Abstract
Macrophages play a critical role in intestinal wound repair. However, the mechanisms of macrophage-assisted wound repair remain poorly understood. We aimed to characterize more clearly the repair activities of murine and human macrophages. Murine macrophages were differentiated from bone marrow cells and human macrophages from monocytes isolated from peripheral blood mononuclear cells of healthy donors (HD) or Crohn's disease (CD) patients or isolated from the intestinal mucosa of HD. In-vitro models were used to study the repair activities of macrophages. We found that murine and human macrophages were both able to promote epithelial repair in vitro. This function was mainly cell contact-independent and relied upon the production of soluble factors such as the hepatocyte growth factor (HGF). Indeed, HGF-silenced macrophages were less capable of promoting epithelial repair than control macrophages. Remarkably, macrophages from CD patients produced less HGF than their HD counterparts (HGF level: 84 ± 27 pg/mg of protein and 45 ± 34 pg/mg of protein, respectively, for HD and CD macrophages, P < 0·009) and were deficient in promoting epithelial repair (repairing activity: 90·1 ± 4·6 and 75·8 ± 8·3, respectively, for HD and CD macrophages, P < 0·0005). In conclusion, we provide evidence that macrophages act on wounded epithelial cells to promote epithelial repair through the secretion of HGF. The deficiency of CD macrophages to secrete HGF and to promote epithelial repair might contribute to the impaired intestinal mucosal healing in CD patients.
Keywords: Crohn's disease, inflammatory bowel diseases, intestinal ulcers, mucosal healing, restitution
Introduction
Wound healing is a vital process characterized by complete restoration of tissue integrity and homeostasis following injury. Different immune and non-immune cell types are implicated in this process. Macrophages have been shown to play major roles in several aspects that are essential for efficient wound repair [1–3]. Indeed, macrophages clear invading microbes, contribute to debris scavenging and critically support wound repair by releasing growth factors such as transforming growth factor (TGF)-β, insulin-like growth factor (IGF)-I, hepatocyte growth factor (HGF) and epidermal growth factor (EGF) [4–6]. Wound repair depends critically upon macrophages, as depletion during skin injury results in delayed re-epithelialization, reduced collagen deposition, impaired angiogenesis and decreased cell proliferation in the healing wounds [2,3]. Similarly, re-epithelialization of intestinal ulcers induced by oral administration of dextran sodium sulphate (DSS) [7] is severely delayed by macrophage depletion [8,9]. Consistently, we have shown that intestinal healing can be stimulated by the administration of granulocyte–macrophage-colony stimulating factor (GM-CSF) [10], a factor that promotes rapid accumulation of macrophages and increases the expression of HGF within the ulcerated mucosa [10]. While these data demonstrate clearly that macrophages play important roles in intestinal mucosal healing, the mechanisms underlying macrophage-assisted intestinal wound repair remain elusive.
The mechanisms underlying the pathogenesis of inflammatory bowel diseases (IBD), such as Crohn's disease (CD) are under intense investigation. Current concepts suggest that CD results from an inappropriate immune response to a subset of enteric bacteria, as well as impaired intestinal mucosal healing in genetically susceptible hosts [11]. In particular, in- vitro studies demonstrated convincingly that macrophages isolated from CD patients are dysfunctional. In a first report, Kamada and co-workers [12] reported that CD monocyte-derived macrophages, differentiated with macrophage colony-stimulating factor and interferon (IFN)-γ produced more interleukin (IL)-23 in response to bacterial stimuli than monocyte-derived macrophages from healthy donors (HD). In a second study, Smith et al. [13] reported that monocyte-derived macrophages from CD, but not ulcerative colitis (UC) patients, differentiated without any exogenous factors, showed impaired secretion of proinflammatory cytokines upon inflammatory stimulation. From these observations it has been suggested that a primary immunodeficiency of macrophages might underlie CD development [14]. However, it is currently unclear whether macrophage dysfunction may contribute to the impaired intestinal mucosal healing in CD patients.
In this study, we made use of in-vitro models of mouse and human intestinal epithelial repair to characterize the mechanisms of macrophage-assisted wound repair. We provide evidence that murine and human macrophages promote epithelial repair through the production of HGF. In addition, we demonstrate that macrophages from CD patients have impaired epithelial repair properties owing, at least in part, to defective HGF production.
Material and methods
Mice
Nine to 12-week-old specific-pathogen free female BALB/c mice were obtained from Harlan (Ad Horst, the Netherlands). All animal procedures were approved by the State Veterinary Office (authorization no. 1748.1).
Mouse splenic CD11b+ isolation and macrophage differentiation
To generate macrophages, 3 × 106 bone marrow cells were seeded in bacteriological Petri dishes with 10 ml of Iscove's modified Eagle's medium (IMDM) GlutaMAX™ (Gibco, Basel, Switzerland) supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), β2-mercaptoethanol (50 μm), 10% heat-inactivated fetal calf serum (FCS) and 10 ng/ml M-CSF (R&D Systems, Minneapolis, MN, USA). On day 3, 10 ml of fresh medium was added to the culture. Macrophages were harvested on day 7 by scraping. Splenic CD11b+ cells were sorted with magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Peripheral blood mononuclear cells (PBMCs) isolation and macrophage differentiation
Peripheral venous blood was collected from CD and UC patients or HD in tubes containing ethylenediamine tetraacetic acid (EDTA). The patients' characteristics are provided in Table 1. PBMCs were isolated by Ficoll density gradient centrifugation and CD14+ cells were positively selected by magnetic sorting (anti-human magnetic particles-DM-clone MφP9; BD Biosciences, San Jose, CA, USA). Cells were plated at density of 0·5–1 × 106 cells/well in 12-well plates in RPMI-1640 medium [25 mM HEPES, 10% FCS, penicillin (50 U/ml), streptomycin (50 μg/ml)]. At days 1 and 4 non-adherent cells were discarded and at day 5 adherent macrophages were recovered by scraping. This study was approved by the human research ethics committee of the Commission cantonale d'éthique de la recherche sur l'être humain (protocol 41/11).
Table 1.
Patients' characteristics.
| Patient no. | Age | Sex | Disease activity | Therapy |
|---|---|---|---|---|
| Crohn's disease patients | ||||
| 1 | 29 | M | Remission | MTX |
| 2 | 49 | M | Remission | IFX |
| 3 | 28 | F | Remission | Steroids |
| 4 | 58 | M | Remission | No TP |
| 5 | 29 | M | Remission | IFX |
| 6 | 22 | M | Remission | IFX |
| 7 | 41 | F | Remission | Un |
| 8 | 35 | M | Remission | IFX |
| 9 | 14 | M | Remission | IFX |
| 10 | 62 | M | Remission | IFX |
| 11 | 31 | F | Remission | IFX |
| 12 | 40 | M | Remission | IFX |
| 13 | 40 | F | Remission | IFX |
| 14 | 46 | M | Remission | IFX |
| 15 | 45 | F | Active | IFX |
| 16 | 25 | F | Active | IFX |
| 17 | 24 | F | Active | IFX |
| 18 | 45 | F | Active | IFX |
| 19 | 56 | M | Active | Un |
| 20 | 56 | M | Active | Un |
| 21 | 29 | M | Active | Un |
| 22 | 46 | F | Active | Steroids + azathioprine |
| 23 | 46 | M | Active | IFX |
| 24 | 24 | M | Active | IFX |
| 25 | 35 | M | Active | Un |
| 26 | 24 | F | Active | IFX |
| 27 | 48 | F | Active | IFX + antibiotics |
| 28 | 64 | M | Active | Steroids + MTX |
| Ulcerative colitis patients | ||||
| 29 | 54 | M | Remission | Mesalasine |
| 30 | 84 | M | Remission | Mesalazine |
| 31 | 43 | F | Remission | Mesalazine |
| 32 | 23 | F | Remission | Mercaptopurine |
| 33 | 62 | F | Remission | No TP |
| 34 | 31 | F | Remission | IFX |
| 35 | 43 | M | Remission | IFX |
| 36 | 48 | M | Remission | No TP |
| 37 | 32 | F | Remission | IFX |
| 38 | 44 | M | Remission | IFX |
| 39 | 49 | M | Remission | No TP |
| 40 | 21 | F | Remission | IFX |
| 41 | 47 | F | Remission | Mesalazine |
| 42 | 26 | F | Active | IFX |
| 43 | 61 | M | Active | IFX |
| 44 | 44 | M | Active | IFX |
| 45 | 34 | M | Active | Un |
| 46 | 71 | F | Active | No TP |
Patients were categorized in remission or in active phase of the disease by the clinician involved in the study (P.M.). MTX: methotrexate; IFX: infliximab; Un: unknown; No TP, no therapy; M: male; F: female.
Isolation of human intestinal macrophages (IMACs)
Healthy parts of surgical pieces of colon cancer patients were collected and washed with phosphate-buffered saline (PBS); the mucosa was released from the muscular layer and then stirred for 30 min in Hanks's buffered salt solution (HBSS) supplemented with dithiothreitol to free it from mucus. The mucosa was then stirred in HBSS + EDTA 0·1 M at 10 g at 37°C for 30 min. After rinsing, epithelial cells were detached by vortexing and vigorous shaking. The mucosa was transferred in Dulbecco's modified Eagle's medium (DMEM) + 10% FCS and kept incubated overnight at 4°C. The following day, after washing with PBS, mucosal slices were digested in PBS + Ca2+ and Mg2+ with collagenase (Sigma, St Louis, MO, USA), hyaluronidase (Sigma) and DNase (Roche, Basel, Switzerland) and stirred for 60 min at 37°C at 20 g. Cell suspension was then poured through a strainer and slices were discarded following further vortexing and vigorous shaking. Intestinal monocytic cells were isolated by Ficoll density gradient centrifugation and CD33+ cells were positively selected by magnetic sorting (mouse anti-human magnetic micro beads, clone AC104.3E3; Miltenyi Biotec) [15].
In-vitro wound repair assays
Mouse assay
CMT-93 cells (5 × 105) grown in 12-well plates were serum-starved (0·1% FCS) for 16 h prior to wounding. Four wounds per well were then performed using a razor blade; two wells were evaluated for each experimental condition. Pictures of each wound were taken on day 0 and 18 h following wounding (Olympus IX81 microscope) in the presence or not of 2·5 × 105 bone marrow-derived macrophages (BMM). In some experiments, BMM were labelled using the PKH26 red fluorescence cell linker kit (Sigma). The wound surfaces were measured using Photoshop software. The percentage of wound closure for cells cultured in DMEM 0·1% FCS [(wounded area t = 0 – wounded area t = 18)/(wounded area t = 0 × 100)] was between 40 and 50%. For each experiment, a repaired area of 1 was attributed arbitrarily to wound closure of epithelial repair in DMEM 0·1% FCS [10].
Human assay
The human intestinal carcinoma cell line Caco2 was grown in DMEM medium supplemented with 10% FCS and 1% penicillin/streptomycin to reach a confluent monolayer in 12-well plates. Caco2 monolayers were starved for 24 h in DMEM 0·5% FCS and then wounded with a p1000 plastic pipette tip connected to a vacuum aspirator. This system allowed us to obtain reproducible circular wounds with an average size of 0·8–1·4 mm2 [16]. Epithelial cells were incubated for 3 days to allow wound healing in the presence or not of 105 human macrophages. On day 3, epithelial cell monolayers were fixed for 10 min with 4% paraformaldehyde and stained for 4 min with Groat haematoxylin. Pictures were taken at day 0 (day of wounding) and at day 3 (×4 objective, Olympus IX81) and the wound area was measured as described above. Wound healing was assessed as the percentage of re-epithelialized area. As negative control, serum-deprived medium (DMEM, 0·5% FCS) was used, whereas DMEM 10% FCS served as positive control.
Inhibition experiments
To inhibit the HGF receptor c-met, PHA-665732 (Tocris Bioscience, Bristol, UK) was used. To inhibit macrophage HGF secretion, non-coding and HGF-specific siRNA (Qiagen, Valencia, CA, USA; sequences available upon request) were electroporated into cells as described by Wiese et al. [17] Briefly, we electropored (4 ms, 250 V) 4 × 106 mouse macrophages in suspension in 400 μl of OPTI-MEM (Gibco) with 6μg of siRNA. Transfection efficacy was evaluated with Alexa-Fluor 488-labelled siRNA; following electroporation, more than 95% of the BMM were fluorescent.
Real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was isolated using the RNeasy Plus Mini kit (Qiagen). Sample quality was tested on agarose gel and the absence of genomic DNA was assessed by PCR using primers specific for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense: 5′-GCTAAGCAGTTGGTGGTGCA-3′, anti-sense: 5′-TCACCACCATGGAGAAGGC-3′). Total RNA samples (1 μg) were then submitted to reverse transcription using the ThermoScript™ RT–PCR system (Invitrogen Life Technologies, Basel, Switzerland), according to the manufacturer's protocol and with oligo-dT as primers. PCR amplification was performed on a MyiQ iCycler (Bio-Rad, Hercules, CA, USA) using the iQ SYBR Green Supermix (Bio-Rad). Primer pairs designed for QuantiTect Primer assays (Qiagen) were used for quantification of HGF. For each individual sample, mRNA quantification was performed by normalizing the number of mRNA copies obtained for the gene of interest per million of mRNA copies obtained for GAPDH [10].
Determination of HGF levels
Human or mouse HGF concentrations were determined by enzyme-linked immunosorbent assay using the Duo Set enzyme-linled immunosorbent assay (ELISA) development systems by R&D Systems (Minneapolis, MN, USA). Total protein levels were determined by bicinchoninic acid assay protein assay (Pierce, Rockford, IL, USA).
F-actin purse string formation
To quantify F-actin purse string formation in epithelial cells, before a complete wound closure, CMT-93 were fixed for 10 min with 4% paraformaldehyde. Cells were permeabilized for 10 min with 0·3% Triton X-100/PBS, incubated in a blocking solution [2% bovine serum albumin (BSA)/PBS] for 30 min and then incubated with Alexa Fluor 488 Phalloidin (Invitrogen A12379) for 20 min. After staining, cells were washed in PBS and microphotographs were taken with a fluorescence microscope (Olympus IX81). At the leading edge of the migration front, F-actin purse string formation was evaluated by quantifying the intensity of phalloidin staining with Photoshop software (Supporting information, Fig. S1). A filamentous-actin (F-actin) purse string formation of 1 was attributed arbitrarily to wound closure in DMEM 0·1% FCS.
Detection of cell proliferation
CMT-93 cells were fixed for 10 min with 4% paraformaldehyde. Cells were permeabilized for 10 min with 0·2% saponin/PBS, incubated in a blocking solution (5% normal goat serum/PBS) for 60 min and then incubated with an anti-Ki67 antibody (clone MM1; Novocastra, Nunningen, Switzerland) for 1 h, followed by washing in PBS and staining with a secondary goat anti-mouse antibody (Alexa Fluor 488; Molecular Probes, Invitrogen AG) for 30 min. After staining, cells were washed in PBS, counterstained with diamidino-2-phenylindole (DAPI) for 5 min and analysed with a fluorescence microscope (Olympus IX81).
Statistical analyses
Data distribution was compared by the Mann–Whitney U-test using Graphpad Prism version 6 software (GraphPad Software, San Diego, CA, USA), with P < 0·05 as the limit of significance. Comparison between several groups to identify a trend was performed by a one-way analysis of variance (anova) test with Trend test as post-test (GraphPad Software).
Results
Murine macrophages promote in-vitro epithelial repair
Using an in-vitro model of epithelial repair [10], we tested whether murine macrophages modulate healing of a wounded colonic epithelial cell monolayer. Remarkably, the addition of BMM to wounded epithelial cells promoted wound repair (Fig. 1a). Similarly, epithelial repair was stimulated to a lesser degree by mouse splenic CD11b+ myeloid cells [10], a heterogeneous cell population containing 30% of cells expressing the F4/80+ macrophage marker (Fig. 1a). In contrast, we did not observe any significant promotion of wound repair after addition of CD4+ T cells [10].
Fig. 1.
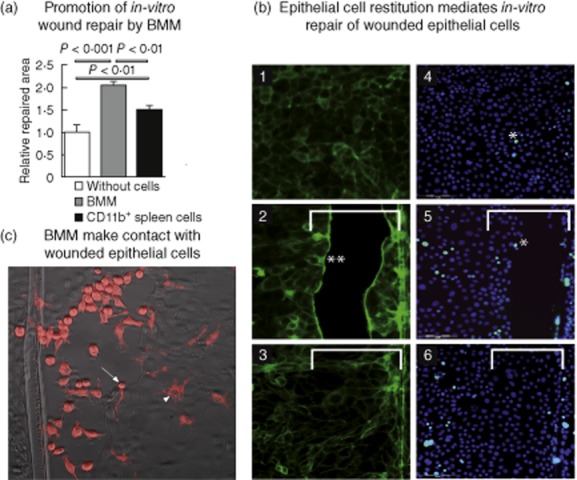
Murine macrophages promote in-vitro wound repair. (a) Wounded CMT-93 epithelial cells were cultivated for 18 h in the absence (without cells) or presence of bone marrow-derived macrophages (BMM) or splenic CD11b+ cells. Six independent experiments were performed; results are represented as mean ± standard error of the mean. P-values were calculated by the Mann–Whitney U-test. (b) Representative images of CMT-93 epithelial cell monolayers stained with Alexa Fluor 488 Phalloidin to detect F-actin (1, 2, 3) or with Syto 60 red fluorescent nucleic acid stain and Alexa Fluor 488 anti-Ki67 antibodies (4, 5, 6) (bars: 100 μm). 1 and 4: epithelial monolayer before wounding, actin microfilaments (green fluorescence) can be detected close to the cell membrane (1) and Ki-67+ proliferating epithelial cells (with light blue nuclei, *, 4) are scarce; 2 and 5: wounded epithelial monolayers after 8 h incubation with BMM placed in cell culture insert, actin microfilaments reorganized and accumulated at the edge of wounded epithelial cell monolayers (2, **), while there is no evident epithelial cell proliferation (*, 5) at the wound edges; 3 and 6: fully repaired wounded epithelial monolayers after 18 h incubation with BMM placed in cell culture insert. Accumulation of actin microfilaments at the edge of wounded epithelial cells disappeared (3) and no increased cell proliferation was detected in the epithelial cells covering the wounded area (6). White bars show the initial wounded area. (c) Representative image of wounded CMT-93 epithelial cells cultivated for 10 h in presence of PKH26-labelled BMM (red cells). Arrow shows BMM making direct contact with epithelial cells at the leading edge of the migration front; arrowhead shows that once epithelial cells covered the wounded area, some adherent BMM can be detected under the epithelial cell monolayer.
In vivo, intestinal epithelial repair relies upon two distinct processes: restitution and proliferation [18,19]. Restitution takes place within minutes from the injury and in this phase epithelial cells reorganize their cytoskeleton to cover the wound bed. This is then followed by a proliferative phase, which allows for complete reconstitution of the initial number of epithelial cells. Restitution is associated with a typical accumulation of F-actin to the leading edge of migrating cells towards the wound centre [16]. To discriminate whether BMM promote epithelial repair by increasing epithelial restitution and/or proliferation, we stained BMM-exposed wounded epithelial cell monolayers for a proliferation marker (Ki67) and F-actin. While epithelial cell proliferation was unchanged, we observed an accumulation of F-actin at the edge of wounded epithelial cells, characteristic of migrating epithelial cells (Fig. 1b). In addition, BMM were labelled with PKH26 fluorescent dye and incubated with unlabelled wounded epithelial cells. Before a complete wound closure, we observed that macrophages are mainly aggregated and adhered to the cell-free wounded area (Fig. 1c). At this time-point, we observed that some BMM make contact with epithelial cells at the leading edge of the migration front (Fig. 1c, arrow). Once migrating epithelial cells covered the wounded area, some adherent BMM could be detected under the epithelial cell monolayer (Fig. 1c, arrowhead). We never detected macrophages between epithelial cells within the repaired monolayer, suggesting that macrophages would probably not replace, even temporally, epithelial cells in a restituted epithelium. Altogether, these results demonstrate that macrophages promote epithelial repair in vitro through a specific effect on epithelial restitution.
Murine macrophages promote wound repair through HGF secretion
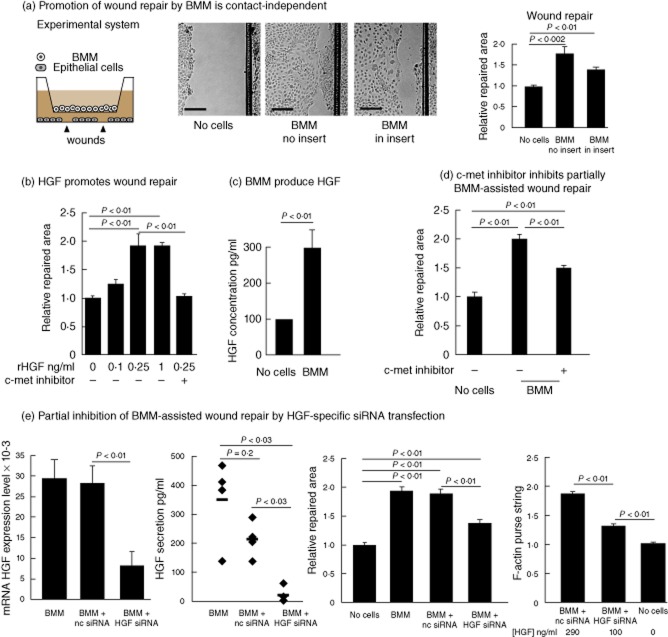
We next asked whether macrophages require cell contact to promote in-vitro wound repair. To address this question, macrophages were co-cultured with wounded epithelial cells in physically separate chambers using cell culture inserts (pore size: 0·4 μm) (Fig. 2a, left panel). Importantly, physical separation of macrophages in cell culture inserts had only modest consequences on their ability to promote wound repair (Fig. 2a). Hence, our observations suggest that macrophage-assisted wound repair can be independent of cell contact and probably depends upon soluble factors.
Fig. 2.
Murine macrophages promote epithelial restitution through hepatocyte growth factor (HGF) secretion. (a) Macrophages do not need cell contact to promote wound repair. Schematic representation of the experimental system (a, left panel). Representative images of wounded areas on t = 18 h of CMT-93 cells cultured in serum-deprived medium (no cells) in the presence of bone marrow-derived macrophages (BMM) added directly on top of wounded cells (BMM no insert) or in the presence of BMM added into a cell culture insert (BMM in insert). (b) Recombinant mouse hepatocyte growth factor (rHGF) promotes epithelial repair. The addition of rHGF to wounded epithelial cells promotes repair in a dose-dependent manner. After addition of 400 nM of the c-met inhibitor PHA-665732, the rHGF-induced wound closure is suppressed. (c) HGF protein concentration measured by enzyme-linked immunosorbent assay (ELISA) in the supernatant of BMM co-cultured with wounded epithelial cells. HGF concentrations were determined at 18 h of culture in Dulbecco's modified Eagle's medium (DMEM) 0·1% fetal calf serum (FCS). (d) Wound closure ability of BMM in the presence of PHA-665732 (400 nM). (e) HGF mRNA expression measured by quantitative polymerase chain reaction (PCR) and HGF protein concentrations in the culture supernatant of BMM left untreated or electroporated with non-coding siRNA (nc siRNA) or HGF specific siRNA (HGF siRNA). Wound closure and F-actin purse string formation abilities of BMM left unmanipulated (BMM) or transfected with non-coding siRNA (BMM + nc siRNA) or HGF-specific siRNA (BMM + HGF siRNA). The data presented show the cumulated results of at least three independent experiments; bars are mean ± standard error of the mean. P-values were calculated by the Mann–Whitney U-test for (b) and (c); for (d) and (e) comparison between several groups to identify a trend was performed by a one-way analysis of variance (anova) test with Trend test as post-test.
We have reported previously that colonic ulcer repair in mice could be ameliorated by administration of GM-CSF [10]. This was associated with a rapid accumulation of macrophages and increased expression of HGF within the ulcerated colon [10]. Hence, we hypothesized that HGF production could be a mechanism for macrophage-assisted wound repair. Addition of recombinant HGF on wounded epithelial cells promoted repair (Fig. 2b). Wound repair depends upon HGF receptor (c-met) signalling, as HGF-assisted repair could be blocked by a specific chemical inhibitor [20] (Fig. 2b). In contrast, the addition of other growth factors such as GM-CSF or irrelevant proteins (rat antibodies) at similar concentrations did not promote epithelial repair (data not shown).
To assess whether HGF is implicated in BMM-induced epithelial repair in vitro, we first measured the concentrations of HGF in the supernatant of macrophages co-cultivated with wounded epithelial cells by ELISA. Concentrations averaging 300 pg/ml of HGF were detected in the supernatant of BMM co-cultivated with wounded epithelial cells (Fig. 2c). As wound repair could be promoted by recombinant HGF at concentrations of 250 pg/ml, the measured concentrations in our co-culture system seemed biologically relevant (Fig. 2b).
To address whether macrophage-assisted wound repair depends on intact HGF-mediated signals, we blocked the HGF signalling pathway with the c-met inhibitor. Macrophage-assisted wound repair was impaired partially but significantly by chemical inhibition of HGF signalling (Fig. 2d). To evaluate specifically whether macrophage-derived HGF secretion is necessary for macrophages to promote epithelial repair, we knocked-down HGF in macrophages using an HGF-specific small interfering RNA (siRNA) [17]. Using this approach, we could decrease HGF expression and secretion efficiently and significantly in macrophages (Fig. 2e). Importantly, HGF-silenced BMM displayed a significant reduction of their properties to assist epithelial repair (Fig. 2e). In contrast, epithelial repair could still be promoted by control macrophages or macrophages electroporated with non-coding siRNA (nc siRNA) (Fig. 2e). In addition, we investigated whether lowered HGF secretion by macrophages is affecting the restitution phase in epithelial cell repair by measuring F-actin purse string formation at the leading edge of migrating epithelial cells. HGF-silenced BMM producing HGF to an average concentration of 100 ± 20 pg/ml and non-coding siRNA-transfected BMM producing 290 ± 40 pg/ml HGF were introduced into inserts and co-cultivated with wounded epithelial cell monolayer. F-actin purse string formation was reduced significantly in HGF-silenced BMM compared to control BMM (Fig. 2e), suggesting that epithelial restitution during repair depends on intact HGF secretion by macrophages.
Because blocking of HGF signalling only partially inhibited macrophage-assisted wound repair (Fig. 2d,e), other macrophage-derived soluble factors such as TGF-β might promote wound repair. However, antibody-mediated neutralization of the anti-inflammatory cytokine TGF-β did not influence significantly the BMM-assisted epithelial repair (Supporting information, Fig. S2). Hence, in our experimental model, TGF-β does not seem to play a critical role in macrophage-induced epithelial repair.
In addition, as macrophages in vivo, especially in inflammatory environments, are likely to be activated by environmental factors when infiltrating tissue, we evaluated how inflammatory stimuli influence HGF production and repair processes. To this end, we added IL-1β (10 ng/ml) or 106 heat-inactivated Escherichia coli to our co-cultures. We observed that, while still present, BMM-assisted epithelial repair in the presence of E. coli tended to be slightly less efficient than co-cultures performed without addition of inflammatory stimuli (P = 0·055, Supporting information, Fig. S3). This correlated with a reduction of HGF production by E. coli-exposed BMM (Supporting information, Fig. S3).
Taken together, these results demonstrate that murine macrophages, independent of inflammatory stimuli, assist epithelial repair in vitro actively through the secretion of soluble factors such as HGF.
Human macrophages promote wound repair
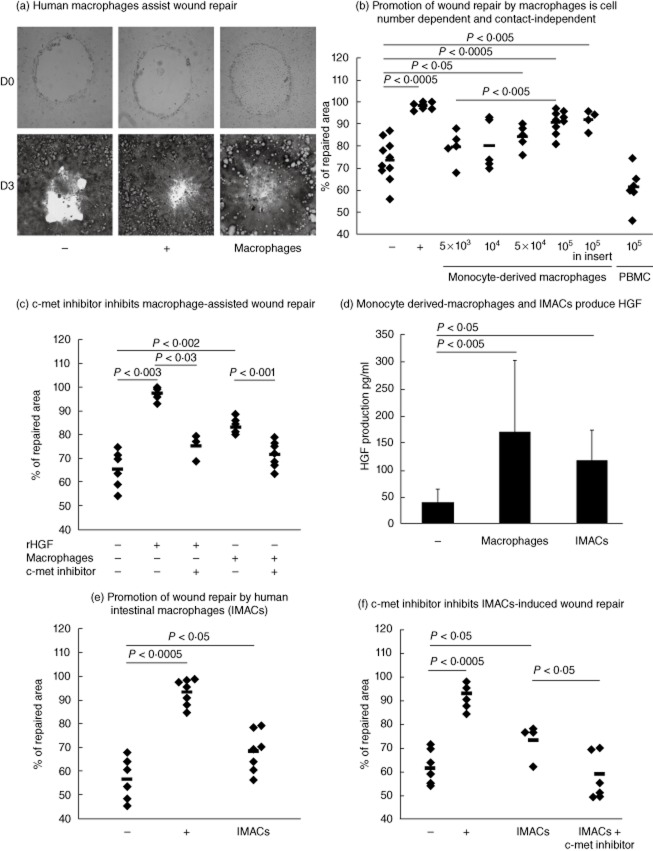
We next evaluated whether our observations using murine macrophages could be translated to human macrophages. Remarkably, monocyte-derived macrophages from healthy donors (HD) promoted repair of a wounded human epithelial cell monolayer in a cell number-dependent manner (Fig. 3a,b). Monocyte-derived macrophages activated with heat-inactivated E. coli promoted repair as efficiently as quiescent counterparts (Supporting information, Fig. S4). In contrast, addition of total peripheral blood mononuclear cells (Fig. 3b) or monocytes (data not shown) of HD to wounded epithelial cells did not assist repair. Similar to our data using murine cells, human macrophages promote wound repair by increasing epithelial cell restitution (data not shown).
Fig. 3.
Human macrophages promote in-vitro wound repair through hepatocyte growth factor (HGF) secretion. (a) Representative images of Caco2 epithelial cell monolayers after wounding (day 0) or after 3 days of incubation. Caco2 cells were seeded with either Dulbecco's modified Eagle's medium (DMEM) 0·5% fetal calf serum (FCS) (–), or DMEM 10% FCS (+) or with 105 human macrophages (macrophages). (b) Wounded Caco2 cells were seeded in DMEM 0·5% FCS either directly with increasing numbers of macrophages, with 105 macrophages into a cell culture insert or with 105 peripheral blood mononuclear cells (PBMC). In negative (–) and positive (+) controls wounded Caco2 cells were seeded with DMEM 0·5% FCS or DMEM 10% FCS, respectively. Comparison between different numbers of macrophages was performed by a one-way analysis of variance (anova) test with Trend test as post-test. (c) c-Met inhibitor inhibits macrophage-assisted repair. Wounded Caco2 cells were seeded in DMEM 0·5% FCS with 300 pg/ml rHGF or with 105 macrophages in the presence or not of PHA-665732 (600 nM). (d) HGF protein concentrations measured by enzyme-linked immunosorbent assay (ELISA) in the culture supernatant of monocytes derived-macrophages or intestinal macrophages (IMACs). HGF concentrations were determined at 72 h of co-culture with wounded epithelial cells in DMEM 0·1% FCS. (e) Wounded Caco2 cells were seeded in DMEM 0·5% FCS with 105 macrophages isolated from human intestinal mucosa (IMACs). In negative (–) and positive (+) controls wounded Caco2 cells were seeded with DMEM 0·5% FCS or DMEM 10% FCS, respectively. (f) c-Met inhibitor inhibits IMACs-assisted repair. Wounded Caco2 cells were seeded in DMEM 0·5% FCS with 105 IMACs in the presence or not of PHA-665732 (600 nM). In negative (–) and positive (+) controls wounded Caco2 cells were seeded with DMEM 0·5% FCS or DMEM 10% FCS, respectively. Each dot represents a healthy donor. Comparison between several groups to identify a trend was performed by a one-way anova test with Trend test as post-test.
We next addressed whether promotion of wound repair by human monocyte-derived macrophages was cell contact-dependent and whether HGF was implicated in this process. Human monocyte-derived macrophages introduced into a cell culture insert still promoted repair (Fig. 3b). Again, HGF seemed to be playing a role in this process, as the addition of human recombinant HGF (rHGF) to wounded intestinal cells promoted repair (Fig. 3c). Consistently, HGF concentration in the supernatant of HD monocyte-derived macrophages co-cultivated with wounded epithelial cells averaged 150–200 pg/ml (Fig. 3d). Moreover, inhibition of the HGF signalling pathway with the c-met inhibitor resulted in a significantly lower promotion of repair by the human monocyte-derived macrophages (Fig. 3c).
It is well established that monocyte-derived macrophages are different phenotypically and functionally from IMACs [15,21]. We next investigated whether IMACs isolated from human colonic mucosa could promote epithelial repair. Importantly, similarly to monocyte-derived macrophages, IMACs were capable of producing comparable amounts of HGF (average 120 pg/ml) (Fig. 3d) and stimulated wound repair (Fig. 3e). Moreover, addition of the c-met inhibitor significantly inhibited the IMACs-mediated promotion of epithelial repair (Fig. 3f).
From these data we can conclude that both human monocyte-derived macrophages and intestinal macrophages promote epithelial repair actively through the secretion of HGF.
Monocyte-derived macrophages from CD patients are unable to promote wound repair and secrete less HGF
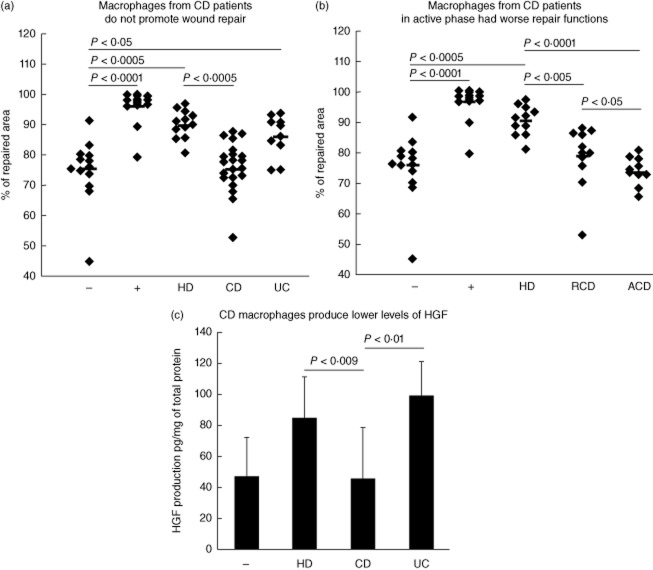
Because CD patients suffer from intestinal healing defects [22] and macrophage dysfunction [12–14], we evaluated the in-vitro repair activity of CD macrophages. Importantly, macrophages obtained from CD patients did not assist epithelial repair (Fig. 4a). This observation was specific to CD, as macrophages from patients suffering from another inflammatory bowel disease, ulcerative colitis (UC), kept their ability to promote wound healing (Fig. 4a). We next evaluated if macrophage repair activity was dependent upon the severity of the disease. Consistently, we found that epithelial repair was reduced more severely when macrophages were recovered from active CD patients (ACD) compared to macrophages recovered from patients in remission (RCD) (Fig. 4b; see Table 1 for patients' characteristics).
Fig. 4.
Macrophages from Crohn's disease (CD) patients are unable to promote wound repair. (a) Wounded Caco2 cells were seeded with Dulbecco's modified Eagle's medium (DMEM) 0·5% fetal calf serum (FCS)with 105 macrophages from healthy donors (HD, n = 14), Crohn's disease patients (CD, n = 22, 11 in remission and 11 in active phase of the disease) or ulcerative colitis patients (UC, n = 10, seven in remission and three in the active phase of the disease). In negative (–) and positive (+) controls wounded Caco2 cells were seeded with DMEM 0·5% FCS or DMEM 10% FCS, respectively. (b) Wounded Caco2 cells were seeded with DMEM 0·5% FCS with 105 macrophages of healthy donors (HD, n = 14), of Crohn's disease patients with disease in remission (RCD, n = 11) or with active disease (ACD, n = 11). (c) hepatocyte growth factor (HGF) protein concentrations measured by enzyme-linked immunosorbent assay (ELISA) and expressed as pg/mg of total protein in the culture supernatant of wounded Caco2 cells seeded with 105 macrophages from healthy donors (HD, n = 9), Crohn's disease (CD, n = 13) or ulcerative colitis (UC, n = 4) patients. HGF concentrations were determined at 72 h of culture. Comparison between several groups to identify a trend was performed by a one-way analysis of variance (anova) test with Trend test as post-test.
Having demonstrated a functional difference between HD and CD monocyte-derived macrophages, we wondered if this was associated with differences in cell survival and/or the phenotype of the two cell populations. Equal numbers of living HD and CD monocyte-derived macrophages were recovered following in-vitro differentiation (Supporting information, Fig. S5a), leading to the conclusion that the survival of HD and CD macrophages is roughly equivalent. We next analysed the expression of a panel of myeloid markers by flow cytometry [CD14, CD11b, CD11c, human leucocyte antigen D-related (HLA-DR), CD33, CD68, CD163 and CD206, the fractalkine receptor CX3CR1 and the GM-CSF receptor (CD116)]. CD14 (HD 24%, CD 42%), CD11b (HD 35%, CD 42%) and HLA-DR (HD 46%, CD 47%) showed the strongest expression, while CD16 was expressed to a lesser degree (HD 1%, CD 4%). Only CD33, CX3CR1 and CD116 were expressed at lower percentages by CD macrophages compared to HD (6, 6 and 13% versus 16, 15 and 24%, respectively), but this difference did not reach statistical significance (Supporting information, Fig. S5b). In addition, CD163 and CD206, two markers of M2 macrophages [6,23] were expressed similarly either by HD and CD macrophages (Supporting information, Fig. S5b). Overall, no phenotypic differences were identified when comparing macrophages from CD and HD patients, suggesting that defective epithelial repair in macrophages from CD patient depends essentially upon cell intrinsic factors.
Having demonstrated that HD macrophages promote epithelial repair through the secretion of HGF (Fig. 3), we next tested whether or not the defect of CD macrophages to promote epithelial repair is associated with a deficient HGF production. While HGF concentrations in the supernatant of HD and UC monocyte-derived macrophages were comparable, HGF concentrations were about 50% lower in the supernatant of CD monocyte-derived macrophages (means: HD: 84 ± 27; UC 99 ± 23; CD 45 ± 34 pg/mg of total protein) (Fig. 4c). We next evaluated whether or not a lower production of other factors such as EGF and/or TGF-β [24,25] might also contribute to the defective macrophage-assisted repair of CD patients. However, there were no statistically significant differences in active TGF-β and EGF concentrations following culture of monocyte-derived macrophages from HD, UC or CD patients (Supporting information, Fig. S6).
These results show that a correlation exists between HGF concentrations in the supernatant and the magnitude of the macrophage-assisted wound repair, and that CD monocyte-derived macrophages are deficient in HGF secretion.
Discussion
Intestinal wound repair is a fundamental process for intestinal homeostasis, and it has been shown to be modulated by macrophages in several in-vivo reports [8,9,26]. However, the mechanisms underlying macrophage-assisted wound repair remain elusive. In this study, we provide evidence that mouse and human macrophages assist repair by promoting epithelial cell restitution. In particular, HGF is identified as a key molecule mediating the macrophage-assisted intestinal epithelial repair. Importantly, we show that macrophages of CD patients are deficient in promoting epithelial repair.
Restitution is a key step in intestinal mucosal healing, as it allows the re-epithelialization of the intestinal epithelium following injury and cell loss [18,19]. Based on our results we can postulate, as has already been proposed for fibroblasts [27], that monocyte-derived macrophages and/or IMACs act on intestinal epithelial cells through the secretion of HGF to promote ulcer re-epithelialization. Several in-vivo and in-vitro reports clearly support the notion that HGF plays a key role in ulcer re-epithelialization [28,29]. Moreover, healing of intestinal ulcers has been reported to be inefficient in mice lacking HGF activity [30] and intestinal ulcer repair could be ameliorated through exogenous HGF therapy [31]. Hence, as IMACs (Fig. 3d) and macrophages infiltrating injured tissues during repair produce HGF [32,33], their ability to promote intestinal wound repair [8,9] might, at least in part, rely upon their capacity to secrete HGF and not TGF-β (Supporting information, Figs S2 and S6). Although we established clearly that macrophage-assisted repair is mediated by HGF secretion, this factor is probably not the only one playing a role in this process. Indeed, physical separation of macrophages from wounded epithelial cells partially inhibits their pro-repair activities, suggesting that a certain degree of cell contact might be beneficial (Fig. 2a). In addition, blocking HGF secretion or HGF signalling pathway only partially inhibited the macrophage-assisted repair (Figs 2d and 3d). Therefore, other uncharacterized factors produced by macrophages also probably contribute to macrophage-assisted repair.
We provide evidence that human macrophages differentiated from monocytes or isolated from the intestine of healthy donors (IMACs) promote wound repair. These observations are consistent with a recent report showing that HD monocyte-derived macrophages assist wound repair in vitro [23]. We observed that IMACs were less effective at promoting restitution than monocyte-derived macrophages (Fig. 3b,e). One possible explanation for this regards differences in the fitness of the cells, as intestinal macrophages, which had undergone extensive isolation procedures, were probably less fit than monocyte-derived macrophages. We observed that, like murine macrophages (Fig. 1), human resident intestinal and monocyte-derived macrophages assist repair through the production of HGF (Fig. 3c,d). These data, generated in an independent model of epithelial wound repair, reinforce our observation that macrophages assist re-epithelialization through the secretion of HGF and demonstrate that this macrophage function is conserved between mice and humans.
The use of the human epithelial restitution model allowed us to explore whether human pathologies characterized by wound healing defects, such as Crohn's disease, are associated with a deficiency of macrophage-assisted repair [34,35]. Consistently, we observed that CD monocyte-derived macrophages did not assist epithelial repair (Fig. 4a). This deficiency was more evident when macrophages came from patients experiencing a flare in their disease activity (Fig. 4b). Importantly, epithelial wound repair was conserved almost completely when monocyte-derived macrophages from UC patients were used (Fig. 4a) independently from disease activity (data not shown). This result suggests that the absence of macrophage-assisted wound repair activity is specific for CD and not a general feature of gastrointestinal inflammatory conditions.
Remarkably, CD macrophages produced lower concentrations of HGF, but similar concentrations of active TGF-β and EGF (Supporting information, Fig. S6Fig. S6), compared with HD and UC counterparts (Fig. 4d), suggesting that decreased macrophage HGF production could be a mechanism for the deficient repair activity of CD macrophages. The underlying molecular mechanisms for defective HGF production are still unclear. However, it is tempting to speculate that this results from aberrant secretion process in macrophages. In fact, Smith and co-authors have already shown that in CD, but not in UC macrophages, an abnormal proportion of cytokines are routed to lysosomes and degraded rather than being released through the normal secretory pathway [13]. As a result, one could hypothesize that secretory defects in macrophages affecting pro-repair factors might predispose CD patients to chronic intestinal ulcerations [35].
To conclude, our study demonstrates for the first time that mouse and human macrophages can assist intestinal epithelial repair by promoting epithelial cell restitution through HGF secretion. In addition, we demonstrate that CD macrophages produce lower concentrations of HGF and do not promote intestinal epithelial repair. We believe that a better characterization of the functioning and differentiation of macrophages promoting tissue repair should pave the way to developing novel treatment strategies for patient suffering from CD.
Acknowledgments
The authors would like to thank Daniel Bachmann, Catherine Pythoud and Julien Barroche for excellent technical support and Professor Gerhard Rogler (Zürich University, Switzerland) for helping us to isolate intestinal macrophages. This work was funded by the Swiss National Foundation grants 3200B0-120717 (to D. Velin), by the Fondation pour les maladies intestinales et hépatiques, Lausanne, Switzerland (to D. Velin) and by an unrestricted grant from UCB Pharma AG (to D. Velin).
Disclosure
None of the authors have a financial interest related to the work presented in this manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. F-actin purse string quantification by Photoshop software. Microphotographs were taken with a fluorescence microscope (Olympus IX81). The fluorescence intensity measured in square sections (arrowhead) defined along the leading edge of the migration front of epithelial cells was measured using Photoshop software. The quantified intensity of phalloidin staining reflects the F-actin purse string formation. At least five wounds per experimental condition were analysed.
Fig. S2. Neutralizing anti-transforming growth factor (TGF)-β antibodies did not reduce the properties of bone marrow-derived macrophages (BMM) significantly to assist epithelial repair. Epithelial repair was performed in the presence of hepatocyte growth factor (HGF) (0·25 ng/ml), TGF-β (1 ng/ml; R&D Systems), BMM (250 000) and/or anti-TGF-β neutralizing monoclonal antibody [20 μg/ml (1D11 clone)]. The data presented show the cumulated results of three independent experiments; bars are means ± standard error of the mean. P-values were calculated by the Mann–Whitney U-test.
Fig. S3. Impact of inflammatory stimuli on macrophage-assisted epithelial repair. One million heat-inactivated Escherichia coli or 10 ng/ml interleukin (IL)-1β were added to 250 000 bone marrow-derived macrophages (BMM) co-incubated with wounded epithelial cell monolayer for 18 h without insert. At the end of the incubation, we measured wound repair and HGF concentrations. The data presented show the cumulated results of three independent experiments; bars are means ± standard error of the mean. P-values were calculated by the Mann–Whitney U-test; n.d.: not done.
Fig. S4. Impact of inflammatory stimuli on human macrophage-assisted epithelial repair. Human monocyte-derived macrophages (MDM) were activated for 24 h with heat-inactivated Escherichia coli at 50 multiplicity of infection (MOI) and 105 were incubated with wounded epithelial cell monolayer. No statistical differences were found between the repair activities of activated or quiescent monocyte-derived macrophages.
Fig. S5. Viability and phenotypical characterization of Crohn's disease (CD) and healthy donor (HD) macrophages. (a) CD14-positive cells were selected by magnetic sorting from peripheral blood mononuclear cells and plated at density of 500 000–1 000 000 cells/ml for 5 days. On days 1 and 4, non-adherent cells were discarded and at day 5 adherent macrophages were recovered by scraping and numerated. The data show the percentage of recovered living macrophages compared to the initial number of plated CD14-positive cells. The data presented show the cumulated results of nine HD and nine CD patients; bars are means ± standard error of the mean. *No statistical difference (P-values were calculated by the Mann–Whitney U-test). (b) Macrophages were surface-stained with monoclonal antibodies to CD14 (clone M5E2; BD Biosciences), CD11b (clone ICRF44; BD Biosciences), CD11c (clone B-ly6; BD Biosciences), human leucocyte antigen D-related (HLA-DR) (clone G46-6; BD Biosciences), CD33 (clone WM53; BD Biosciences), CD68 (clone Y1/82A; BD Biosciences), CD116 (clone hGMCSFR-M1; BD Biosciences), CD163 (clone GHI/61; Biolegend), CD206 (clone 15.2; Biolegend), CX3CR1 (clone 2A9-1, MBL Nagoya, Japan). Data were acquired using a fluorescence activated cell sorter (FACS)scan flow cytometer (BD Biosciences) and analysed with CellQuest software (BD Biosciences). Dead cells were excluded by propidium iodide staining.
Fig. S6. Active transforming growth factor (TGF)-β and epidermal growth factor (EGF) levels in human co-cultures. Active TGF-β and EGF protein concentrations measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems) in the culture supernatant of monocytes derived-macrophages co-cultivated for 72 h with wounded epithelial cells in Dulbecco's modified Eagle's medium (DMEM) 0·1% fetal calf serum (FCS). No differences between TGF-β and EGF levels for healthy donors (HD) (eight individuals), Crohn's disease (CD) (11 individuals) and ulcerative colitis (UC patients) (four individuals) can be observed. Comparison between several groups to identify a trend was performed by a one-way analysis of variance (anova) test with Trend test as post-test.
References
- 1.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 4.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 5.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 6.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 8.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 9.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernasconi E, Favre L, Maillard MH, et al. Granulocyte–macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–441. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova JL, Abel L. Revisiting Crohn's disease as a primary immunodeficiency of macrophages. J Exp Med. 2009;206:1839–1843. doi: 10.1084/jem.20091683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogler G, Hausmann M, Vogl D, et al. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205–215. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vongsa RA, Zimmerman NP, Dwinell MB. CCR6 regulation of the actin cytoskeleton orchestrates human beta defensin-2- and CCL20-mediated restitution of colonic epithelial cells. J Biol Chem. 2009;284:10034–10045. doi: 10.1074/jbc.M805289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiese M, Castiglione K, Hensel M, Schleicher U, Bogdan C, Jantsch J. Small interfering RNA (siRNA) delivery into murine bone marrow-derived macrophages by electroporation. J Immunol Methods. 2010;353:102–110. doi: 10.1016/j.jim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50:S34–38. doi: 10.1007/s10620-005-2804-5. [DOI] [PubMed] [Google Scholar]
- 19.Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Christensen JG, Schreck R, Burrows J, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 21.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009;15:1295–1301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- 23.Vos AC, Wildenberg ME, Arijs I, et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis. 2012;18:401–408. doi: 10.1002/ibd.21818. [DOI] [PubMed] [Google Scholar]
- 24.Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock GE, Kaplan G, Cohn ZA. Keratinocyte growth regulation by the products of immune cells. J Exp Med. 1988;168:1395–1402. doi: 10.1084/jem.168.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am J Physiol. 1998;274:G809–818. doi: 10.1152/ajpgi.1998.274.5.G809. [DOI] [PubMed] [Google Scholar]
- 28.Nusrat A, Parkos CA, Bacarra AE, et al. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest. 1994;93:2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai M, Takahashi N, Miyazawa K, Kawaguchi M, Chijiiwa K, Kataoka H. Activation of MET receptor tyrosine kinase in ulcer surface epithelial cells undergoing restitution. Pathol Int. 2008;58:462–464. doi: 10.1111/j.1440-1827.2008.02255.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanayama M, Takahara T, Yata Y, et al. Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293:G230–239. doi: 10.1152/ajpgi.00068.2007. [DOI] [PubMed] [Google Scholar]
- 31.Numata M, Ido A, Moriuchi A, et al. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm Bowel Dis. 2005;11:551–558. doi: 10.1097/01.mib.0000164192.71381.5c. [DOI] [PubMed] [Google Scholar]
- 32.Amano H, Morimoto K, Senba M, et al. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol. 2004;172:398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 33.Lalive PH, Paglinawan R, Biollaz G, et al. TGF-beta-treated microglia induce oligodendrocyte precursor cell chemotaxis through the HGF-c-Met pathway. Eur J Immunol. 2005;35:727–737. doi: 10.1002/eji.200425430. [DOI] [PubMed] [Google Scholar]
- 34.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 35.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.