Abstract
Common variable immunodeficiency (CVID) is a primary immunodeficiency characterized by hypogammaglobulinaemia and recurrent infections. Although the underlying cause is unknown, B cells from most CVID patients fail to differentiate to memory or plasma cells. We investigated if increased apoptosis could influence the fate of B cells. For this purpose we activated purified B lymphocytes of CVID patients with a surrogate T-dependent (anti-CD40) or T-independent [cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) or anti-immunoglobulin (Ig)M)] stimulus with or without interleukin (IL)-21. We found that CD27+ B cells were more sensitive than CD27– B cells to spontaneous apoptosis and less sensitive to rescue from apoptosis. The addition of IL-21 down-modulated the protective effect of all the stimuli on CD27– B cells and the protective effect of CpG-ODN and anti-IgM on CD27+ B cells. In contrast, IL-21 rescued unstimulated CD27– B cells and improved the rescue of anti-CD40-stimulated CD27+ B cells. When we compared patients and controls, mainly CD27+ B cells from MB0 patients were less sensitive to rescue from apoptosis than those from MB1 patients and controls after activation, irrespective of the IL-21 effect. Increased apoptosis during an immune response could result in lower levels of immunoglobulin production in these patients.
Keywords: apoptosis, CD27+ B cells, CVID, IL-21, TRAIL
Introduction
Common variable immunodeficiency (CVID) is the most frequent symptomatic primary humoral immunodeficiency. It includes a heterogeneous group of disorders of unknown aetiology characterized by deficient antibody production, recurrent respiratory infections by encapsulated bacteria, mainly Streptococcus pneumoniae and Haemophilus influenzae, and poor response to vaccination. Patients benefit from immunoglobulin replacement therapy [1–4]. Several genetic mutations and polymorphisms [inducible T cell co-stimulator (ICOS), tumour necrosis factor receptor superfamily, member 13b (TNFRS13B/TACI), CD19, CD20, CD81, B cell-activating factor receptor (BAFF-R) and CD21] have been described in fewer than 10% of CVID patients, while the underlying molecular defect remains unknown for most of them [5–7]. A vast array of immunological defects have been described in CVID patients, of which the most outstanding is abnormal late B cell differentiation to CD27+ memory B cells, switched-memory B cells and plasma cells. Accordingly, patients have been classified depending on their number of naive, memory and switched-memory B cells [8,9]. Furthermore, a low percentage of memory B cells in CVID patients has been associated with a worse clinical presentation and poor response to vaccines [10–12]. Loss of memory B cells also occurs from the onset of acute HIV infection. Recently, low frequencies of CD27+ memory B cells and decreased production of antibodies have been described in successfully treated HIV patients in spite of drug-suppressed viraemia. Surface expression levels of TNF-related apoptosis-inducing ligand (TRAIL) on memory B cells correlated negatively with their peripheral blood frequency [13].
The generation of memory B cells and plasma cells is essential to establish efficient humoral immune responses. Co-operation of B cell receptor (BCR)-activated B cells with helper T cells is relevant and occurs through contact between T cell membrane molecules (CD40L, ICOS, etc.) and their corresponding B cell ligands [14]. The importance of several of these components of the immune system has been exemplified by naturally occurring immunodeficiencies [15]. Furthermore, secretion of cytokines by T cells also instruct the differentiation of B cells, including interleukin (IL)-21 as one of the more potent cytokines for human B cell proliferation and differentiation [16–20]. Following antigenic stimulation, Toll-like receptor (TLR) can provide an additional signal for the differentiation of B cells and even substitute T cell-derived signals [21,22].
Apart from their effect on proliferation and differentiation, several of these stimuli also influence B cell survival. BCR activation has been shown to induce B cell apoptosis in the absence of survival signals such as that provided through CD40. Mainly produced by activated CD4+ follicular T cells [19,23,24], IL-21 is a type I cytokine that belongs to a family that uses the common cytokine receptor γ-chain as a component of their receptors [25,26]. The stimulatory or inhibitory effect of IL-21 depends on the maturation and activation status of the B cell, the co-stimulatory accompanying signal and the presence of other cytokines. In humans, IL-21 is a potent inductor of plasma cell differentiation if combined with anti-CD40 [16], induces class-switch recombination and secretion of immunoglobulin (Ig)G and IgA in pre-switched IgM memory B cells [19,27] and is able to induce plasma cell differentiation and immunoglobulin production even by naive B cells [16]. However, IL-21 triggers B cell death when BCR is ligated [16,28]. A balance between apoptosis-inducing and survival signals must exist to preserve B cell homeostasis.
We have shown previously that, although acting through different pathways, neither cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) nor anti-CD40 with IL-21 stimulation are able to induce B cells from CVID patients to produce normal levels of immunoglobulins, and this defect is not corrected by the addition of anti-IgM [29]. This could imply a signalling defect in both pathways or, alternatively, it could indicate that B cells from CVID patients die in culture after stimulation. In this study we evaluated the effect of IL-21 on spontaneous and TLR-9-, CD40- or BCR-induced apoptosis or proliferation of CD27– and CD27+ B cells from CVID patients. The aim of the study was to ascertain if differences in response between controls and patients could determine a different fate of CD27– and CD27+ B cells and explain the imbalanced B cell homeostasis and finally immune deficiency in CVID patients.
Methods
Patients
Twenty-two CVID patients were selected according to diagnostic criteria of the International Union for Immunological Societies scientific group for primary immunodeficiency diseases. Patients were classified into three groups according to Piqueras et al. [8]: (i) CVID patients with a low percentage of CD27+ (memory phenotype) B cells or MB0; (ii) patients with normal IgD+CD27+ (non-switched-memory phenotype) and a low percentage of IgD–CD27+ (switched-memory phenotype) B cells or MB1; and (iii) patients with normal percentages of CD27+ B cells or MB2. Patients received intravenous gamma globulin therapy every 21 days and did not suffer from infections at the time of the study. Peripheral blood samples were collected before gamma globulin replacement. Table 1 summarizes the patients' age, gender and percentages of B cell subpopulations. Twenty-two age- and sex-matched healthy blood donors were included as controls. The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by CEIC (Balearic Islands Clinical Research Ethics Committee; IB 1564/11 PI). Informed consent was obtained from all subjects.
Table 1.
Age, gender and B lymphocyte subpopulations of common variable immunodeficiency (CVID) patients
| Patient | Age (years) | Gender (male/female) | CD19 (%) | CD19+ (%) | Group | ||
|---|---|---|---|---|---|---|---|
| Naive IgD+CD27− | Unswitched memory IgD+ CD27+ | Switched memory IgD−CD27+ | |||||
| 1 | 43 | M | 5 | 94 | 5 | <1 | MB0 |
| 2 | 61 | M | 8 | 96 | 3 | <1 | MB0 |
| 3 | 29 | M | 15 | 95 | <1 | <1 | MB0 |
| 4 | 29 | F | 9 | 87 | 6 | 2 | MB0 |
| 5 | 61 | F | 19 | 84 | 5 | 5 | MB0 |
| 6 | 60 | F | 5 | 95 | 1 | 1 | MB0 |
| 7 | 60 | M | 2 | 92 | 3 | <1 | MB0 |
| 8 | 67 | F | 5 | 96 | 3 | <1 | MB0 |
| 9 | 34 | F | 16 | 70 | 2 | 3 | MB0 |
| 10 | 38 | M | 4 | 90 | 5 | 1 | MB0 |
| 11 | 23 | M | 14 | 91 | 4 | <1 | MB0 |
| 12 | 27 | F | 8 | 97 | 2 | <1 | MB0 |
| 13 | 73 | F | 27 | 72 | 27 | <1 | MB1 |
| 14 | 67 | F | 14 | 64 | 25 | 4 | MB1 |
| 15 | 67 | F | 7 | 73 | 18 | 5 | MB1 |
| 16 | 46 | F | 16 | 77 | 20 | 1 | MB1 |
| 17 | 47 | F | 9 | 75 | 17 | 5 | MB1 |
| 18 | 33 | F | 9 | 84 | 8 | 4 | MB1 |
| 19 | 60 | F | 26 | 76 | 7 | 6 | MB1 |
| 20 | 67 | F | 16 | 84 | 12 | 1 | MB1 |
| 21 | 82 | F | 2 | 39 | 14 | 33 | MB2 |
| 22 | 44 | F | 20 | 52 | 10 | 20 | MB2 |
Current age (years), gender (M: male, F: female); percentage of peripheral blood B lymphocytes (CD19+) and percentages of naive [immunoglobulin (Ig)D+CD27−], memory unswitched (IgD+CD27+) and memory-switched (IgD−CD27+) B cell subpopulations (referred to total CD19+ B lymphocytes) and Piqueras classification of CVID patients.
B lymphocyte purification, CD27–and CD27+ B cell sorting and cell culture
Peripheral blood mononuclear cells (PBMC) were isolated from 40 ml of heparinized blood by density gradient centrifugation. B lymphocytes were obtained from PBMC by negative selection using the Dynabeads Untouched™ human B cells separation kit (Dynal; Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. CD27– and CD27+ B cells were sorted from 4 × 106 purified B cells using a Coulter Epics Altra HypersortTM system (Beckman Coulter, Brea, CA, USA). Purified B cells or sorted CD27– and CD27+ B cells were resuspended in RPMI-1640 medium supplemented with 10% heat inactivated fetal calf serum (FCS), glutamine (2 mM) and antibiotics (penicillin and streptomycin). Purified B cells (1 × 106/ml) were labelled during 5 min at room temperature (RT) (25°C) with 1 μg carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen), following the manufacturer's instructions.
CFSE-free purified B cells, 5 × 104, or sorted CD27– and CD27+ B cells and CFSE-labelled purified B cells were cultured in 96-well plates and stimulated with CpG-ODN (0·6 μg/ml; CpG-ODN type B; InvivoGen, San Diego, CA, USA), F(ab)2 goat anti-human IgM (5 μg/ml; Jackson ImmunoResearch, West Grove, PA, USA), anti-human CD40/TNFRSF5 antibody (1 μg/ml; R&D Systems, Abingdon, UK) without or with human recombinant IL-21 (100 ng/ml; Biosource, Cambridge, MA, USA). Cultures were maintained for 3 days at 37°C in a 5% CO2 atmosphere.
Flow cytometry
B cell purity, apoptosis, proliferation and surface marker expression were analysed by flow cytometry using an Epics FC500 flow cytometer and the CXP software (Beckman Coulter).
Cell purity was assessed using the following monoclonal antibody combinations: anti-CD45 fluorescein isothiocyanate (FITC), anti-CD19 phycoerythrin cyanin 5 (PCy5) (both from Coulter Immunotech) and anti-CD3 phycoerythrin (PE) (Becton Dickinson, Franklin Lakes, NJ, USA) for purified B cells and anti-CD19 PCy7 plus anti-CD27 PCy5 (both from Coulter Immunotech) for sorted CD27– and CD27+ B cells. Purity was always superior to 95%.
Annexin V and propidium iodide staining protocol (Becton Dickinson) was performed to evaluate apoptosis of CSFE-free purified (Fig. 1a) and sorted CD27– and CD27+ B cells (Fig. 1b,c), following the manufacturer's instructions. Briefly, 1 × 105 cultured CFSE-free cells were harvested, stained with anti-CD19 PCy7 and anti-CD27 PCy5, washed with cold phosphate-buffered saline (PBS), resuspended in 100 μl binding buffer and stained with 5 μl of a 1·2 μg/ml solution of annexin V-FITC and 5 μl of a 50 μg/ml solution of propidium iodide. Cells were incubated for 15 min at RT (25°C) in the dark, resuspended in 400 μl of binding buffer and analysed. Propidium iodide positivity was used to exclude necrotic CD19+ cells and percentage of apoptotic cells (annexin V-FITC-positive) was calculated from the resulting population. Rescue from apoptosis was expressed as [(% baseline apoptosis − % post-stimulation apoptosis)/% baseline apoptosis] × 100, to indicate the decrease in apoptosis induced by each stimulus related to baseline apoptosis.
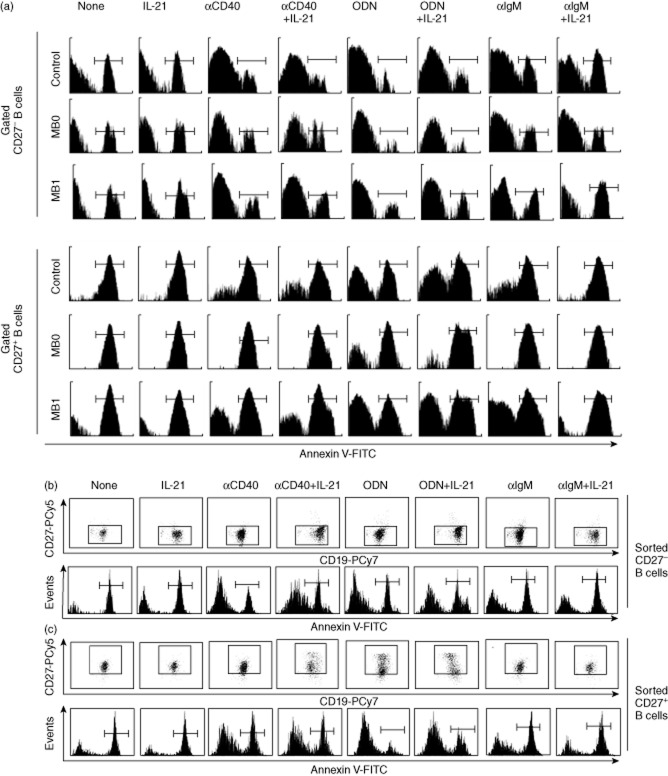
Fig. 1.
Representative experiments of spontaneous and post-activation apoptosis of CD27– and CD27+ B cells. Purified B cells from common variable immunodeficiency (CVID) patients and controls or sorted CD27– and CD27+ B cells from controls were stimulated with different combinations of CD40, Toll-like receptor (TLR)-9, B cell receptor (BCR) ligands or interleukin (IL)-21. (a) Purified B cells: markers in histograms differentiate apoptotic [annexin V-fluorescein isothiocyanate (FITC)-positive] from viable (annexin V-FITC-negative) cells on propidium iodide-free CD27– (upper panel) or CD27+ (lower panel) gated cells. Histograms show a representative experiment from a healthy control (upper row) and CVID MB0 (middle row) and CVID MB1 (lower row) patients. (b–c) Sorted cells: CD27 expression (upper dot-plots rows) and apoptosis (lower histograms rows) of propidium iodide-free CD27– (b) or CD27+ (c) sorted B cells. Dot plots and histograms are representative from three independent experiments performed on healthy controls.
A CFSE dilution protocol was used to evaluate the proliferation of CFSE-labelled cultured purified B cells. Proliferation index was calculated on CD19+CD27– or CD19+CD27+ stained B cells attending to the number of divisions and the percentages of cells in each round of division, as described previously by Quah et al. [30].
TRAIL expression was evaluated in whole blood samples stained with anti-CD19 energy-coupled dye (ECD), anti-CD27 PCy7 (both from Coulter Immunotech) and anti-TRAIL-PE (Becton Dickinson)-conjugated monoclonal antibodies. TRAIL median fluorescence intensity (MFI) was measured in previously gated CD19+CD27– and CD19+CD27+ B cells.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4·0 software (San Diego, CA, USA). Data are expressed as median and 25th and 75th percentiles. The Mann–Whitney U-test was used to compare differences between B cells subpopulations. The Kruskal–Wallis test was used to compare differences between CVID patients groups and controls. The Wilcoxon test was used to compare differences between two paired groups of treatments (each stimulus with or without IL-21). A statistical test based on measures of central tendency comparison was not applicable to the particular case of anti-IgM combined with IL-21. A P-value less than 0·05 was considered statistically significant.
Results
CD27+ B cells are less sensitive to apoptosis rescue by single stimulus than CD27– B cells
B cells die from apoptosis if maintained unstimulated in culture [31]. After 3 days, spontaneous apoptosis was higher in CD27+ than in CD27– B cells (79·2 versus 57·6%, P < 0·001) (Fig. 2a).
Fig. 2.
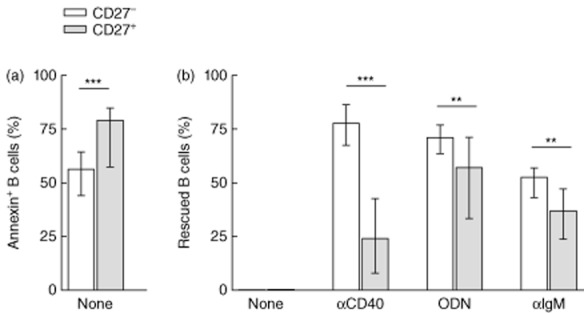
Spontaneous apoptosis and anti-CD40, cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) or anti-immunoglobulin (Ig)M activation-induced rescue from apoptosis of peripheral CD27– and CD27+ B cells from healthy controls. (a) Percentage of apoptotic [annexin V-fluorescein isothiocyanate (FITC)-positive/propidium iodide (PI)-negative) CD27– (white bars) and CD27+ (grey bars) unstimulated B cells. (b) Percentage of rescued CD27– and CD27+ B cells upon stimulation with anti-CD40, CpG-ODN or anti-IgM. Data are given as median and 25th to 75th percentiles from 22 independent experiments (Mann–Whitney U-test P-values: P < 0·01**; P < 0·001***).
When B cells are stimulated, they are rescued from apoptosis. The effectiveness of the rescue depends upon both the kind of stimulus used and the subpopulation of B cells. For CD27– B cells, the strongest rescue effect was induced by anti-CD40 followed by CpG-ODN and to a lesser extent by anti-IgM, whereas for CD27+ B cells, CpG-ODN appeared to be the strongest rescue stimulus (Fig. 2b). Nevertheless, all the stimuli evaluated were more efficient in the CD27– than in the CD27+ population: anti-CD40 (77·9 versus 23·9%, P < 0·001), CpG-ODN (71·4 versus 57·3%, P < 0·01) and anti-IgM (52·7 versus 36·9%; P < 0·01) (Fig. 2b).
Proliferation was evaluated simultaneously. Anti-CD40 and anti-IgM did not induce proliferation of either CD27– or CD27+ B cells while CpG-ODN induced proliferation of both subpopulations (Table 2). Although CpG-ODN induced a lower level of proliferation on CD27– than CD27+ B cells (PI = 0·1 versus PI = 1·8, respectively; P < 0·001) (Table 2), it induced higher rescue from apoptosis in the CD27– population (Fig. 2b). These aforementioned results suggest that proliferation and rescue from apoptosis are two independent processes.
Table 2.
Effect of interleukin (IL)-21 on CD27− and CD27+ B cell proliferative responses
| CD27− B cells | P-value | CD27+ B cells | P-value | CD27− versus CD27+ P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Lone | + IL-21 | Lone | + IL-21 | Lone | + IL-21 | |||
| Anti-IgM | 0·0 | 0·0 | n.s. | 0·0 | 0·0 | n.s. | n.s. | n.s. |
| Anti-CD40 | 0·0 | 0·4 | *** | 0·0 | 1·0 | *** | n.s. | ### |
| CpG-ODN | 0·1 | 0·2 | ** | 1·8 | 1·1 | *** | ### | ### |
Proliferation index after anti-immunoglobulin (Ig)M, anti-CD40 or cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) activation with or without IL-21 co-stimulation in CD27− and CD27+ B cells from healthy controls (n = 19). Proliferation index was calculated as described in the flow cytometry section and takes into account both the number of divisions and the percentage of cells in each round of division. Wilcoxon's test (left grid) was applied to evaluate the effect of IL-21 addition to each stimulus on CD27− or CD27+ B cells (P values: **P < 0·01; ***P < 0·001; n.s.: not significant). Mann-Whitney test (right grid) was used to compare differences between B cell subpopulations proliferative responses to single or IL-21-combined stimuli (P-values: ###P < 0·001; n.s.: not significant).
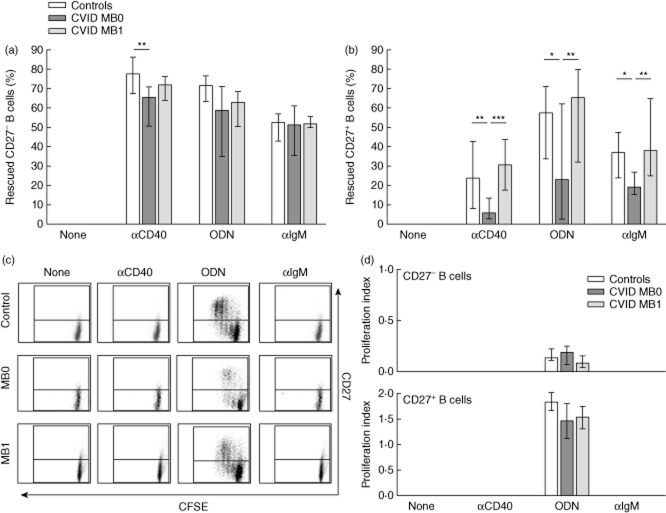
CD27– and CD27+ B cells from CVID MB0 patients are less sensitive to apoptosis rescue by single stimulus
CD27– B cells from CVID MB0 patients were less sensitive to rescue from apoptosis when stimulated with a T-dependent stimulus (anti-CD40) than control subjects (65·4 versus 77·9%, P < 0·05) (Fig. 3a). They were also less sensitive to rescue from apoptosis when stimulated with a T-independent stimulus (CpG-ODN) than control subjects or CVID MB1 patients, although differences did not reach statistical significance (58·8 versus 71·4 and 63·0%, respectively, P = 0·075). CD27– B cells from CVID MB1 patients were rescued from apoptosis similarly to controls, regardless of the stimulus used (Fig. 3a). After BCR engagement with anti-IgM CD27– B cells from both CVID MB0 and MB1, patients were rescued equally from apoptosis than healthy controls.
Fig. 3.
Activation-induced rescue from apoptosis and proliferation of peripheral CD27– and CD27+ B cells from common variable immunodeficiency (CVID) patients and healthy controls. (a) Percentage of rescued CD27– and (b) CD27+ B cells upon stimulation with anti-CD40, cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) or anti-immunoglobulin (Ig)M in healthy controls (n = 22; white bars), CVID MB0 patients (n = 12; dark grey bars) and CVID MB1 patients (n = 8; light grey bars). (c) Representative dot-plots with dividing and non-dividing CD19+CD27–carboxyfluorescein succinimidyl ester (CFSE)+ (lower quadrants) and CD19+CD27+CFSE+ (upper quadrants) B cells from a healthy control (upper row), one CVID MB0 patient (middle row) and one CVID MB1 patient (lower row) after stimulation with anti-CD40, CpG-ODN or anti-IgM. (d) Proliferation index after anti-CD40, CpG-ODN or anti-IgM activation of CFSE-labelled CD27– (upper panel) and CD27+ (lower panel) B cells from healthy controls (n = 19; white bars), CVID MB0 patients (n = 8; dark grey bars) and CVID MB1 patients (n = 6; light grey bars). No proliferation was detected with anti-CD40 and anti-IgM. Data are given as median and 25th to 75th percentiles (Kruskal–Wallis test P-values: P < 0·05*; P < 0·01**; P < 0·001***).
CD27+ B cells from CVID MB0 patients, stimulated with either a T-dependent (anti-CD40) or a T-independent stimulus (CpG-ODN), were less sensitive to apoptosis rescue than control subjects (6·0 versus 23·9%, P < 0·01; and 23·2 versus 57·3%, P < 0·05, respectively) and CVID MB1 patients (6·0 versus 30·6%, P < 0·001; and 23·2 versus 65·7%, P < 0·01, respectively). They were also less sensitive to rescue from apoptosis after BCR engagement with anti-IgM than control subjects (19·2 versus 36·9%, P < 0·05) or CVID MB1 patients (19·2 versus 38·2%, P < 0·01) (Fig. 3b). With either stimulus CD27+ B cells from CVID MB1 patients were rescued from apoptosis similarly to controls (Fig. 3b).
Thus, CD27+ B cells from CVID MB0 patients appear to be resistant to apoptosis rescue irrespective of the stimulus. This was not linked to differences in proliferation because both CD27– and CD27+ B cells from CVID MB0 patients proliferated similarly to controls and CVID MB1 patients (Fig. 3c,d).
IL-21 modulates apoptosis rescue of co-stimulated CD27– and CD27+ B cells
IL-21 alone was able to rescue CD27– (16·9%) but not CD27+ B cells from spontaneous apoptosis (Figs 1a and 4). In spite of this, the addition of IL-21 down-modulated the protective effect of anti-CD40 (77·9 versus 75·9%, P < 0·01) and CpG-ODN (71·4 versus 42·7%, P < 0·001) on CD27– B cells.
Fig. 4.
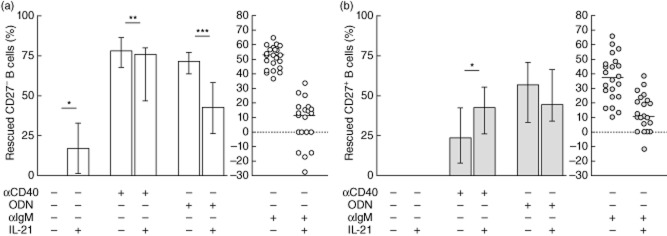
Interleukin (IL)-21 effect on spontaneous apoptosis and activation-induced rescue from apoptosis of peripheral CD27– and CD27+ B cells from healthy controls. (a) Percentage of rescued CD27– and (b) CD27+ B cells upon stimulation with IL-21 alone or in combination with anti-CD40, cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) or anti-immunoglobulin (Ig)M. Data are given as median and 25th to 75th percentiles from 22 independent experiments (Wilcoxon's test P-values: P < 0·05*; P < 0·01**; P < 0·001***).
In CD27+ B cells IL-21 tended to reduce the CpG-ODN rescue effect but increased the protective effect of anti-CD40 significantly (23·9 versus 42·8%, P < 0·05) (Figs 1a and 4b).
IL-21 not only reverted the protective effect of anti-IgM on CD27– and CD27+ B cells, but in some cases even increased apoptosis above spontaneous baseline values (Fig. 1a and scatter-plots in Fig. 4).
Similar results were obtained when we evaluated activation induced rescue from apoptosis on sorted CD27– and CD27+ B lymphocytes stimulated with the same stimuli (histograms in Fig. 1b,c). Moreover, we did not find increased CD27 expression when we stimulated CD27– B cells with any of the stimuli (dot-plots in Fig. 1b), which validates the gating strategy when using purified total B cells.
Effect of IL-21 on apoptosis is not linked to proliferation
IL-21 modulates proliferation induced by co-stimulation on CD27– and CD27+ B cells. This effect has to be taken into account when analysing the apoptosis rate.
Neither CD27– nor CD27+ B cells proliferated in response to anti-IgM combined with IL-21 (Table 2). However, both subpopulations proliferated in response to IL-21 with anti-CD40, although the proliferation index was higher in CD27+ B cells. Remarkably, IL-21 increased proliferation of CpG-ODN-activated CD27– B cells but decreased proliferation of CpG-ODN-activated CD27+ B cells (Table 2).
In CD27+ B cells, IL-21 reduction of CpG-ODN apoptosis rescue is accompanied by a reduction in the proliferative response. In contrast, the increase in anti-CD40 apoptosis rescue is accompanied by a proliferation enhancement (Fig. 4b and Table 2). However, IL-21 reduction in apoptosis rescue induced by anti-CD40 or CpG-ODN on CD27– B cells is not due to a negative effect on proliferation (Fig. 4a and Table 2). Furthermore, in spite of the higher proliferative response induced by IL-21 combined with anti-CD40 or CpG-ODN on CD27+ versus CD27– B cells (Table 2), the rescue from apoptosis is not higher in CD27+ B cells for any of the stimulus (Fig. 4). Thus, although we cannot rule out that the effect of IL-21 on apoptosis is linked to proliferation, our results support the independence of these processes.
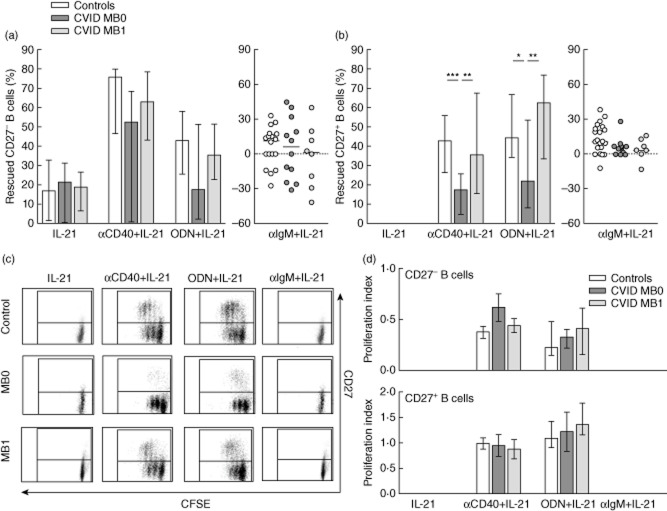
CD27– and CD27+ B cells from CVID MB0 patients are less sensitive to apoptosis rescue by IL-21 combined with a single stimulus
IL-21 alone rescued both CVID MB0 and MB1 CD27– B cells similar to controls. CD27– B cells from CVID MB0 were less sensitive to apoptosis rescue by anti-CD40 plus IL-21 or CpG-ODN plus IL-21 than controls or CVID MB1 patients, although these differences were not statistically significant (Fig. 5a).
Fig. 5.
Interleukin (IL)-21 effect on apoptosis rescue and proliferation of activated peripheral CD27– and CD27+ B cells from common variable immunodeficiency (CVID) patients. (a) Percentage of rescued CD27– and (b) CD27+ B cells upon stimulation with IL-21 alone or in combination with anti-CD40, cytosine–phosphate–guanosine oligodeoxynucleotides (CpG-ODN) or anti-immunoglobulin (Ig)M in healthy controls (n = 22; white bars/dots), CVID MB0 patients (n = 12; dark grey bars/dots) and CVID MB1 patients (n = 8; light grey bars/dots). (c) Representative dot-plots of dividing and non-dividing CD19+CD27–carboxyfluorescein succinimidyl ester (CFSE)+ (lower quadrants) and CD19+CD27+CFSE+ (upper quadrants) B cells from a healthy control (upper row), one CVID MB0 (middle row) and one MB1 (lower row) patient, after stimulation with IL-21 alone or in combination with anti-CD40, CpG-ODN or anti-IgM. (d) Proliferation index of CFSE-labelled CD27– (upper panel) and CD27+ (lower panel) B cells from healthy controls (n = 19; white bars), CVID MB0 (n = 8; dark grey bars) and MB1 (n = 6; light grey bars) patients after stimulation with IL-21 alone or in combination with anti-CD40, CpG-ODN or anti-IgM. No proliferation was detected with IL-21 and anti-IgM + IL-21. Data are given as median and 25th to 75th percentiles (Kruskal–Wallis test P-values: P < 0·05*; P < 0·01**; P < 0·001***).
CD27+ B cells from CVID MB0 patients were less sensitive to apoptosis rescue when stimulated with anti-CD40 and IL-21 or CpG-ODN and IL-21 than control subjects (17·6 versus 42·8%, P < 0·001; and 21·9 versus 44·4%, P < 0·05, respectively) and CVID MB1 patients (17·6 versus 35·8%, P < 0·01; and 21·9 versus 62·5%, P < 0·01, respectively).
CD27– and CD27+ B cells from CVID MB1 (Fig. 5b) patients were rescued from apoptosis similarly to controls.
IL-21 not only abrogated the protective effect induced by anti-IgM, but increased the percentage of apoptotic B cells both in controls and CVID patients irrespective of their group (Fig. 5a,b).
When we evaluated the proliferation index, we did not find differences between CVID patients and controls (Fig. 5c,d). Thus, again, differences of apoptosis rescue between CD27+ B cells from CVID MB0 patients and controls cannot be attributed to differences on B cell proliferation (Fig. 5).
CD27+ B cells from MB0 patients show higher TRAIL expression than controls and MB1 patients
Higher expression of TRAIL has been related to apoptosis and loss of peripheral memory B cells (identified as CD27+) in successfully treated aviraemic HIV patients. We evaluated if differences in TRAIL expression on CD27+ B cells from CVID MB0 patients could explain the observed resistance to apoptosis rescue. CD27– B cells from CVID MB0 and MB1 patients showed similar TRAIL expression than controls (Fig. 6). However, CD27+ B cells from CVID MB0 patients showed higher TRAIL expression than controls (2·8 versus 1·6 MFI; P < 0·001) or MB1 patients (2·8 versus 1·7 MFI, P < 0·001). We did not find differences in CD27+ B cells from CVID MB1 when compared to controls (Fig. 6).
Fig. 6.
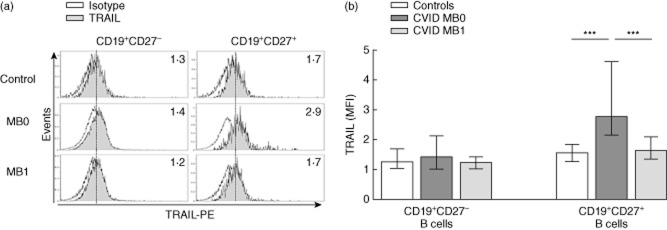
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) surface expression on peripheral blood B cells from common variable immunodeficiency (CVID) patients and healthy controls. (a) Representative histograms of TRAIL expression (grey) on CD19+CD27– (left columns) and CD19+CD27+ (right columns) B cells from a healthy control (upper row), one CVID MB0 (middle row) and one CVID MB1 (lower row) patients. Numbers indicate TRAIL median fluorescence intensity (MFI) evaluated on a 3-decade log scale. (b) TRAIL MFI of CD19+CD27– and CD19+CD27+ B cells from healthy controls (n = 18; white bars), CVID MB0 (n = 9; dark grey bars) and MB1 (n = 6; light grey bars) patients. Data are given as median and 25th to 75th percentiles (Kruskal–Wallis test P-values: P < 0·001***).
Discussion
The B cell fate is determined by the nature of the antigen encountered and a combination of signals provided through membrane co-receptors or by secreted interleukins encountered in the lymphoid compartment. Unsuccessfully stimulated B cells die from apoptosis. Survival, growth and differentiation signals are required to maintain B cell homeostasis and to induce their differentiation into effector subsets.
In this study, we show that CD27+ B cells are less sensitive to rescue from apoptosis than CD27– B cells, irrespective of the stimulus used. Although IL-21 rescues unstimulated CD27– B cells from spontaneous apoptosis and increases the protective effect of anti-CD40 in CD27+ B cells, it reduces the protective effect of most stimuli used in both CD27– and CD27+ B cells. When we evaluate CVID patients, we observe that CD27+ B cells from MB0 patients are less sensitive to rescue from apoptosis than B cells from MB1 patients and normal controls after anti-CD40 or CpG-ODN stimulation. These differences are not restored by the addition of IL-21. This is in agreement with the higher TRAIL expression observed in CVID MB0 patients.
The effect of IL-21, one of the most important cytokines for B cell differentiation, depends upon the subset of B cell studied and accompanying co-stimulus [28]. Jin et al. [32] demonstrated that besides strain differences in mice, the context in which B cells were activated influenced their fate. IL-21-driven apoptosis and inhibition of proliferation were dominant when B cells were activated through TLR-4 and TLR-9. Co-stimulation and low apoptosis were observed in B cells stimulated with anti-IgM or anti-IgM plus anti-CD40, whereas both apoptosis and co-stimulation were detected when IL-21 acted on anti-CD40 previously activated B cells. This raised the possibility that different subsets of B cells responded differentially to IL-21. In our hands, although IL-21 rescues unstimulated CD27– B cells from spontaneous apoptosis, it reduces the protective effect of most of the stimuli both in CD27– and CD27+ B cells. On the contrary, IL-21 increases the protective effect of anti-CD40 in CD27+ B cells. This suggests that IL-21 per se increases survival of CD27– (mostly naive and transitional) B cells, but this effect is lost after these cells are activated. However, CD27+ B cells become sensitive to rescue from apoptosis if they are prestimulated with a surrogate T-dependent stimulus (anti-CD40). Stimulation through the BCR or with a T-independent stimulus (CpG-ODN) renders CD27+ B cells insensitive to the protective effect of IL-21. IL-21 acts as a checkpoint for a productive B cell response. Only memory and marginal zone B cells (contained in the CD27+ population) that receive cognate T cell help in the presence of IL-21 would be protected from apoptosis and directed to proliferation and eventually differentiation to antibody secreting cells. We also report that rescue from apoptosis is independent of proliferation. This is particularly evident with anti-CD40 that, although it does not induce proliferation, it rescues most CD27– B cells from apoptosis.
Our present results support that the inability of CVID B cells to produce normal levels of immunoglobulins in vitro (and in vivo) can be the consequence of an increased susceptibility to apoptosis upon stimulation. That would result in a reduced number of cells during an immune response. CD27–, but particularly CD27+ B cells, from our CVID MB0 patients are less sensitive to rescue from apoptosis than MB1 patients and controls.
Moreover, CD27+ B cells from CVID MB0 patients showed significantly higher expression of TRAIL than controls or CVID MB1 patients. TRAIL is a member of the TNF superfamily of cytokines able to induce programmed cell death in tumour cells. Different subpopulations of B cells show distinct sensitivity to TRAIL-mediated apoptosis. BCR triggering sensitizes peripheral blood memory, but not naive human B cells, to TRAIL-mediated apoptosis [33] and TRAIL promotes death of normal plasma cells [34]. In agreement with our results, van Grevenynghe et al. [13] demonstrated that memory B cell survival was decreased significantly in aviraemic successfully treated (ST) HIV subjects compared with uninfected controls. Memory B cells (identified as CD19+CD27+) from ST subjects showed specifically higher expression levels of TRAIL that correlated negatively with the frequency of these cells. Production of immunoglobulins was lower in ST subjects as a result of reduced survival and not lower proliferation of B cells.
Increased apoptosis of B cells in the MB0 group can result in fewer cells developing into antibody-secreting cells upon stimulation, hypogammaglobulinaemia and poor humoral response to antigens. For CVID MB1 patients a different mechanism should be responsible, because their B cells behave like control B cells in their sensitivity to apoptosis. This holds true for the two evaluated CVID MB2 patients. Their B cell apoptosis rescue was similar to CVID MB1 patients and controls (data not shown). In a recent paper, Borte et al. [35] suggested that IL-21 restores immunoglobulin production in patients with CVID. Using purified B cells, they found that IL-21 reduced apoptosis from naive and memory B cells from 14 CVID patients. However, no CVID group distinction was made; stimulation with anti-CD40 and IL-21 also included IL-4, and they considered only the CD27– naive and CD27+ IgD– memory B cell populations (excluding CD27+IgD+). The proportion of MB1/MB2 to MB0 patients in their studied cohort might have influenced the final result and explain the apparently distinct conclusions.
We cannot exclude the possibility that the peripheral blood B cells with increased apoptosis found in CVID MB0 could be the result of incomplete activation by follicular CD4+ T cells. In keeping with this, Hagn et al. [36] have demonstrated that human B cells co-cultured with incompletely activated CD4 T cells that secrete IL-21, but do not express CD40L, differentiate into granzyme B (GzmB)-secreting and potentially cytotoxic cells, able to induce slowly developing apoptosis of several cell lines. Activation of human B cells by IL-21 and BCR engagement in the absence of CD40 ligation results in their differentiation into GzmB-secreting cytotoxic cells rather than into plasma cells.
In summary, our findings reinforce the fact that (in humans) the net effect of different stimuli on B cells depends upon both the B cell subpopulation studied and the activation status of the B cell and underscore the relevance of these features in CVID physiopathology. We suggest that higher levels of apoptosis of CVID MB0 CD27+ B cells during an immune response can result in lower levels of immunoglobulin production, irrespective of their proliferation. The results highlight the heterogeneity among CVID patients, where distinct molecular mechanisms underlie common clinical symptoms, and highlight the need to classify and study CVID patients separately when evaluating B cell responses.
Acknowledgments
A.C., J.P., N.L. and J.M.F. designed and performed the experiments and analysed the data. N.M. and J.P. contributed to patient selection. All authors contributed to writing the manuscript. We thank Guillem Frontera for his helpful statistical support, Catalina Crespí for her sorting technical support and Cristina Martínez and Mónica Portell for their technical assistance. We are also grateful to the Hospital Universitari Son Espases Ambulatory Care Unit nursing staff for their continued support and to the patients for their generous collaboration. This work has been supported by the Fondo de Investigación Sanitaria from the Spanish Government (grants FIS PI08/0362 and FIS PI11/0160).
Disclosure
None of the authors has any potential financial conflict of interest related to this manuscript.
References
- 1.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 2.Wood P, Stanworth S, Burton J, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149:410–423. doi: 10.1111/j.1365-2249.2007.03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salzer U, Unger S, Warnatz K. Common variable immunodeficiency (CVID): exploring the multiple dimensions of a heterogeneous disease. Ann NY Acad Sci. 2012;1250:41–49. doi: 10.1111/j.1749-6632.2011.06377.x. [DOI] [PubMed] [Google Scholar]
- 4.Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency – an update. Arthritis Res Ther. 2012;14:223. doi: 10.1186/ar4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacchelli C, Buckridge S, Thrasher AJ, Gaspar HB. Translational mini-review series on immunodeficiency: molecular defects in common variable immunodeficiency. Clin Exp Immunol. 2007;149:401–409. doi: 10.1111/j.1365-2249.2007.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuijpers TW, Bende RJ, Baars PA, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zelm MC, Smet J, Adams B, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–1274. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 9.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(–)IgD(–)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 10.Carsetti R, Rosado MM, Donnanno S, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115:412–417. doi: 10.1016/j.jaci.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120:310–318. doi: 10.1016/j.clim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 13.van Grevenynghe J, Cubas RA, Noto A, et al. Loss of memory B cells during chronic HIV infection is driven by FoxP3a- and TRAIL-mediated apoptosis. J Clin Invest. 2011;121:3877–3888. doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop GA, Hostager BS. B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr Opin Immunol. 2001;13:278–285. doi: 10.1016/s0952-7915(00)00216-8. [DOI] [PubMed] [Google Scholar]
- 15.Davies EG, Thrasher AJ. Update on the hyper immunoglobulin M syndromes. Br J Haematol. 2010;149:167–180. doi: 10.1111/j.1365-2141.2010.08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 17.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 18.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 19.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 20.Recher M, Berglund LJ, Avery DT, et al. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118:6824–6835. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 22.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 23.Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 24.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 25.Asao H, Okuyama C, Kumaki S, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettinger R, Sims GP, Robbins R, et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Clemente A, Pons J, Matamoros N, Iglesias J, Ferrer JM. B cells from common variable immunodeficiency patients fail to differentiate to antibody secreting cells in response to TLR9 ligand (CpG-ODN) or anti-CD40+IL21. Cell Immunol. 2011;268:9–15. doi: 10.1016/j.cellimm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 31.Husain Z, Holodick N, Day C, Szymarnski I, Alper CA. Increased apoptosis of CD20+ IgA+ B cells is the basis for IgA deficiency: the molecular mechanism for correction in vitro by IL-10 and CD40L. J Clin Immunol. 2006;26:113–125. doi: 10.1007/s10875-006-9001-y. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 33.Guerreiro-Cacais AO, Levitskaya J, Levitsky V. B cell receptor triggering sensitizes human B cells to TRAIL-induced apoptosis. J Leukoc Biol. 2010;88:937–945. doi: 10.1189/jlb.0510246. [DOI] [PubMed] [Google Scholar]
- 34.Ursini-Siegel J, Zhang W, Altmeyer A, et al. TRAIL/Apo-2 ligand induces primary plasma cell apoptosis. J Immunol. 2002;169:5505–5513. doi: 10.4049/jimmunol.169.10.5505. [DOI] [PubMed] [Google Scholar]
- 35.Borte S, Pan-Hammarstrom Q, Liu C, et al. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood. 2009;114:4089–4098. doi: 10.1182/blood-2009-02-207423. [DOI] [PubMed] [Google Scholar]
- 36.Hagn M, Sontheimer K, Dahlke K, et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol Cell Biol. 2012;90:457–467. doi: 10.1038/icb.2011.64. [DOI] [PubMed] [Google Scholar]