Abstract
Sitagliptin, a dipeptidyl-peptidase 4 (DPP-4) inhibitor, improves blood glucose control in patients with type 2 diabetes by blocking cleavage of glucagon-like peptide 1 (GLP-1). In type 2 diabetes patients sitagliptin use is associated with an increase in minor infections, and in new-onset type 1 diabetes patients the ability of sitagliptin to dampen autoimmunity is currently being tested. DPP-4, also known as CD26, is expressed on leucocytes and can inactivate many chemokines important for leucocyte migration, as well as act as a co-stimulatory molecule on T cells. Therefore, this study was conducted to test whether sitagliptin is immunomodulatory. In this randomized, placebo-controlled trial, healthy volunteers were given sitagliptin or placebo daily for 28 days, and blood was drawn for immune assays. No significant differences were observed in the percentage of leucocyte subsets within peripheral blood mononuclear cells (PBMCs), plasma chemokine/cytokine levels or cytokines released by stimulation of PBMCs with either lipopolysaccharide (LPS) or anti-CD3. Individuals taking sitagliptin displayed increases in the percentage of cells expressing higher levels of CD26 at early time-points compared to placebo controls, but these differences resolved by day 28 of treatment. Therefore, in healthy volunteers, treatment with sitagliptin daily for 28 days does not overtly alter systemic immune function.
Keywords: cytokine, dipeptidyl-peptidase 4, GLP-1, immune function
Introduction
Dipeptidyl-peptidase 4 (DPP-4) inhibitors, such as sitagliptin, improve glycaemia by increasing active glucagon-like peptide 1 (GLP-1) levels and are prescribed frequently for the treatment of type 2 diabetes. DPP-4 normally cleaves GLP-1, and sitagliptin inhibits the peptidase activity of DPP-4 [1]. DPP-4 is also involved in other biological processes that could potentially alter immune function, but it is not clear how inhibition of DPP-4 enzymatic activity affects human immune function. Several clinical observations suggest that sitagliptin might affect immune function. Sitagliptin has been associated with an increase in minor infections, such as nasopharyngitis [2–4]. A case study has also been reported in which an individual with type 2 diabetes and psoriasis had marked improvement of this autoimmune skin condition after treatment with sitagliptin [5]. These studies are consistent with the possible inhibition of immune activation after DPP-4 blockade. A current clinical protocol in patients with type 1 diabetes is testing the effects of sitagliptin along with lansoprazole on preserving beta cell insulin secretion. The investigators hypothesized that this drug combination could dampen the autoimmune response and directly enhance beta cell mass and function [6] (NCT01155284).
A membrane-bound form of DPP-4 is found on leucocytes including T cells, where it is called CD26. Immune activation increases DPP-4/CD26 expression; CD45RO+ memory T cells express more CD26 [7,8]. Inhibition of CD26 activity results in reduced T cell activity [9]. Interestingly, CD26 can increase T cell activation by increasing the co-stimulator CD86 on antigen-presenting cells in a process that requires enzymatic activity [10]. CD26 associates with other membrane proteins on T cells, including the tyrosine phosphatase CD45 and the ectoenzyme adenosine deaminase (ADA), which might be important for the co-stimulatory activity of CD26 [8,11]. However, inhibition of DPP-4 enzymatic activity may not block all these immune activities; the ability of soluble CD26 to bind ADA and enhancement of T cell proliferation can usually occur even when the active site of DPP-4 has been mutated [12,13]. CD26 is also expressed on myeloid cells, and enzymatic inhibition decreased macrophage activation and migration into adipose tissue [14].
In addition to GLP-1, DPP-4 also cleaves immune peptides, including neuropeptide Y (NPY) and chemokines such as interferon gamma-induced protein (IP)-10, stromal cell-derived factor (SDF)1-alpha and regulated upon activation normal T cell expressed and secreted (RANTES) [15]. DPP-4 cleavage can affect chemokine activity or receptor specificity; therefore, inhibition of DPP-4 could alter leucocyte chemotaxis [16]. In humanized mice, human haematopoetic stem cells show enhanced engraftment with DPP-4 inhibition, which may be due to altered migration of these cells [17]. Clinical trials are now under way to test if sitagliptin can improve cord blood transplant outcomes (NCT00862719).
In mouse models of T cell-mediated autoimmunity, inhibitors of DPP-4 can reduce disease severity and are associated with increases in transforming growth factor (TGF)-β levels and improvements in immune tolerance induction [18,19]. Interestingly, in human autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, increased CD26 levels on leucocytes are observed, yet there is decreased DPP-4-associated peptidase activity [20–22]. The reason for the discrepancy between activity and membrane CD26 levels is unclear, but this could be due to decreased shedding of CD26 from the membrane or decreased levels of other peptidases that cleave the same substrate.
Despite evidence that sitagliptin might alter immune activity, few direct measurements of immune function after sitagliptin treatment in humans have been undertaken [23]. Therefore, we set up a double-blind clinical protocol in which healthy individuals were given either sitagliptin or placebo daily for 4 weeks. We chose to enrol healthy volunteers to separate effects of sitagliptin from disease effects on immune readouts (e.g. in type 2 diabetes). We measured many aspects of the immune system including plasma chemokine and cytokine expression, the percentage of regulatory T cells and other immune subsets and CD26 levels by flow cytometry, gene expression in whole blood and stimulation of peripheral blood mononuclear cells (PBMCs) with both innate, lipopolysaccharide (LPS) and adaptive, anti-CD3, immune stimuli. Our primary outcome measure was plasma TGF-beta levels.
Materials and methods
Study design and protocol
This study (NCT00813228) is a double-blind, randomized placebo-controlled trial approved by the institutional review board of NIDDK. Healthy individuals were recruited to the NIH Clinical Center. Seventy-six individuals who passed an initial telephone screening provided informed consent and were assessed further for eligibility. Inclusion criteria included age > 18 years, fasting blood glucose < 100 mg/dl and HbA1c < 5·7%. Exclusion criteria included pregnancy, recently active allergy, malignancy or infection or history of autoimmune disease or other immune abnormalities, anaemia, pancreatitis or hypersensitivity to sitagliptin. Forty-one healthy subjects were randomized at a ratio of 3:1 into two groups: sitagliptin or placebo (Fig. 1a). Both patients and researchers were blinded in this study. The randomization was performed by the NIH pharmacy. The sitagliptin and placebo groups in this study had similar demographic characteristics (Supporting information, Table S1).
Fig. 1.
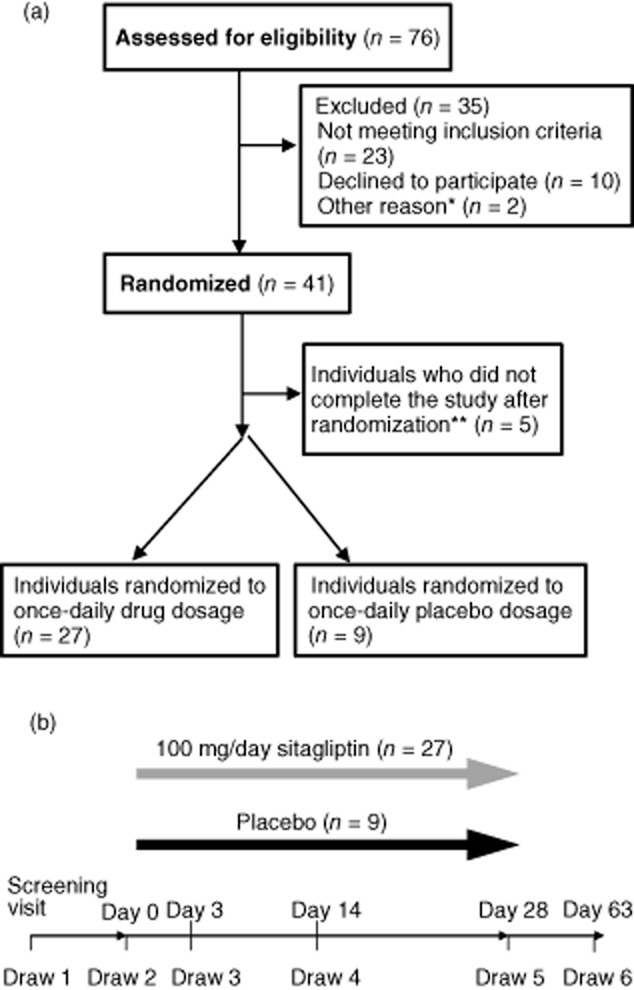
Study overview. (a) Patient flow diagram. (b) Overview of study visits and 4-week drug treatment. *Two individuals consented after enrolment was complete; **two individuals were unable to make study appointments, one had a positive serum human chorionic gonadotrophin (HCG) at 4 weeks, one had a potential allergic reaction to the drug and one was non-compliant with taking study medication.
Participants took 100 mg sitagliptin or placebo once daily for 28 days. Drug compliance was assessed by tablet counts. Six study visits were scheduled: the screening visit and visits at day 0 (before starting on drug or placebo), days 3, 14 and 28 (during drug or placebo treatment) and day 63 (5 weeks after stopping drug or placebo treatment) (Fig. 1b). For each visit, a brief history and physical examination was performed and fasting blood samples were obtained. Grades 1 and 2 adverse events occurred at similar rates in subjects in the sitagliptin and placebo groups (data not shown). No grade 3 or higher adverse events were observed.
Measurements
Blood collection and assays
Complete blood counts with differential were measured at all time-points. Plasma was processed from blood drawn into sodium citrate vacutainers as described previously to minimize platelet activation, thus preventing release of TGF-β [24]. Plasma TGF-β levels were assessed by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). Levels of other cytokines were also assessed in plasma and culture supernatant as indicated. These were measured using the Bioplex Pro 27-plex group I human cytokine array, following the manufacturer's instructions (Biorad, Hercules, CA, USA). For GLP-1 measurement, blood was drawn into K2 ethylenediamine tetraacetic acid (EDTA) vacutainers supplemented with DPP-4 inhibitor (10 μl/ml of blood) (EMD Millipore, Billerica, MA, USA). Active GLP-1 (7-36 and 7-37) was measured by ELISA (EMD Millipore). DPP-4 activity levels were measured from plasma samples using the DPP-4/CD26 activity kit (Enzo Life Sciences, Farmingdale, NY, USA). Maximum velocity (Vmax) values were measured after 60 min at room temperature, and values were measured every minute to ensure linearity. Values reported are the percentage of the day 0 value for each individual. For PBMC isolation, blood drawn into sodium heparin vacutainers was combined 1:1 with phosphate-buffered saline (PBS). The blood was then centrifuged over a Ficoll gradient (GE Healthcare, Pittsburgh, PA, USA). The buffy layer was collected and washed twice with PBS.
Flow cytometry
Freshly isolated PBMCs were stained with the following panels: immune cell subsets (CD3, CD19, CD56, CD14 and CD26), T cells (CD3, CD4, CD8, CD45RA, CD45RO and CD26) and regulatory T cells [CD4, CD25, CD127, forkhead box protein 3 (FoxP3)]. The following lymphocyte populations were gated: monocytes (CD14+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD19+), natural killer (NK) cells (CD3–CD56+) and NK T cells (CD3+CD56+). T cell populations were also gated as naive (CD45RA+) or memory (CD45RO+). CD26 levels were assessed in all lymphocyte populations, and CD4 and CD8 T cells (total, naive and memory) were gated on CD26 negative, low and high populations, as shown in Fig. 3c. Regulatory T cells were gated as CD4+CD25+FoxP3+ cells, and were confirmed as having lower interleukin (IL)-7Rα (CD127). CD3, CD25 and CD127 antibodies were purchased from Biolegend (San Diego, CA, USA). CD3, CD4, CD8, CD14, CD19, CD26, CD45RA, CD45RO and CD56 and FoxP3 antibodies were purchased from BD Biosciences (San Jose, CA, USA). Cell fixation and permeabilization for intracellular staining for FoxP3 was accomplished with FoxP3 fixation/permeabilization buffers (eBioscience, San Diego, CA, USA). Both Foxp3 and CD26 gates were set using fluorescence minus one (FMO) controls in which a stain was performed with all fluorphore-conjugated antibodies, except the one specific for either Foxp3 or CD26.
Fig. 3.
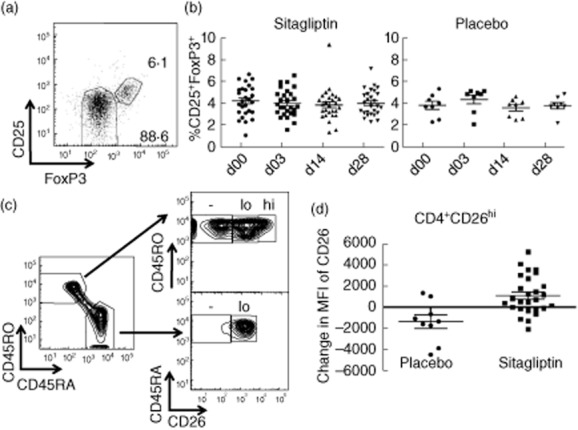
Lymphocyte subset analysis. (a) Fresh peripheral blood mononuclear cells (PBMCs) were collected from each donor at the indicated time-points, flow cytometry was performed, and CD3+CD4+ lymphocytes were further gated on CD25 and forkhead box protein 3 (FoxP3) as shown. Isotype and fluorescence minus one controls were used to set this gate. (b) The percentage of CD25+FoxP3+ (regulatory T cells) among CD3+CD4+ cells at the indicated time-points for individuals in the sitagliptin (left) or placebo (right) groups. (c) CD26 expression and gating (CD26–, CD26lo, CD26hi) on naive (CD45RA+) and memory (CD45RO+) CD4+ T cells. (d) The change in CD26 geometric mean fluorescence intensity on CD4+CD26hi within individuals in the placebo (circles) and sitagliptin (squares) groups from days 0 to 3.
Microarray analysis
RNA was isolated from whole blood using Tempus Tubes following the manufacturer's instructions (Life Technologies, Grand Island, NY, USA). Gene expression profiling was performed with days 0 and 28 samples using Affymetrix arrays.
LPS stimulation
Isolated PBMCs were cultured at 2 × 105 cells/well in 96-well flat-bottomed plates in defined, serum-free X-Vivo15 media (Lonza, Basel, Switzerland) with or without 0·5 mg/ml of LPS (Sigma, St Louis, MO, USA) for 24 h. Supernatants were collected and assessed for cytokine levels by TGF-β ELISA and 27-plex human cytokine array, as described above.
Anti-CD3 stimulation
This assay was performed on only 11 individuals known to be in the sitagliptin group, after unblinding. Frozen PBMCs were thawed and rested overnight in X-Vivo15 media. Cells were then labelled with 1 μM 5,6-carboxyfluorescein succinimidyl ester (CFSE; Life Technologies) and plated in X-Vivo15 media at 2 × 105 cells/well in 96-well round-bottomed plates with or without 0·02 mg/ml anti-CD3 (BD Biosciences). CD4+ and CD8+ T cell proliferation was measured by flow cytometry analysis of CFSE dilution after 4 days of stimulation, and activation of T cells was assessed by CD25 up-regulation.
Statistical analysis
This study's primary outcome was change in TGF-β protein levels in plasma, calculated by subtracting the value of TGF-β at day 0 from the value at day 28. Sample size was calculated based on previously published reports of plasma TGF-β in healthy subjects; we estimated a 50% increase in plasma TGF-β, with an > 88% power to detect this difference with a sample size of 40 subjects (30 treatment, 10 control) and estimating a 10% dropout rate. The two-sided two-sample t-test was used to compare the mean change in TGF-β between the treatment group and the control group. The significance level was 0·05. For secondary outcomes, the change from baseline (day 0) to values at days 3, 14, 28 and 63 was compared by group. Secondary measurements included expression of CD26 on lymphocyte subsets in PBMCs, percentages of lymphocyte subsets within PBMCs, cytokine and chemokine concentrations in plasma, cytokine and chemokine concentrations in LPS-stimulated PBMCs, clinical complete blood count (CBC) values, gene expression in whole blood, proliferation and production of cytokines and chemokines (including TGF-β) in supernatants from anti-CD3-stimulated PBMCs. Comparison of the two groups at specific time-points was performed with t-test or Mann–Whitney U-test for quantitative variables and χ2 or Fisher's exact test for categorical variables. The primary analysis of these secondary variables included day 28 only, and did not adjust for baseline. Subsequent analyses used generalized linear models to investigate changes over time and a Bonferroni's correction was applied to account for the multiple time-points. A P-value of 0·0125 was used. These analyses included an adjustment for baseline and log transformation as needed. The analysis of correlations between the change in activity level of DPP-4 and changes in immune parameters was performed using Pearson's correlations using GraphPad Prism. Each individual's percentage change in DPP-4 activity level was calculated from their individual day 0 value and each subsequent on-drug time-point (days 3, 14, 28); Pearson's correlations were then calculated between this percentage baseline DPP4 activity and immune parameters (calculated as change from baseline at each time-point, as indicated above).
Results
A significant increase in active GLP-1 levels was observed in the sitagliptin group but not the placebo group, indicating that this group was taking active drug (Fig. 2a). As expected, because participants were fasting at the time of the blood draws, GLP-1 levels were low, with an average of 4·9 pg/ml at baseline (day 0). In addition, DPP-4 enzyme activity levels were measured, and a significant drop (P < 0·0001) in the percentage activity compared to day 0 was observed in the sitagliptin group, but not the placebo group (Fig. 2b). On average, while taking sitagliptin, this group showed 50–60% inhibition of activity.
Fig. 2.
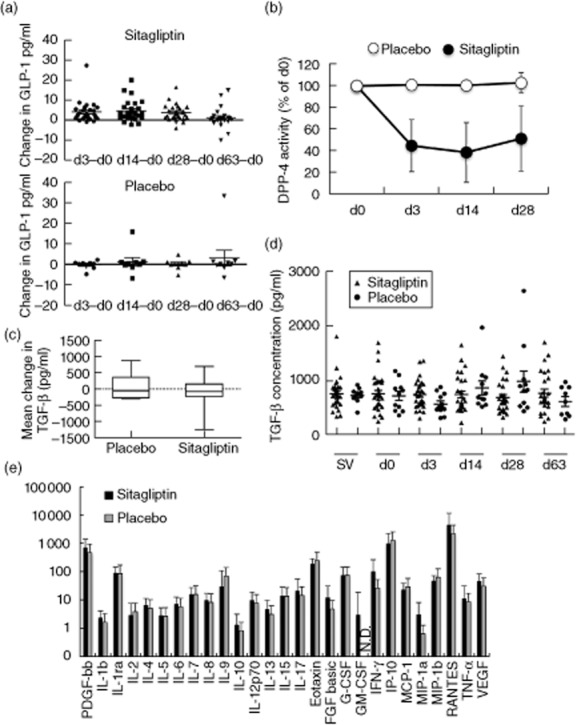
Plasma glucagon-like peptide (GLP)-1 and cytokine levels. (a) The change in GLP-1 levels from baseline (day 0) within an individual at the indicated time-points for both the sitagliptin group (top) or placebo group (bottom). (b) The percentage of baseline (day 0) dipeptidyl-peptidase (DPP)-4 enzyme activity was calculated for each individual at the indicated time-points with averages for the sitagliptin group (closed circles) or placebo group (open circles) (mean ± standard deviation). (c) The mean change in plasma transforming growth factor (TGF)-β from days 0 to 28 for individuals randomized to placebo or sitagliptin. P = 0·4691. (d) Concentration of plasma TGF-β in all individuals randomized to either the sitagliptin group (triangles) or placebo group (circles) for each study visit as indicated. (e) Concentrations of 27 cytokines and chemokines in plasma at day 3. The mean level (pg/ml) of each protein in either sitagliptin (black) or placebo (grey) groups is shown. A log scale is used on the y-axis so that all analytes can be visualized on one graph. Error bars show standard deviation. PDGF: platelet-derived growth factor; IL: interleukin; FGF: fibroblast growth factor; G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon; IP: interferon gamma-induced protein; MCP: monocyte chemoattractant protein; MIP: macrophage inflammatory protein; RANTES: regulated upon activation normal T cell expressed and secreted; VEGF: vascular endothelial growth factor; N.D.: not detected.
The primary outcome for this study was the change in total plasma TGF-β levels from baseline (day 0) to day 28, comparing the group that received sitagliptin and the placebo group. Plasma TGF-β levels were similar at baseline [737 ± 287 pg/ml (mean ± standard deviation) for the placebo group and 723 ± 338 pg/ml for the sitagliptin group] and at day 28 (822 ± 374 pg/ml for the placebo group and 695 ± 286 pg/ml for the sitagliptin group). We observed no significant change in this measurement (Fig. 2c, P = 0·4691). Plasma TGF-β levels were relatively stable over time in both groups (Fig. 2d).
We next measured plasma cytokine and chemokine levels in both groups using multiplex assays. Twenty-seven analytes were measured, and no significant differences were found in the change from baseline between the placebo and sitagliptin groups at any of the time-points. The levels of cytokines and chemokines in both the drug and placebo groups at day 3 are shown in Fig. 2e. Similar results were obtained at other time-points (data not shown).
The percentage of lymphocyte subsets in PBMCs were measured by flow cytometry. The frequency of major lymphocyte subsets (B cells, T cells: both CD4+ and CD8+, NK, NKT cells and monocytes) was measured, and no significant differences were found between groups (data not shown). In addition, numbers of regulatory T cells (CD4+CD25+FoxP3+) were assessed. In mice, increases in regulatory T cells with DPP-4 inhibition have been reported [18]. However, we observed no significant changes in the percentage of regulatory T cells with sitagliptin treatment (Fig. 3a,b). A small increase in neutrophil and total white blood cell count after sitagliptin treatment was reported to the Federal Drug Administration. In our study, CBC values were also measured, and no significant differences were found between groups in any measure, including white blood cells (WBC) and neutrophils (data not shown and Supporting information, Fig. S1).
CD26/DPP-4 is expressed differentially on naive (CD45RA+) and memory (CD45RO+) T cells [25]. Therefore, we measured the percentage of naive and memory T cells in both the CD4+ and CD8+ T cell compartment. The percentage of CD8+CD45RO+ cells increased significantly on day 3 in the sitagliptin group compared to the placebo (P = 0·0104) and was also higher on day 14 (P = 0·0351) (Table 1). We also measured CD26 expression, gating on three populations: CD26– cells defined by fluorescence-1 controls, CD26lo cells, corresponding to the low level found on most naive CD45RA+ cells and CD26hi cells found primarily among the memory CD45RO+ population (Fig. 3c). We observed changes consistent with an increase in CD26 expression early after sitagliptin treatment compared to placebo treatment, including increases in the percentages of CD4+CD45RO+CD26hi and CD8+CD26hi cells, and in the fluorescence levels of CD26 on CD4+CD26hi, CD3+CD26hi and CD3+CD45RO+CD26hi populations (Fig. 3d and Table 1). A significant decrease in the percentage of CD8+CD26lo cells was also observed in sitagliptin-treated individuals compared to placebo, which is consistent with increased CD26, as these cells probably shifted to the CD8+CD26hi population. These significant (P < 0·015) changes were observed primarily at day 3, but some of these populations were also different at day 14, with P-values < 0·05 (Table 1).
Table 1.
Differences in T cell subsets between placebo control and sitagliptin-treated individuals
| Population variable | Time-point | Placebo† (mean ± s.d.) | Sitagliptin† (mean ± s.d.) | P-value | Change with sitagliptin |
|---|---|---|---|---|---|
| P < 0·015 | |||||
| CD8+ CD45RO+, % | Day 3 | −3·8 ± 7·0 | 1·3 ± 3·9 | 0·0104 | Up |
| CD4+ CD45RO+ CD26hi, % | Day 3 | −2 ± 2·3 | 0·4 ± 2·0 | 0·0038 | Up |
| CD8+ CD26lo, % | Day 3 | 2·6 ± 7·5 | −2·8 ± 4·0 | 0·0085 | Down |
| CD8+ CD26hi, % | Day 3 | −2·9 ± 6·0 | 1·5 ± 2·4 | 0·0036 | Up |
| CD26lo, CD26 geo. mean | Day 3 | −325·2 ± 338·4 | 37 ± 348·2 | 0·0102 | Up |
| CD4+ CD26hi, CD26 geo. mean | Day 3 | −1350 ± 1894·3 | 1084·9 ± 1766·5 | 0·0013 | Up |
| CD4+ CD45RO+ CD26hi, % | Day 14 | −2·7 ± 5·1 | 0·9 ± 2·4 | 0·0084 | Up |
| P < 0·05 | |||||
| CD8+ CD45RA+, % | Day 3 | 3·6 ± 6·8 | −1·6 ± 6·0 | 0·0333 | Down |
| CD8+ CD45RO+ CD26lo, % | Day 3 | 4·3 ± 10·3 | −2·4 ± 5·8 | 0·0198 | Down |
| CD8+ CD45RA+, % | Day 14 | 2 ± 6·1 | −1·4 ± 3·0 | 0·0353 | Down |
| CD8+ CD45RO+, % | Day 14 | −1·3 ± 5·0 | 1·7 ± 2·8 | 0·0351 | Up |
| CD3+ CD8+, % | Day 14 | −2 ± 2·6 | −0·2 ± 1·6 | 0·0172 | Up |
| CD8+ CD26lo, % | Day 14 | 1·5 ± 5·2 | −1·4 ± 2·0 | 0·0217 | Down |
| CD8+ CD26hi, % | Day 14 | −1 ± 4·1 | 1 ± 1·7 | 0·0423 | Up |
| CD8+ CD45RA+ CD26hi, % | Day 14 | −7·2 ± 15·0 | 2·1 ± 9·5 | 0·0375 | Up |
| CD8+ CD45RO+ CD26hi, % | Day 14 | −8·5 ± 20·2 | 2·2 ± 6·4 | 0·0211 | Up |
| CD4+ CD26hi, CD26 geo. mean | Day 14 | −1167 ± 1867·7 | 628·3 ± 2311·2 | 0·0436 | Up |
Listed as the mean change from day 0; s.d.: standard deviation.
Gene expression changes induced by the sitagliptin treatment were assessed from whole blood samples taken at days 0 and 28. Paired analysis was performed to identify changes within individuals. In the sitagliptin group, a group of 86 transcripts was identified as significantly changed between days 0 and 28 (paired t-test, P < 0·001). Sixteen transcripts changed in the placebo group (P < 0·001) and none overlapped with those changed in the sitagliptin group, indicating the specificity of the genes identified in the treatment group. Although these changes were statistically significant, with a stringent P-value cut-off, the magnitude of these observed changes was small (most with a fold change < 1·2), indicating that the changes observed might not be biologically relevant. Shown in Supporting information, Table S2, are transcripts changed significantly with either sitagliptin or placebo treatment (P < 0·001) that had a fold change > 1·2. One of the transcripts with the highest significance and fold change was matrix metallopeptidase 9, a protein important for leucocyte trafficking that is up-regulated in many autoimmune diseases [26]. Another gene changed significantly in the sitagliptin group was small ubiquitin-related modifier (SUMO-1), that can modify other proteins via sumoylation. SUMO-1 interacts with dipeptidyl peptidase 9 (DPP-9), a protein with structural and functional similarity to DPP-4, yet sitagliptin is specific for DPP-4 and does not inhibit DPP-9 [27].
Some alterations in immune function may not be directly observable ex vivo, and may require an immune stimulus to reveal differences. Therefore, we treated PBMCs with LPS as an innate immune stimulus, and measured cytokine and chemokine levels. TGF-β levels were measured by ELISA, and did not differ before and after drug treatment or between the sitagliptin and placebo groups (data not shown). The same 27 cytokines and chemokines measured in plasma were also measured in supernatants with and without LPS treatment, and no significant differences were observed between placebo and sitagliptin groups (Fig. 4 and data not shown). Shown in Fig. 4 are the expression levels of proteins from this panel that were induced with LPS treatment of day 3 samples. Although individuals from the sitagliptin group exhibit moderately higher levels of certain cytokines in PBMCs cultured without LPS [for example, IL-6 and macrophage inflammatory protein (MIP)-1α], this difference was not statistically significant.
Fig. 4.

Cytokine/chemokine levels from lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells (PBMCs). Fresh PBMCs were cultured overnight in media with or without LPS, and supernatants were then collected for measurement of 27 different cytokines or chemokines. Blue: sitagliptin plus LPS, red: placebo plus LPS, black: sitagliptin no LPS, white: placebo no LPS. Only those cytokines that changed expression with LPS treatment are presented. A log scale is used on the x-axis so that all analytes can be visualized on one graph. Error bars show standard deviation. TNF: tumour necrosis factor; RANTES: regulated upon activation normal T cell expressed and secreted; MIP: macrophage inflammatory protein; MCP: monocyte chemoattractant protein; IP: interferon gamma-induced protein; G-CSF: granulocyte colony-stimulating factor; IL: interleukin.
In order to elicit an adaptive immune response and activate T cells, PBMCs from participants were stimulated with anti-CD3 for 4 days. Samples were obtained from 11 individuals who received sitagliptin (this part of the study was not blinded). T cell activation was measured by up-regulation of CD25 and T cell proliferation was measured via CFSE dilution (Fig. 5). Both parameters were measured in CD4+ and CD8+ T cells. Supernatants were also collected for measurement of cytokine and chemokine levels. No significant differences were observed comparing baseline values to levels observed after drug treatment (Fig. 5 and data not shown).
Fig. 5.
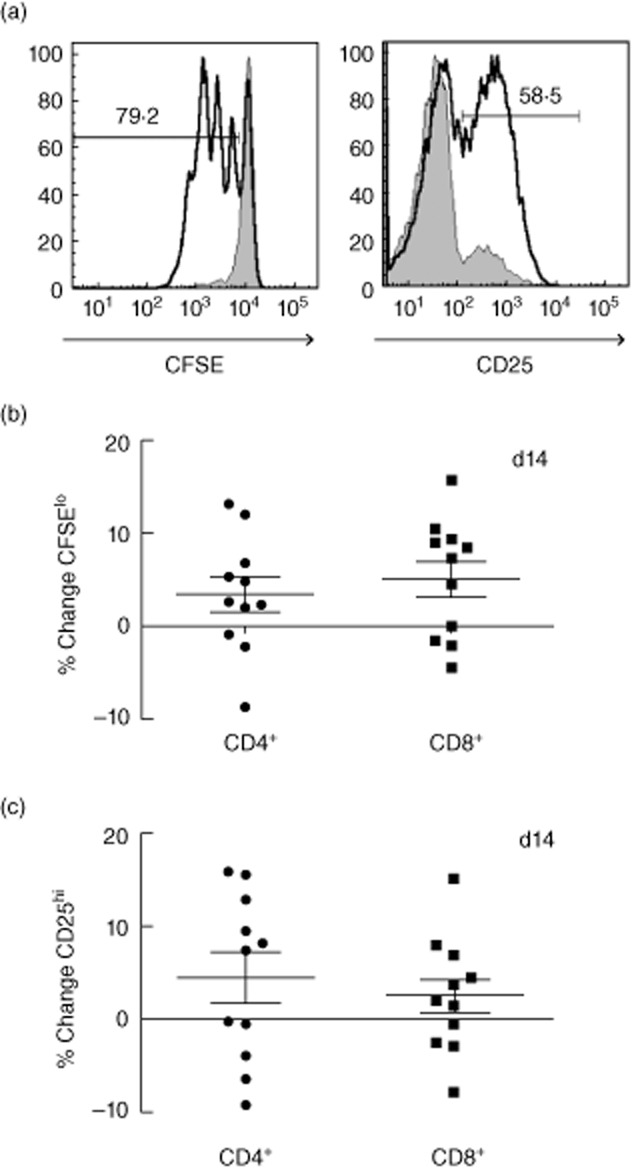
T cell activation after anti CD3 treatment. Peripheral blood mononuclear cells (PBMCs) collected on days 0, 3, 14 and 28 from 11 donors in the sitagliptin group were thawed, labelled with 5,6-carboxyfluorescein succinimidyl ester (CFSE) and stimulated with anti-CD3 for 4 days. Flow cytometry was performed to assess proliferation and activation of T cells. After gating on CD4 and CD8 T cells, the percentage of CFSElo or CD25hi cells at each time-point was determined. An example of gating with (open histogram) or without (shaded histogram) anti-CD3 is shown in (a); numbers represent percentage of stimulated sample. The percentage change from baseline (day 0) was then calculated. Shown is the percentage change in CFSElo (b) or CD25hi (c) populations in either CD4+ T cells (left) or CD8+ T cells (right).
In order to determine if level of drug activity correlated with change in immune function, we performed an additional post-hoc statistical analysis. The sitagliptin group was tested for significant correlations between the change in each immune parameter and the percentage baseline DPP-4 activity for each time-point. This would allow us to observe any immune changes that may be missed because of variance within the sitagliptin group for level of DPP-4 inhibition. However, in individuals taking sitagliptin, no biologically relevant correlations were found between change in DPP-4 activity and change in immune function. This lends strength to the conclusion that sitagliptin does not induce sustained systemic immune effects.
Discussion
Although numerous previous studies point to the possibility that DPP-4 inhibition could potentially be immunomodulatory [9,28], this is the first study to measure systematically a wide variety of immune readouts in humans taking sitagliptin. Here, we have shown that individuals given sitagliptin daily for 28 days do not have significantly altered immune readouts. GLP-1 levels were higher in the sitagliptin group and DPP-4 activity was lower, indicating that this group was taking active drug. Importantly, the dose given here (100 mg/day) is the standard dose prescribed to most patients with type 2 diabetes. These data support the safety of the drug for patients with type 2 diabetes, and have implications for the use of sitagliptin in immune diseases. Several investigators have suggested that sitagliptin might down-modulate immune responses but our study results suggest that this is unlikely, at least for effects that can be observed systemically. However, sitagliptin could have relevant immune effects in individuals undergoing chronic immune activation, such as individuals with autoimmune diseases. Future studies to assess immune readouts in patients with type 1 diabetes or other autoimmune diseases could be informative.
We observed an increase in CD26 levels early after sitagliptin treatment, but these changes were not observed at the 28-day time-point. Therefore, DPP-4 inhibition may increase CD26/DPP-4 levels transiently on T cells, but this is unlikely to lead to clinically relevant alterations in immune function because the effect is not maintained. A small but significant increase in the percentage of memory CD8+ T cells from days 0 to 3 suggests that sitagliptin might activate T cells, but this effect was also not sustained.
Interestingly, even chemokines known to be substrates of DPP-4 such as RANTES and IP-10 show no change in level with sitagliptin treatment. However, the chemokine measurements performed here cannot distinguish the active form from the DPP-4 cleaved form, so it is possible that changes in active chemokine levels occurred with sitagliptin treatment. Future studies using assays that measure both cleaved and full-length forms of these chemokines would be informative. In addition, as we were only able to measure changes in peripheral blood it is possible that sitagliptin, via effects on chemokine activity, could alter migration of leucocytes within tissues, thus altering immune responses in these locations with potential effects on infection or autoimmunity.
Taken together, no sustained differences in the immune readouts were observed between the sitagliptin and placebo groups in the 4-week study period, and therefore we conclude that sitagliptin is not overtly systemically immunomodulatory in healthy individuals.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). We would like to thank Michelle Ashmus for coordinating patient recruitment, Dr Monica Skarulis for serving as the medically responsible investigator, Drs Xiongce Zhao and Elizabeth Wright for help with statistical analysis, the NIH Center for Human Immunology, specifically Phil McCoy and Angélique Biancotto for flow cytometry and multiplex support, and Dr Francesco Marincola, Ena Wang and Hui Liu for help with gene expression analysis. In addition, Mary Walter and the NIDDK Central Laboratory helped with GLP-1 assays, DPP-4 activity assays, sample storage and database maintenance. NIH pharmacy, including Judith Starling provided the drug with matching placebo and performed randomization.
Disclosure
The authors have nothing to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Change in neutrophil percentage (top) and absolute count per µl (bottom) were measured in participants in both the sitagliptin group (left) and placebo group (right). No significant changes were observed between placebo and sitagliptin groups (P = 0·41 for percentage and P = 0·59 for absolute number change from days 0 to 28).
Table S1. Demographic characteristics of study subjects (n = 36).
Table S2. Significant (P < 0·001) genes changed greater than 1·2-fold after sitagliptin or placebo treatment.
References
- 1.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–768. doi: 10.2147/vhrm.s1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD006739.pub2. CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishioka T, Shinohara M, Tanimoto N, Kumagai C, Hashimoto K. Sitagliptin, a dipeptidyl peptidase-IV inhibitor, improves psoriasis. Dermatology. 2012;224:20–21. doi: 10.1159/000333358. [DOI] [PubMed] [Google Scholar]
- 6.Suen CS, Burn P. The potential of incretin-based therapies in type 1 diabetes. Drug Discov Today. 2012;17:89–95. doi: 10.1016/j.drudis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Fox DA, Hussey RE, Fitzgerald KA, et al. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133:1250–1256. [PubMed] [Google Scholar]
- 8.Ishii T, Ohnuma K, Murakami A, et al. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci USA. 2001;98:12138–12143. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30:600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Ohnuma K, Munakata Y, Ishii T, et al. Soluble CD26/dipeptidyl peptidase IV induces T cell proliferation through CD86 up-regulation on APCs. J Immunol. 2001;167:6745–6755. doi: 10.4049/jimmunol.167.12.6745. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Navio JM, Casanova V, Pacheco R, et al. Adenosine deaminase potentiates the generation of effector, memory, and regulatory CD4+ T cells. J Leukoc Biol. 2011;89:127–136. doi: 10.1189/jlb.1009696. [DOI] [PubMed] [Google Scholar]
- 12.Kirby M, Yu DM, O'Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci. 2010;118:31–41. doi: 10.1042/CS20090047. [DOI] [PubMed] [Google Scholar]
- 13.Yu DM, Slaitini L, Gysbers V, et al. Soluble CD26/dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand J Immunol. 2011;73:102–111. doi: 10.1111/j.1365-3083.2010.02488.x. [DOI] [PubMed] [Google Scholar]
- 14.Shah Z, Kampfrath T, Deiuliis JA, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 16.Iwata S, Yamaguchi N, Munakata Y, et al. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int Immunol. 1999;11:417–426. doi: 10.1093/intimm/11.3.417. [DOI] [PubMed] [Google Scholar]
- 17.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Gao J, Hao J, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151:3049–3060. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Nian C, Doudet DJ, McIntosh CH. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes. 2009;58:641–651. doi: 10.2337/db08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagihara M, Ohhashi M, Nagatsu T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in mice with lupus erythematosus-like syndrome and in patients with lupus erythematosus and rheumatoid arthritis. Clin Chem. 1987;33:1463–1465. [PubMed] [Google Scholar]
- 21.Krakauer M, Sorensen PS, Sellebjerg F. CD4(+) memory T cells with high CD26 surface expression are enriched for Th1 markers and correlate with clinical severity of multiple sclerosis. J Neuroimmunol. 2006;181:157–164. doi: 10.1016/j.jneuroim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Ellingsen T, Hornung N, Moller BK, Hjelm-Poulsen J, Stengaard-Pedersen K. In active chronic rheumatoid arthritis, dipeptidyl peptidase IV density is increased on monocytes and CD4(+) T lymphocytes. Scand J Immunol. 2007;66:451–457. doi: 10.1111/j.1365-3083.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 23.White PC, Chamberlain-Shea H, de la Morena MT. Sitagliptin treatment of patients with type 2 diabetes does not affect CD4+ T-cell activation. J Diabetes Complications. 2010;24:209–213. doi: 10.1016/j.jdiacomp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold D, Bank U, Buhling F, et al. A detailed protocol for the measurement of TGF-beta1 in human blood samples. J Immunol Methods. 1997;209:203–206. doi: 10.1016/s0022-1759(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 25.de Meester I, Scharpe S, Vanham G, et al. Antibody binding profile of purified and cell-bound CD26. Designation of BT5/9 and TA5.9 to the CD26 cluster. Immunobiology. 1993;188:145–158. doi: 10.1016/S0171-2985(11)80494-8. [DOI] [PubMed] [Google Scholar]
- 26.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 27.Pilla E, Moller U, Sauer G, Mattiroli F, Melchior F, Geiss-Friedlander R. A novel SUMO1-specific interacting motif in dipeptidyl peptidase 9 (DPP9) that is important for enzymatic regulation. J Biol Chem. 2012;287:44320–44329. doi: 10.1074/jbc.M112.397224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


