Abstract
Macaques provide important animal models in biomedical research into infectious and chronic inflammatory disease. Therefore, a proper understanding of the similarities and differences in immune function between macaques and humans is needed for adequate interpretation of the data and translation to the human situation. Dendritic cells are important as key regulators of innate and adaptive immune responses. Using a new whole blood assay we investigated functional characteristics of blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes in rhesus macaques by studying induction of activation markers and cytokine expression upon Toll-like receptor (TLR) stimulation. In a head-to-head comparison we observed that rhesus macaque venous blood contained relatively lower numbers of pDC than human venous blood, while mDC and monocytes were present at similar percentages. In contrast to humans, pDC in rhesus macaques expressed the interleukin (IL)-12p40 subunit in response to TLR-7/8 as well as TLR-9 stimulation. Expression of IL-12p40 was confirmed by using different monoclonal antibodies and by reverse transcription–polymerase chain reaction (RT–PCR). Both in humans and rhesus macaques, TLR-4 stimulation induced IL-12p40 expression in mDC and monocytes, but not in pDC. The data show that, in contrast to humans, pDC in macaques are able to express IL-12p40, which could have consequences for evaluation of human vaccine candidates and viral infection.
Keywords: animal models – non-human primates, cytokine/interleukin/chemokine receptors, dendritic cells (myeloid, plasmacytoid, monocyte-derived), Toll-like receptors (TLRs)
Introduction
Non-human primates (NHP) provide essential models for biomedical research and have been crucial in understanding the pathogenesis of infectious diseases such as acquired immunodeficiency syndrome (AIDS), influenza, malaria and tuberculosis [1]. The close phylogenetic relationship with humans and consequential significant biological, immunological and genetic similarities make NHP a highly relevant animal model in preclinical safety, immunogenicity and efficacy evaluation of vaccines and therapies.
Dendritic cells (DCs) play an essential role in the induction and regulation of immune responses [2]. Hence, appropriate triggering of DC function, including antigen presentation, migration, expression of co-stimulatory molecules and cytokines, is critically important for induction of adaptive immune responses during natural infection as well as during vaccination. DC function is modulated by infection with viruses such as HIV, hepatitis C virus and dengue virus [3–7]. For instance, chronic HIV infection in humans is associated with a reduced number of DC in blood and lymphoid tissues and decreased DC-mediated interferon (IFN)-α production [8–13]. A similar depletion and loss of function of plasmacytoid DC (pDC) is seen in the simian immunodeficiency virus (SIV) infection model of AIDS in macaques, while for myeloid (mDC) both a decrease as well as an increase has been reported [14–18]. Depletion of pDC in the blood may, in part, be a result of migration to the lymphoid tissues, where increased numbers have been reported both in SIV-infected macaques [19–21] as well as in HIV-1 infected humans [22].
The important role of DC in vaccination as well as in inflammation and infectious disease implies that the appropriate interpretation of results obtained in NHP disease models requires a proper understanding of phenotypic and functional characteristics of NHP DC in comparison with human DC. Several studies have shown that although NHP DC do not completely recapitulate the human DC system, they reflect it more closely than murine DC models [23]. As in humans, two populations of circulating DCs have been characterized, i.e. mDC, defined as negative for the lineage markers (CD3, CD8, CD14, CD20), human leucocyte antigen D-related (HLA-DR)+, CD11c+, CD123– and pDC, which are lineage–, HLA-DR+, CD11c–, CD123+ [2,16,24]. Both human and NHP mDC mature upon granulocyte–macrophage colony-stimulating factor (GM-CSF) and CD40L stimulation, have potent allostimulatory and interleukin (IL)-12-producing capacity and express the innate Toll-like receptors (TLRs) -3, -4, -7 and -8 [24,25]. Instead, human and rhesus pDC are sensitive to IL-3 stimulation, are the main type I interferon (IFN)-producing cells and express TLR-7 and -9 [24–28].
Previous studies on DC function in rhesus macaques have all been performed on isolated peripheral blood mononuclear cells (PBMC) or on cells isolated from lymphoid organs or other tissues [24–28]. However, these purification techniques can lead to a loss of specific subsets or result in some activation due to the reagents used and hence introduce artefacts. Recently, a whole blood (WB) stimulation assay was developed to study TLR-mediated activation of human peripheral blood DC [29]. Subsequent staining with a panel of monoclonal antibodies (mAb) to discriminate the pDC and mDC subsets in combination with either CD83, CD80 maturation markers or tumour necrosis factor (TNF)-α, IL-12, IFN-α intracellular cytokines allowed for simultaneous analysis of the response in these defined subsets upon stimulation [29]. In this study we performed this assay to study DC function in peripheral blood of rhesus macaques in a direct comparison with whole blood samples of human volunteers. Surprisingly, we observed that pDC in macaques express IL-12p40 upon TLR-7/8 stimulation, in contrast to human pDC exposed to the same ligand. Similar results were obtained following TLR-9 [cytosine–phosphate–guanosine (CpG-C)] stimulation, while TLR-4 [lipopolysaccharide (LPS)] did not induce IL-12p40 expression in pDC, in agreement with reported TLR expression profiles [25]. Induction of IL-12p40 expression was confirmed further by polymerase chain reaction (PCR) using purified fluorescence activated cell sorted (FACS) pDC.
Our results show that in rhesus macaques pDC in peripheral blood express IL-12p40 upon TLR-7/8 and TLR-9 stimulation, which could potentially affect their response to vaccination and viral infection.
Materials and methods
Animals
This study was performed in mature captive-bred Indian origin rhesus monkeys (Macaca mulatta) that were housed at the Biomedical Primate Research Center, Rijswijk, the Netherlands. All procedures were in accordance with the international guidelines for non-human primate care and use (The European Council Directive 2010/63/EU and Convention ETS 123, including the revised Appendix A). The Institutional Animals Care and Use Committee (DEC-BPRC) approved the study protocols developed according to strict international ethical and scientific standards and guidelines. Human peripheral blood was obtained from informed healthy volunteer donors via the Netherlands Red Cross Blood Bank.
Monoclonal antibodies
The following mAb were used; CD20V450 (clone L27), CD45V500 (clone TU116), CD3FITC (clone SP34), CD16FITC (clone 3G8), CD80PE (clone L307·4), anti-IL12p40/70PE (clone C11·5), anti-TNF-αPE (clone Mab11), CD123PerCP-Cy5 (clone 7G3), CD11cAPC (clone S-HCL3), anti-TNF-αPE-Cy7 (clone Mab11) and HLA-DRAPC-CY7 (clone L243), all from Becton Dickinson (San Jose, CA, USA), CD8FITC (clone DK25; Dako, Glostrup, Denmark), CD14PE-TxRed (clone RM052; Beckman Coulter, Brea, CA, USA), IL-12p40/70PE (clone C8·6; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and CD83PE (clone HB15a; Beckman Coulter). For detection of IFN-α, the mAb MMHA2 was used (Invitrogen, Breda, the Netherlands).
Whole blood stimulation assay
Heparinized venous blood was used within 3 h of collection. The assay was performed in 5-ml polypropylene tubes (Becton Dickinson), to which 200 μl of whole blood was added. The stimulation assay was performed by adding to all tubes 800 μl RPMI-1640 medium (Gibco, Carlsbad, CA, USA), 15 U/ml heparin, 0·1% fetal calf serum (FCS) (Gibco), β-mercaptoethanol (50 μM; Gibco), penicillin (50 U/ml) and streptomycin (50 mg/ml) and 10 ng/ml recombinant human IL-3 (Peprotech, London, UK). The tubes were incubated either without further stimulus or in the presence of TLR-7/8 (1 μg/ml CL097; Invivogen, San Diego, CA, USA), TLR-9 (5 μM CpG-C, M362; Girundus, Cincinnatti, OH, USA) or TLR-4 (1 μg/ml LPS, serotype 026:B6; Sigma L8274, St Louis, MO, USA) agonists at 37°C for 8 h. Golgiplug (1:1000; Becton Dickinson) was added after 2 h of incubation, to prevent protein secretion. The kinetics of induction of CD83, CD80 and cytokine expression was determined by incubating the blood for 3, 5, 8 or 16 h with TLR ligands, with Golgiplug added after 1, 1, 2 and 2 h, respectively.
FACS analysis
To establish the absolute number of pDC, mDC and monocytes, 200 μl of heparinized blood was stained with a mixture of mAb, consisting of: CD45V500, CD3FITC, CD8FITC, CD16FITC, CD20FITC, CD14PE-TxRed, CD123PerCP-Cy5, CD11cAPC and HLA-DRAPC-CY7. A fixed amount of Flow-Count Fluorospheres (Beckman Coulter) was added to each tube. Absolute number of monocytes, pDC and mDC was established by selecting CD45-positive cells and then the respective subsets by using the gating strategy described below. Absolute number per ml was calculated as: number of recorded monocytes, pDC or mDC × bead concentration/number of recorded beads.
For the time–course experiments, the stimulated blood samples were first washed with PBS and incubated with 50 μl of live/dead fixable violet dead cell stain kit (Molecular Probes, Eugene, OR, USA; cat. no. L34955), diluted in PBS for 15 min at 4°C in the dark. After washing the samples were incubated for 20 min at 4°C with a mixture of mAb for surface staining, consisting of: CD45V500, CD3FITC, CD8FITC, CD16FITC, CD20FITC, CD14PE-TxRed, CD123PerCP-Cy5, CD11cAPC and HLA-DRAPC-CY7, supplemented with CD83PE or with CD80PE. Subsequently, cells were washed once with 1 ml cold PBS and 2 ml lysing solution (Becton Dickinson) was added for 10 min at room temperature. Cells were pelleted and fixed in PBS with 2% paraformaldehyde or incubated with anti-IFN-α−phycoerythrin (PE) conjugate or a mixture of anti-IL-12PE and anti-TNF-αPE-Cy7 diluted in Becton Dickinson perm/wash solution for 30 min at 4°C in the dark. After washing with 1 ml of perm/wash solution, cells were fixed in PBS with 2% paraformaldehyde.
For detection of IFN-α in rhesus macaques, the commercially available unlabelled mAb (MMHA2) was labelled with PE using Zenon labelling technology (Zenon mouse IgG1 kit; Molecular Probes). Flow cytometry was performed on a LSRII or FACSaria using Diva software (Becton Dickinson).
The analysis strategy of the FACS data is depicted in Fig. 1. In brief, the forward-scatter (FSC)-A was plotted against the side-scatter (SSC)-A and an extended lymphocyte gate was drawn to select lymphocytes as well as monocyte and DC populations. Then, cells negative for live/dead (L/D) stain and positive for CD45 were gated. Subsequently, the fluorescein isothiocyanate (FITC) signal (consisting of a combination of CD3, CD8, CD16 and CD20) was plotted against HLA-DR. Lineage-negative/HLA-DR-positive cells were selected and CD14 was used to identify CD14-positive monocytes and a population of negative cells containing DC. Within the DC population, CD123 was plotted against CD11c to select the CD11c–/CD123+ pDC and CD11c+/CD123– mDC subpopulations. Fluorescence minus one (FMO) controls, containing all mAb except for the PE or PE-Cy7-labelled mAb, showed the same level of expression as CD83 or CD80 on fresh cells. Background expression was not increased after stimulation.
Fig. 1.
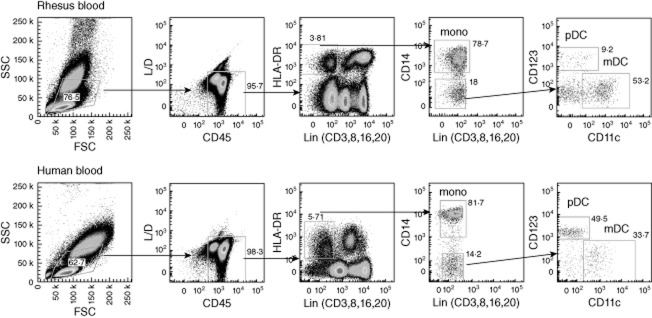
Analysis of rhesus versus human peripheral blood dendritic cell (DC) subsets. For analysis of monocytes and DC in rhesus macaques (top row) and humans (bottom row), an extensive lymphogate is drawn in the forward-scatter (FSC)/side-scatter plot (SSC), encompassing all lymphocyte, monocyte and DC subsets. Subsequently, cells negative for live/dead (L/D) stain and positive for CD45 are gated and expression of lineage markers (combination of CD3, CD8, CD16, CD20) is plotted against human leucocyte antigen D-related (HLA-DR). Within this plot the Lin–, HLA-DR+ cells are selected and expression of CD14 is used to identify the monocytes. The CD14– cells are then divided into a CD11c+/CD123– mDC and a CD11c–/CD123+ pDC subset. Figures in the graphs indicate per gate the number of cells as a percentage of the parental population. The relative difference in plasmacytoid dendritic cell (pDC) percentages between the rhesus and human sample is striking.
Because the data showed that regardless of stimulation condition, after 8 h >95% of the cells were still found within the live/CD45+ gate, these markers were not included in subsequent experiments. Instead, CD20 was used in the V450 detection channel to allow separate analysis of the B cells, as described previously [30]. The minimal number of white blood cells analysed per tube was 200 000. The minimal number of pDC, mDC and monocytes analysed were 75, 500 and 3000, respectively.
Detection of IL-12p40 mRNA expression
A multi-step procedure was used to measure IL-12p40 mRNA expression in purified peripheral blood pDC and mDC upon TLR stimulation. First, PBMC were isolated from peripheral blood using lymphocyte separation medium (LSM) density gradient centrifugation (Organon-Teknica, Durham, NC, USA). Subsequently, partial purification of DC and monocytes was performed by depletion of CD2-, CD3-, CD8-, CD16-, CD19- and CD20-expressing cells, using a cocktail of PE-labelled mAb, followed by incubation with BD anti-PE beads and collection of the supernatant after placing the labelled cells for 8–10 min in a BD-Imagnet. These partially purified cell preparations were stimulated with either CL097 (1 μg/ml) or LPS (1 μg/ml) for 6 h at 37°C with Golgiplug present during the last 5 h. Finally, the cells were stained with a mixture of CD20V450, CD8AmCyan, CD14ECD, CD123PerCPCy5, CD11cAPC, CD3AF700 and HLA-DRAPCCy7 mAb and pDC and mDC subpopulations were sorted on a FACSAria cell sorter, using the gate setting described above. In each experiment, between 3000 and 5000 pDC and between 5000 and 10 000 mDC were obtained. Sorted pDC and mDC fractions contained between 5–15% and 10–20% granulocytes, respectively, as examined by Giemsa staining. Sorted monocytes contained fewer than 1% granulocytes. FACS analysis on sorted populations showed monocytes to be about 90% pure with fewer than 1% pDC and fewer than 5% mDC present. Sorted pDC were 60–75% pure, with 10–20% monocytes and less than 4% mDC contamination. Sorted mDC were 70–80% pure, with 5–20% monocytes and less than 1% pDC contamination.
Polymerase chain reaction (PCR)
Probe-based quantitative PCRs were designed using the human Universal Probe Library design center (Roche Applied Science, Penzberg, Germany). Real-time quantitative PCRs (qPCRs) were performed on the CFX96™ real-time PCR detection system (Biorad, Herts, UK) using iTaq Supermix with Rox (Biorad) and the following primer (Invitrogen/Life Technologies, Paisley, UK) and probe (human Exiqon probe library; Roche, Woerden, the Netherlands) combinations: IL-12p40 5′-CCACATTCCTACTTCTCCCTGA-3′ and 5′-ACCGTGGCTGAGGTCTTGT-3′ with TCCAGGTC fluorescent probe, TNF-α 5′-AAGCCTGTAGCCCATGTTGT-3′ and 5′-GCTGGTTATCTGTCAGCTCCA-3′, with CCAGGAGG fluorescent probe and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 5′-CAACGAATTTGGCTACAGCA-3′ and 5′-GTGGTCCGGGGGTCTTAC-3′ with CCACCACC fluorescent probe. IL-12p40 and TNF-α mRNA expression levels were standardized to reference gene GAPDH mRNA expression levels using the Pfafll method [31].
Statistical analysis
The non-parametric Mann–Whitney U-test was used to determine the statistical significance of cell numbers and TLR-induced cytokine expression in rhesus macaque versus human DC subsets and monocytes.
Results
Rhesus macaques have relatively low numbers of pDC
As published previously [16,24], pDC and mDC subsets can be distinguished in peripheral blood of rhesus macaques on the basis of CD11c versus CD123 expression in HLA-DR-positive cells, which are negative for lineage markers CD3, CD8, CD16, CD20 and CD14 (Fig. 1). However, comparison of the dot-plots shown in Fig. 1 (right graphs) reveals a striking difference in the percentage of pDC relative to mDC in the lineage–, HLA-DR+ cells in human versus rhesus macaque blood. As shown in Fig. 2, analysis of a larger cohort showed that the absolute number of pDC was significantly lower in rhesus macaques (3020 ± 1357 cells/ml) than in humans (10 495 ± 4353 cells/ml), while there was no difference in the number of mDC (20 811 ± 14 361 versus 17 178 ± 5671 cells/ml) or monocytes (324 000 ± 161 000 versus 217 000 ± 107 000 cells/ml).
Fig. 2.
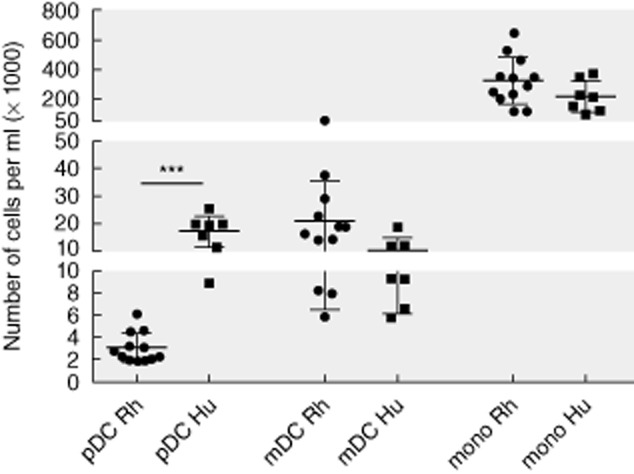
Absolute number of plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes in rhesus (n = 12) and human (n = 7) peripheral blood. Indicated is the number of cells (×1000)/ml blood. ***P < 0·001.
pDC in rhesus macaques express IL-12p40 upon TLR-7/8 and TLR-9 stimulation
In order to evaluate the function of peripheral blood DC subsets in rhesus macaques without interference of cell isolation procedures, a whole blood stimulation assay was used, analogous to the previously described assay for human blood DC [29]. In brief, heparin blood was diluted 1:5 in RPMI-1640 medium with 0·1% bovine serum albumin (BSA), heparin and β-mercaptoethanol. Samples were exposed for 8 h to different TLR ligands and the cells were then stained and analysed by flow cytometry for the induction of maturation markers and cytokine expression. This procedure has the advantage that it allows detection of the response of different DC subsets simultaneously in one tube. Time–course experiments showed optimal induction of cytokine expression after 5–8 h incubation with all selected TLR-7/8 (CL097), TLR-9 (CpG-C) and TLR-4 (LPS) ligands (Fig. 3). All reagents were used at saturating concentrations, as determined in dose–response experiments (not shown).
Fig. 3.
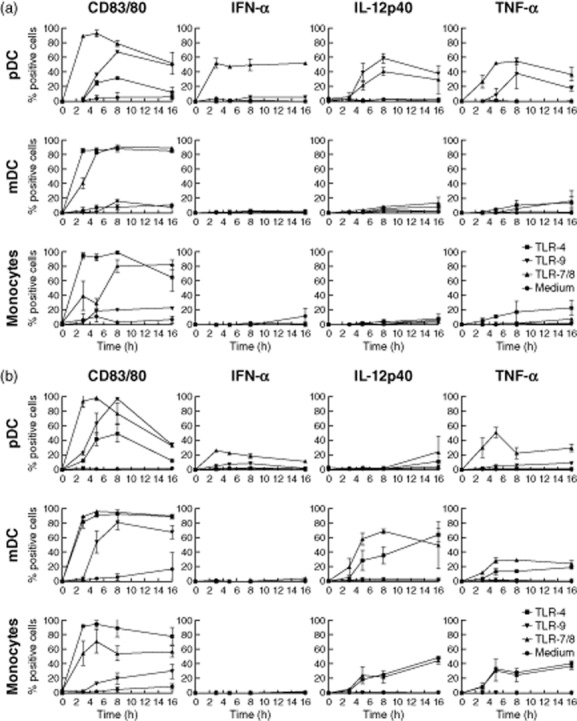
Kinetics of Toll-like receptor (TLR)-7/-8-, TLR-9- and TLR-4-induced CD83 or CD80 and cytokine expression in rhesus (a) and human (b) peripheral blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes. For each subset the percentage of cells expressing CD83 (pDC and mDC) or CD80 (monocytes), interferon (IFN)-α, interleukin (IL)-12p40 or tumour necrosis factor (TNF)-α after 3, 5, 8 or 16 h stimulation with TLR-7/8 (1 μg/ml CL097), TLR-9 [5 μM class C cytosine–phosphate–guanosine (CpG-C)] or Toll-like receptor (TLR)-4 (1 μg/ml lipopolysaccharide) is shown. Golgiplug was added after 1, 1, 2 and 2 h of incubation, respectively. Indicated is mean expression with error of two independent experiments.
Binding of CL097 to TLR-7/8 ligands, which are expressed on pDC and mDC as well as monocytes, resulted in human samples in induction of CD83/CD80 expression on all subsets, IFN-α and TNF-α expression in pDC and IL-2p40 and TNF-α expression in mDC and monocytes, as described previously [2,29,32] (Fig. 4). Surprisingly, in rhesus macaques we observed IL-12p40 expression in pDC, while mDC and monocytes showed relatively low levels of IL-12p40 as well as TNF-α induction by CL097. No cytokine expression was seen in non-stimulated blood cell cultures. Similar results, with regard to induction of IL-12p40 expression on rhesus pDC, mDC and monocytes, were obtained with other TLR-7/8 ligands, including imiquimod and R848 (not shown). As neutrophils can express HLA-DR and CD11c and could potentially have been included in the analysis, the experiments were repeated on LSM-separated PBMC. Induction of CD83 and CD80 as well as IFN-α and IL-12p40 cytokine expression in the PBMC were comparable to the whole blood cell cultures, while TNF-α expression was higher in CL097-stimulated PBMC than in whole blood cell cultures (results not shown).
Fig. 4.
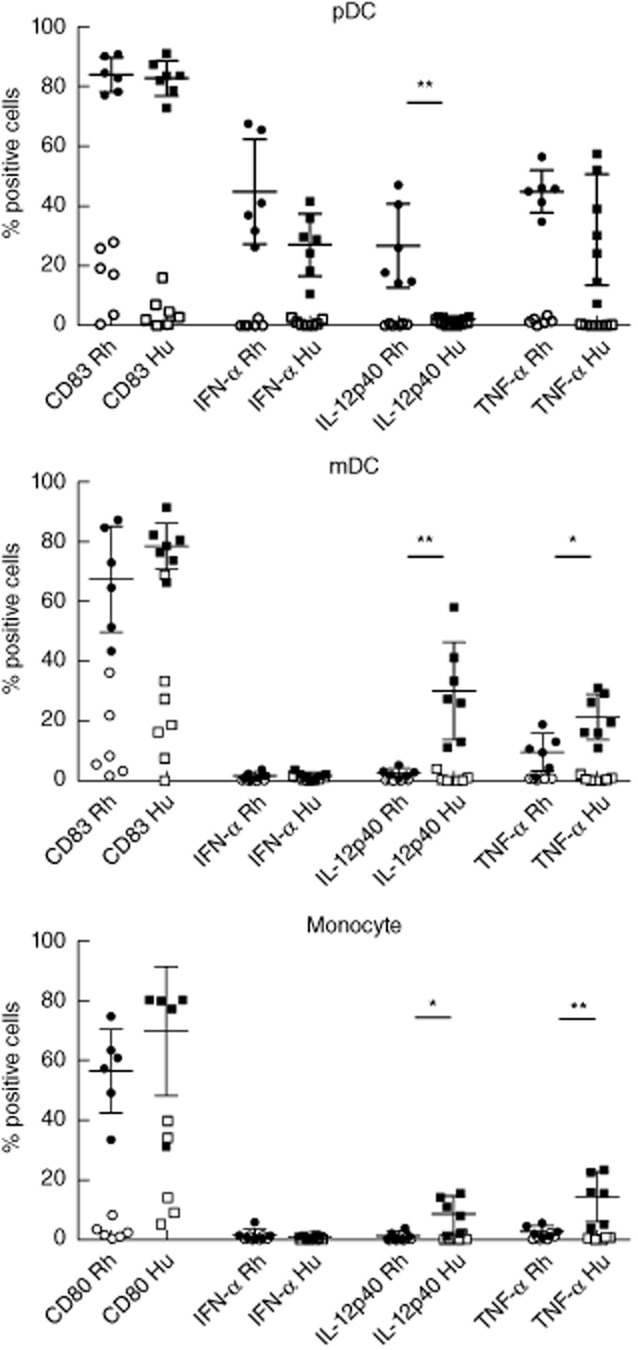
Toll-like receptor (TLR)-7/-8-induced CD83 or CD80 and cytokine expression in rhesus versus human peripheral blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes. For each subset the percentage of cells expressing CD83 (pDC and mDC) or CD80 (monocytes), interferon (IFN)-α, interleukin (IL)-12p40 or tumour necrosis factor (TNF)-α after 8 h stimulation with 1 μg/ml CL097 is shown (filled symbols); background expression as observed in cultured non-stimulated cells (open symbols) was not subtracted. *P < 0·05; **P < 0·01.
We next sought to confirm this observation by using TLR-9 triggering as a more specific pDC stimulus [32]. Figure 5 shows that stimulation with class C CpG (CpG-C) resulted in similar distinct induction of IL-12p40 expression by rhesus but not human pDC, while CD83, IFN-α and TNF-α induction was observed both in rhesus and human pDC. As expected, both rhesus and human mDC and monocytes did not produce cytokines upon CpG stimulation, although there was some up-regulation of CD83 on mDC, possibly as a bystander effect of stimulation of pDC and possibly B cells. Analysis of supernatants from CpG-stimulated cultures showed IL-12p40 production in rhesus macaques (14 pg/ml, n = 5), while human samples (n = 2) were negative. TNF-α production was comparable in rhesus and human samples; i.e. 13 and 10 pg/ml, respectively. Finally, stimulation with the TLR-4 ligand LPS did not induce any cytokine production in rhesus or human pDC, but activated mDC and monocytes in both species (Fig. 6). It should be noted that, similar to TLR-7/8 stimulation, in this study the percentage of IL-12p40-positive mDC and monocytes was also lower in rhesus macaques relative to humans. There was some induction of CD83 on pDC with TLR-4 which is, again, possibly a bystander effect of the activation of other cells.
Fig. 5.

Toll-like receptor (TLR)-9-induced CD83 and cytokine expression in rhesus (n = 6) versus human (n = 2) peripheral blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes. For each subset the percentage of cells expressing CD83 (pDC and mDC), interferon (IFN)-α, interleukin (IL)-12p40 or tumour necrosis factor (TNF)-α after 8 h stimulation with 5 μM class C cytosine–phosphate–guanosine (CpG-C) (M362) is shown (filled symbols); background expression as observed in cultured non-stimulated cells (open symbols) was not subtracted; n.d.: not done.
Fig. 6.
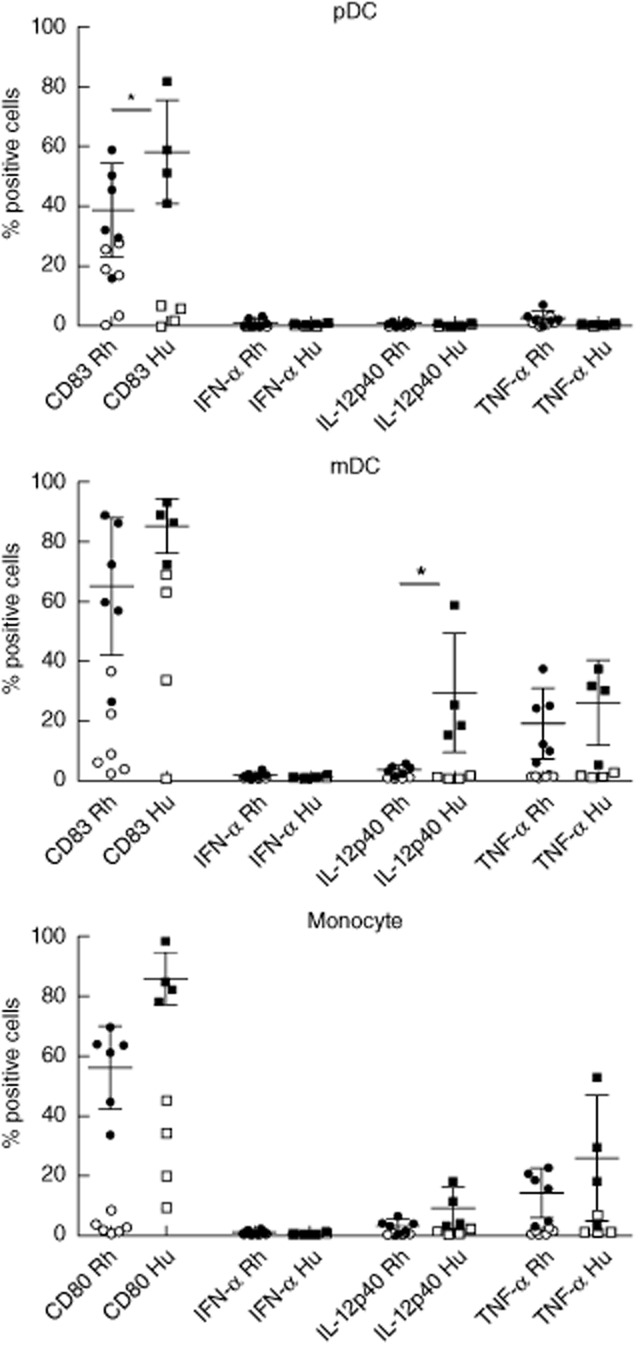
Toll-like receptor (TLR)-4-induced CD83 and cytokine expression in rhesus (n = 6) versus human (n = 4) peripheral blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes. For each subset the percentage of cells expressing CD83 (pDC and mDC) or CD80 (monocytes), interferon (IFN)-α, interleukin (IL)-12p40 or tumour necrosis factor (TNF)-α after 8 h stimulation with 1 μg/ml lipopolysaccharide is shown (filled symbols); background expression as observed in cultured non-stimulated cells (open symbols) was not subtracted. *P < 0·05.
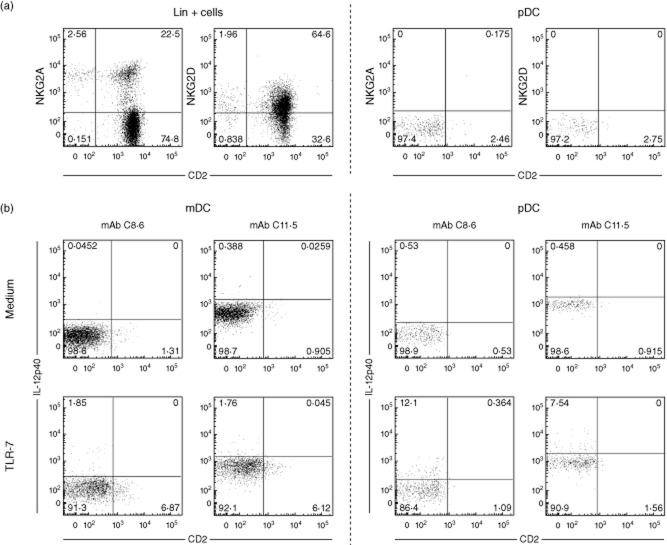
Recently a so-called ‘interferon-producing killer dendritic cell’ (IK-DC) has been described in mice, which was reported to produce both type I IFN as well as IL-12 and IFN-γ [33] upon TLR-9 stimulation, although their relation to the DC lineage remains somewhat obscure [34]. In humans a CD2-expressing pDC subset was described [35], with similar characteristics to IK-DCs in mice. However, pDC in rhesus macaques do not seem to qualify as IK-DC, because they were found to be negative for CD2 as well as natural killer (NK) cell markers NKG2A and NKG2D (Fig. 7a).
Fig. 7.
Phenotypic analysis of rhesus plasmacytoid dendritic cells (pDC) and induction of interleukin (IL)-12p40 expression. (a) Shown is expression of CD2 versus natural killer (NK) type G2A (NKG2A) or NKG2D on lineage positive cells (two left graphs) or pDC [lineage–/human leucocyte antigen-D-related (HLA-DR)+/CD11c–/CD123+) (two right graphs). (b) Expression of IL-12p40 (vertical axis) in mDC and pDC in unstimulated (medium) versus Toll-like receptor (TLR)-7/-8 (CL097)-stimulated cells, detected with either monoclonal antibody (mAb) C8·6 or mAb 11·5.
Induction of IL12p40 expression on rhesus pDC, as observed with mAb C8·6, was confirmed by using another anti-IL-12p40/70 mAb (clone C11·5), which gave similar percentages of positive cells for pDC as well as mDC upon TLR-7/8 stimulation (Fig. 7b). Finally, analysis of IL-12p40 mRNA in TLR-7/8 (CL097)-stimulated purified pDC, mDC and monocyte populations showed similar high expression levels in pDC relative to mDC and monocytes and no induction in pDC upon TLR-4 stimulation (Fig. 8a), thus confirming the FACS expression data. Both mDC and monocytes up-regulated TNF-α mRNA expression upon TLR-4 (LPS) as well as TLR-7/8 (CL097) stimulation, underscoring the functional capacity of these purified cell populations (Fig. 8b). In agreement with the FACS analysis, TNF-α mRNA expression in pDC was up-regulated only upon TLR-7/8 and not TLR-4 stimulation.
Fig. 8.
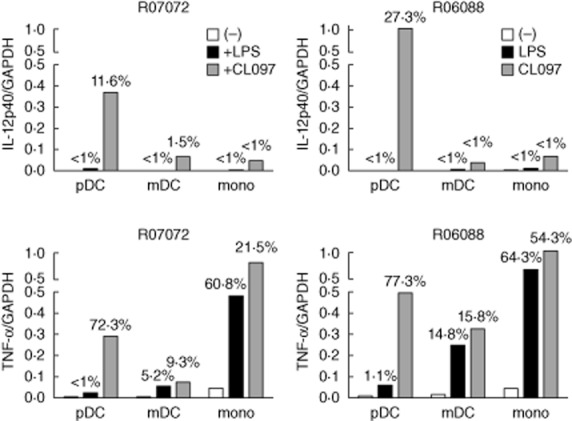
Interleukin (IL)-12p40 and tumour necrosis factor (TNF)-α mRNA expression in Toll-like receptor (TLR)-4 (lipopolysaccharide) and TLR-7/8 (CL097) stimulated purified rhesus macaque plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes. Shown are expression levels of IL-12p40 (top graphs) and TNF-α (bottom graphs) relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in pDC, mDC and monocytes as well as percentage of positive cells as detected by fluorescence activated cell sorter (FACS) analysis on the same cells (number at top of bars). Results from two individual rhesus macaques are shown.
While the mDC and pDC preparations were only 60–75% pure the other cells present were either granulocytes or monocytes and this could not have affected the IL-12p40 expression data, as monocytes were observed to have only very low IL-12p40 expression and the monocyte fraction itself was >90% pure with <5% mDC and <1% pDC contamination.
Discussion
In this work, we adapted a whole blood stimulation assay to study functional characteristics of peripheral blood DCs and monocytes in macaques and performed a direct comparison with human blood samples. Most responses of the different subsets were very similar between macaques and humans and in agreement with previous studies, in which purified cell populations instead of whole blood stimulation had been used [2,17,25–28,32]. However, we observed that, in contrast to humans, rhesus pDC expressed IL-12p40 upon stimulation with TLR-7/8 or TLR-9. Preliminary data suggest a similar IL-12p40 expression pattern in cynomolgus macaques (V.S., to be published elsewhere). We also observed that relative to humans, mDC and monocytes in rhesus macaques responded less well to TLR-7/8 stimulation when expressed as percentage of IL-12p40- and TNF-α-positive cells. Of note is that a similar relatively lower level of IL-12 induction has been reported previously for macaque monocyte-derived DC [23]. The capacity of rhesus pDC to produce IFN-α as well as IL-12p40 may potentially modify their response to viral infections, where pDC are known to play an important role [36].
Previous studies either did not include IL-12 in their analysis [23] or measured IL-12 cytokine production by enzyme-linked immunosorbent assay (ELISA) on either stimulated total PBMC or lineage-negative cell cultures [25–27]. Others used FACS analysis, but studied IL-12 expression only in LPS-stimulated PBMC [17], which would have given no expression in pDC. Hence, our observation was made possible by the use of FACS analysis to detect TLR-induced cytokine expression in all subsets simultaneously.
At present the mechanism underlying this difference in IL12p40 induction between macaque and human pDC is not yet understood. Macaque and human pDC were shown to have similar TLR expression profiles [25], which is in agreement with the response patterns observed by us. Also TLR-7, TLR-9 and myeloid differentiation primary response gene 88 (MYD88) sequences were shown to be identical, whereas there were important differences for interferon regulatory factor 7 (IRF-7) [26]. Other regulatory pathways still need to be explored [37]. Beside TLRs, the C-type lectin receptor (CLR) family plays an important role in the modulation of innate immune responses [38,39]. Human pDC express the CLRs blood dendritic cell antigen 2 (BDCA2) and dendritic cell immunoreceptor (DCIR) [40]. Cross-linking of DCIR was shown to result in reduced IFN-α induction upon TLR-9 stimulation [40], and similar inhibitory effects were reported following incubation with the CLR ligand mannan [41]. Interestingly, BDCA2 [our unpublished observation and documented at the NIH non-human primate reagent resource portal (http://nhpreagents.bidmc.harvard.edu/NHP)] and DCIR [42] were shown to be absent on pDC in rhesus macaques. Although not investigated here, a difference in the balance between activating TLRs and inhibitory CLRs could lead to different levels of pDC activation, possibly translating into a difference in cytokine production pattern.
A direct comparison between the absolute numbers of pDC, mDC and monocytes in rhesus versus human blood showed that rhesus macaques had a lower number of pDC, while there was no difference in the abundance of the other subsets. The number of pDC observed, i.e. 3020 ± 1357 cells/μl, is in agreement with several reports on rhesus macaques [16,18,24,25,43] and considerably less than in humans [44]. In contrast, two other studies, where a direct head-to-head comparison was made, showed no difference in pDC number [17,28], although it must be noted that in those studies the quantification was either performed on PBMC or cynomolgus monkeys imported from Mauritius were used, which have a more limited genetic diversity and might differ from rhesus macaques.
The strong IL-12p40 expression in rhesus pDC may have implications for preclinical evaluation of vaccines in this model. For instance, TLR-7/8 containing adjuvants might trigger different responses in macaques than in humans and involve pDC as IL-12 producing cells. Also TLR-9 agonists could be expected to induce an IL-12 response in rhesus macaques, in contrast to humans. Simultaneous production of IFN-α and the inflammatory cytokines TNF-α and T helper type 1 (Th1)-skewing cytokine IL-12 might also lead to a slightly different response pattern to bacterial and viral infection and have consequences for the induction of CD8 responses [45,46].
Acknowledgments
We would like to thank Dr F. Verreck for critical reading of the manuscript, Dr S.B. Geutskens for organizing the collection of the human blood samples and H. van Westbroek for preparing the figures. This work was supported by the Collaboration for AIDS Vaccine Discovery (CAVD) Comprehensive T Cell Vaccine Immune Monitoring Consortium (grant number 38650) from the Bill & Melinda Gates Foundation.
Disclosure
None.
References
- 1.Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Sanchez E, Altmeyer R, Amara A, et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, et al. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem. 2003;278:20358–20366. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells [see comments] Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 6.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanto T, Inoue M, Miyatake H, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 8.Macatonia SE, Patterson S, Knight SC. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989;67:285–289. [PMC free article] [PubMed] [Google Scholar]
- 9.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(–) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 11.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 12.Feldman S, Stein D, Amrute S, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 13.Grassi F, Hosmalin A, McIlroy D, et al. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 14.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 15.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koopman G, Niphuis H, Haaksma AG, et al. Increase in plasmacytoid and myeloid dendritic cells by progenipoietin-1, a chimeric Flt-3 and G-CSF receptor agonist, in SIV-infected rhesus macaques. Hum Immunol. 2004;65:303–316. doi: 10.1016/j.humimm.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Malleret B, Karlsson I, Maneglier B, et al. Effect of SIVmac infection on plasmacytoid and CD1c+ myeloid dendritic cells in cynomolgus macaques. Immunology. 2008;124:223–233. doi: 10.1111/j.1365-2567.2007.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39:235–242. doi: 10.1111/j.1600-0684.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malleret B, Maneglier B, Karlsson I, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 20.Kwa S, Kannanganat S, Nigam P, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves RK, Evans TI, Gillis J, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis. 2012;206:1462–1468. doi: 10.1093/infdis/jis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foussat A, Bouchet-Delbos L, Berrebi D, et al. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1-infected patients. Blood. 2001;98:1678–1686. doi: 10.1182/blood.v98.6.1678. [DOI] [PubMed] [Google Scholar]
- 23.Jesudason S, Collins MG, Rogers NM, Kireta S, Coates PT. Non-human primate dendritic cells. J Leukoc Biol. 2012;91:217–228. doi: 10.1189/jlb.0711355. [DOI] [PubMed] [Google Scholar]
- 24.Coates PT, Barratt-Boyes SM, Zhang L, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 25.Ketloy C, Engering A, Srichairatanakul U, et al. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Mandl JN, Barry AP, Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 27.Teleshova N, Kenney J, Jones J, et al. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004;173:1647–1657. doi: 10.4049/jimmunol.173.3.1647. [DOI] [PubMed] [Google Scholar]
- 28.Gujer C, Sundling C, Seder RA, Karlsson Hedestam GB, Lore K. Human and rhesus plasmacytoid dendritic cell and B-cell responses to Toll-like receptor stimulation. Immunology. 2011;134:257–269. doi: 10.1111/j.1365-2567.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ida JA, Shrestha N, Desai S, et al. A whole blood assay to assess peripheral blood dendritic cell function in response to Toll-like receptor stimulation. J Immunol Methods. 2006;310:86–99. doi: 10.1016/j.jim.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Tel J, Beenhakker N, Koopman G, et al. Targeted delivery of CpG ODN to CD32 on human and monkey plasmacytoid dendritic cells augments IFNalpha secretion. Immunobiology. 2012;217:1017–1024. doi: 10.1016/j.imbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 34.Guimont-Desrochers F, Boucher G, Dong Z, et al. Redefining interferon-producing killer dendritic cells as a novel intermediate in NK-cell differentiation. Blood. 2012;119:4349–4357. doi: 10.1182/blood-2011-11-395954. [DOI] [PubMed] [Google Scholar]
- 35.Matsui T, Connolly JE, Michnevitz M, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon [see comments] Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 37.Moynagh PN. TLR signalling and activation of IRFs: revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005;26:469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 39.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 41.Seeds RE, Mukhopadhyay S, Jones IM, Gordon S, Miller JL. The role of myeloid receptors on murine plasmacytoid dendritic cells in induction of type I interferon. Int Immunopharmacol. 2011;11:794–801. doi: 10.1016/j.intimp.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romain G, van Gulck E, Epaulard O, et al. CD34-derived dendritic cells transfected ex vivo with HIV-Gag mRNA induce polyfunctional T-cell responses in nonhuman primates. Eur J Immunol. 2012;42:2019–2030. doi: 10.1002/eji.201242478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autissier P, Soulas C, Burdo TH, Williams KC. Immunophenotyping of lymphocyte, monocyte and dendritic cell subsets in normal rhesus macaques by 12-color flow cytometry: clarification on DC heterogeneity. J Immunol Methods. 2010;360:119–128. doi: 10.1016/j.jim.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 45.Di Pucchio T, Chatterjee B, Smed-Sorensen A, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol. 2012;90:492–497. doi: 10.1038/icb.2012.7. [DOI] [PubMed] [Google Scholar]