+IgM+IgDlo) and T2 (green; CD10+IgM+IgD+)]. (b) This chart displays the proportion of CD24hiCD38hi cells in the T1 and T2 B cell populations. The mean (± s.e.m.) frequency of T1 and T2 B cells that were CD24hiCD38hi in the normal BM was 85·2% (± 2·7) and 69% (± 4·1), respectively (n = 19). The lines join the two values in the same sample. CD24 and CD38 expression is significantly lower in the T2 B cells (P < 0·001).
Furthermore, CD21 expression was examined on these T1 and T2 B cell populations. The results displayed a significantly higher CD21 expression on T2 B cells when compared to T1 B cells (Fig. 4). These phenotypes are consistent with the CD21lo (T1) and CD21hi (T2) transitional B cell subsets described recently in PB [7].
Fig. 4.
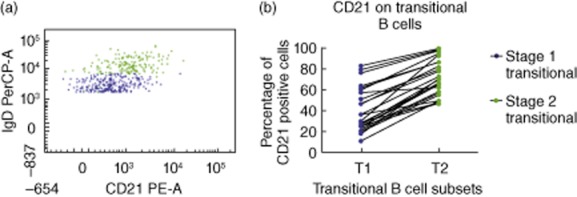
CD21 expression on bone marrow (BM) transitional B cell subsets. (a) A representative normal BM sample is presented here, and only transitional B cell subsets are displayed [T1 (blue; CD10+IgM+IgDlo) and T2 (green; CD10+IgM+IgD+)]. (b) This chart displays the proportion of CD21+ cells in the T1 and T2 B cell populations. The means [± standard error of the mean (s.e.m.)] of CD21+ T1 and CD21+ T2 B cells in the normal BM were 40·9% (± 4·2) and 72·4% (± 3·4), respectively (n = 25). A higher percentage of T2 B cells express CD21 compared with T1 B cells (P < 0·0001).
Interestingly, CD5 was not expressed uniformly on BM transitional B cells, as was expected in light of current definitions of transitional cells in human PB [22,23]. A significantly greater proportion of T2 B cells expressed CD5 compared to T1 B cells (Fig. 5).
Fig. 5.
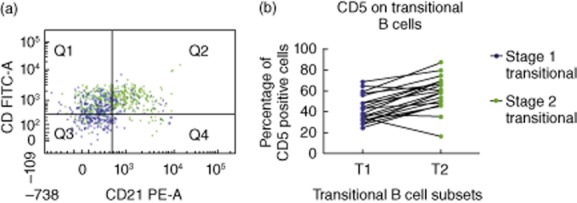
CD5 expression on bone marrow (BM) transitional B cell subsets. (a) A representative normal BM sample is presented here, and only transitional B cell subsets are displayed [T1 (blue; CD10+IgM+IgDlo) and T2 (green; CD10+IgM+IgD+)]. (b) This chart illustrates CD5 expression on T1 and T2 B cells. The mean [± standard error of the mean (s.e.m.)] of CD5+ T1 and CD5+ T2 B cells in the normal BM was 43·1% (± 2·8) and 58·2% (± 3·6), respectively (n = 20). CD5 expression is significantly higher on T2 cells than T1 cells (P < 0·001).
Discussion
Our understanding of murine B cell lymphopoiesis is robust; however, this is not the case for human B cell development. In this study, transitional B cells were characterized in normal human BM. Obtaining normal human BM is challenging on both a practical and ethical front, which explains the low numbers of publications in this field [22]. In the few studies which have been performed, transitional B cells were identified as CD24hiCD38hi B cells [5,10], with a later addition of CD10 positivity [9]. The experiments performed in the present study utilized an alternative classification (CD45+CD19+CD10+IgM+IgDlo/+), as guided by research on PB [7,8].
In agreement with recent publications on PB cells, IgD was gained progressively on transitional B cells, thus enabling the identification of T1 (IgDlo) and T2 (IgDhi) B cell subsets (Fig. 1). Furthermore, CD21 expression was increased significantly on T2 B cells compared to T1 B cells (Fig. 4), providing compelling support for the recently published report that in human PB, T1 cells are CD21lo and T2 cells are CD21hi [7]. In another study, T1 B cells were noted to be the most abundant transitional subtype in bone marrow, and were reported to express dim CD21 [9]. However, detailed data on the BM T2 B cell phenotype was not presented, and only four BM samples were analysed.
In PB, approximately 75% of transitional B cells were reported to be of T2 (CD21hi) stage [7]. These authors proposed that CD21lo T1 cells migrate from the BM and develop subsequently into CD21hi T2 cells in the periphery. This prompted the hypothesis that T1, and not T2, B cells would be found in the BM. Surprisingly, the results of the present study identified similar proportions of T1 and T2 B cell subsets in normal human BM (Fig. 2). These data suggest that transitional B cells might have the capacity to depart the BM at either the T1 or T2 stage, and that some T1 cells might be able to differentiate into T2 cells in the BM. Furthermore, it is possible that transitional cells departing the BM at different stages could differentiate into different B cell subsets. Moreover, transitional B cells could play a significant and currently unknown role in the human BM.
A majority of the transitional B cells identified in the BM displayed a CD24hiCD38hi phenotype (Fig. 3), consistent with the initial phenotypic criteria proposed for human transitional B cells [12]. Interestingly, the results showed that a significantly greater proportion of BM T1 B cells displayed this phenotype compared with BM T2 B cells (Fig. 3). Moreover, when the markers were analysed individually, a significantly greater proportion of T1 B cells were CD24hi and CD38hi when compared to T2 B cells. These data are consistent with an earlier report on T1 and T2 cells in BM [9], and indicate that CD24 and CD38 are down-regulated simultaneously in the T2 B cell population.
Two recent publications have characterized a third (T3) CD10–/lo transitional B cell subset (CD24+CD38+IgM+IgD+ABCB1–) [9,24]. In the present paper, positive staining for CD10 was utilized as a criterion for transitional B cell identification. As the published T3 populations exhibited reduced CD24 and CD38 expression, it is possible that the classifications used in the present study included the CD10lo T3 B cells with the T2 B cells. This could explain why a reduced number of BM T2 B cells displayed a CD24hiCD38hi phenotype when compared to T1 B cells.
The BM phenotypic data generated by the present study correspond well with published human PB transitional B cell data, with the notable exception that CD5 expression was found to be lower in the BM in the present study (Fig. 5) compared with published reports on PB [5,9]. A limitation of the present study is that data for transitional cells in PB are not included. CD5 is expressed on certain human B cell subsets and functions to regulate B cell receptor (BCR) signalling negatively [25,26]. Moreover, CD5 is induced on B cells as a consequence of BCR engagement and cytokine-mediated activation [27]. Our results demonstrate that approximately 43% of BM T1 B cells and 58% of BM T2 B cells express CD5. It must be considered, however, that transitional B cells might only express CD5 adequately in the periphery after BCR engagement and/or interaction with microenvironmental factors.
A large proportion of the B cells identified in normal BM samples were of a naive B cell phenotype, which is due probably to the recirculating tendency of this population [28]. A small subset of unswitched memory B cells known as natural effector B cells is likely to have been identified as naive B cells because IgD, rather than CD27, was used to distinguish naive from memory B cells. It should be considered, however, that a proportion of B cells might not leave the BM until they have developed into naive B cells [17]. The CD4 : CD8 T cell ratio can be used as an indication of blood contamination, because CD8 T cells home preferentially to the BM [29]. In the 13 BM samples in which a PB sample was available for immunophenotyping at the time of BM investigation, the CD4 : CD8 T cell ratio was 1·1:1 in the BM and 1·9:1 in the corresponding PB sample. These data confirm the presence of BM tissue in the BM samples, although the possibility of blood admixture cannot be eliminated entirely.
Although the definition of ‘normal’ samples in this study is not absolutely ideal, the use of clinically indicated bone marrow investigations that showed no pathological abnormalities overcomes many practical and ethical issues, and has been employed by numerous other investigators [9,30–36]. Although patients receiving chemotherapy were excluded from this study, many variables such as oral medications and other medical conditions were not controlled for. Young and healthy volunteers provide a solution to this problem, and normal haematopoietic stem cell transplant donors have been recruited by certain centres [37–39].
These findings warrant further research into normal human transitional B cells. Transitional B cell definitions are being refined continuously, and studies are hence required to confirm their presence in normal human BM. Moreover, further PB characterization is essential, and other sites including the spleen, cord blood and coelomic cavities should also be analysed. The identification of differing transitional B cell frequencies in infants and adults could provide more clues about human B cell development. Finally, functional studies on transitional B cells will determine whether or not they play a distinct role in the BM, or indeed any other anatomical sites.
Acknowledgments
The authors thank Anthony Dodds, Maria Fahd, Keith Fay, David Ma, Samuel Milliken, John Moore, Rebecca Walsh and Barbara Withers, of the Haematology Department, St Vincent's Hospital, for collection of the BM samples. This study was funded by St Vincent's Clinical School and the Faculty of Medicine, University of New South Wales, and supported by the Royal College of Pathologists of Australasia Scholarship for Medical Schools.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Hoebeke I, De Smedt M, Stolz F, et al. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(–)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia. 2007;21:311–319. doi: 10.1038/sj.leu.2404488. [DOI] [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 3.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 5.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 7.Suryani S, Fulcher DA, Santner-Nanan B, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 8.Marie-Cardine A, Divay F, Dutot I, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127:14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Palanichamy A, Barnard J, Zheng B, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 11.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M(+)IgD(+) peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 13.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anolik JH, Friedberg JW, Zheng B, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.van Lochem EG, van der Velden VHJ, Wind HK, te Marvelde JG, Westerdaal NAC, van Dongen JM. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B Clin Cytom. 2004;60B:1–13. doi: 10.1002/cyto.b.20008. [DOI] [PubMed] [Google Scholar]
- 16.McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4-color flow cytometry. Leuk Lymph. 2004;45:277–285. doi: 10.1080/1042819031000151950. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Andres M, Paiva B, Nieto WG, et al. Human peripheral bood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78:S47–60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 18.Berkowska MA, Driessen GJA, Bikos V, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 20.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood. 1997;90:2863–2892. [PubMed] [Google Scholar]
- 22.Suryani S, Tangye SG. Therapeutic implications of advances in our understanding of transitional B-cell development in humans. Exp Rev Clin Immunol. 2010;6:765–775. doi: 10.1586/eci.10.55. [DOI] [PubMed] [Google Scholar]
- 23.Vossenkamper A, Spencer J. Transitional B cells: how well are the checkpoints for specificity understood? Arch Immunol Ther Exp (Warsz) 2011;59:379–384. doi: 10.1007/s00005-011-0135-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 25.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100:4537–4543. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- 26.Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol. 2011;23:310–318. doi: 10.1016/j.coi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–353. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Cyster JG. Chemokines – chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 29.Rego EM, Garcia AB, Viana SR, Falcao RP. Age-related changes of lymphocyte subsets in normal bone marrow biopsies. Cytometry. 1998;34:22–29. doi: 10.1002/(sici)1097-0320(19980215)34:1<22::aid-cyto4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Ghia P, ten Boekel E, Sanz E, de la Hera A, Rolink A, Melchers F. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain gene loci. J Exp Med. 1996;184:2217–2229. doi: 10.1084/jem.184.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Jain P, Deo SVS, Sharma A. Flow cytometric analysis of CD5+B cells – a frame of reference for minimal residual disease analysis in chronic lymphocytic leukemia. Am J Clin Pathol. 2004;121:368–372. doi: 10.1309/T5EM-9BQU-B9CM-8F57. [DOI] [PubMed] [Google Scholar]
- 33.Vaskova M, Fronkova E, Starkova J, Kalina T, Mejstrikova E, Hrusak O. CD44 and CD27 delineate B-precursor stages with different recombination status and with an uneven distribution in nonmalignant and malignant hematopoiesis. Tissue Antigens. 2008;71:57–66. doi: 10.1111/j.1399-0039.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 34.Fuda FS, Karandikar NJ, Chen W. Significant CD5 expression on normal stage 3 hematogones and mature B lymphocytes in bone marrow. Am J Clin Pathol. 2009;132:733–737. doi: 10.1309/AJCPU5E3NXEKLFIY. [DOI] [PubMed] [Google Scholar]
- 35.Lutherborrow M, Bryant A, Jayaswal V, et al. Expression profiling of cytogenetically normal acute myeloid leukemia identifies microRNAs that target genes involved in monocytic differentiation. Am J Hematol. 2011;86:2–11. doi: 10.1002/ajh.21864. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson A, de Milito A, Mowafi F, et al. Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp Hematol. 2005;33:1500–1507. doi: 10.1016/j.exphem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Lucio P, Parreira A, van den Beemd MWM, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia. 1999;13:419–427. doi: 10.1038/sj.leu.2401279. [DOI] [PubMed] [Google Scholar]
- 38.Brooimans RA, Kraan J, van Putten W, Cornelissen JJ, Lowenberg B, Gratama JW. Flow cytometric differential of leukocyte populations in normal bone marrow: influence of peripheral blood contamination. Cytometry B Clin Cytom. 2009;76B:18–26. doi: 10.1002/cyto.b.20439. [DOI] [PubMed] [Google Scholar]
- 39.van Zelm MC, van der Burg M, de Ridder D, et al. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol. 2005;175:5912–5922. doi: 10.4049/jimmunol.175.9.5912. [DOI] [PubMed] [Google Scholar]