Abstract
There is increasing interest in the role of T cell exhaustion and it is well known that the natural history of chronic hepatitis C virus infection (HCV) is modulated by CD8+ T cell immunobiology. There are many pathways that alter the presence of exhaustive T cells and, in particular, they are functionally impaired by inhibitory receptors, such as programmed death-1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3). We obtained spleen, liver and peripheral blood (before and after splenectomy) lymphoid cells from 25 patients with HCV-related cirrhosis undergoing liver transplantation for end-stage disease or splenectomy for portal hypertension. In all samples we performed an extensive phenotypic study of exhaustion markers [PD-1, Tim-3, interferon (IFN)-γ) and their ligands (PD-L1, PD-L2, galectin-9] in CD8+ T cell subpopulations (both total and HCV-specific) and in antigen-presenting cells (APC; monocytes and dendritic cells). In the spleen, total and HCV-specific CD8+ T cells demonstrated enhanced markers of exhaustion, predominantly in the effector memory subpopulation. Similarly, splenic APC over-expressed inhibitory receptor ligands when compared to peripheral blood. Finally, when peripheral blood CD8+ T cells were compared before and after splenectomy, markers of exhaustion were reduced in splenic CD8+ T cells and APC. Our data in HCV-related cirrhosis suggest that CD8+ T cells in the spleen manifest a significantly higher exhaustion compared to peripheral blood and may thus contribute to the failure to control HCV. Counteracting this process may contribute to inducing an effective immune response to HCV.
Keywords: hepatitis C, liver cirrhosis, PD-1, portal hypertension, splenectomy, Tim-3
Introduction
During the course of chronic viral infection, T cells may undergo both memory and exhaustion as part of the natural history in the modulation of infection, as well exemplified by hepatitis C virus (HCV) in both liver and peripheral blood [1,2]. In this respect, the spleen is often ignored despite its key role in the regulation of the immune response to infectious agents [3,4]. Importantly, a significant proportion of HCV-infected patients develop hypersplenism, which is a contributing factor to their co-morbidities of liver cirrhosis and portal hypertension [5–7]. We note an increasing role for CD8+ T cells in clearing infection, as a mediator in autoimmunity and, in particular, the observation that 70% of infected individuals are unable to clear HCV [8,9]. These observations suggest that T cell exhaustion is part of the natural history of HCV. CD8+ T cell exhaustion is co-regulated by multiple inhibitory receptors, and the interaction between cellular receptors and inhibitory receptor ligands on antigen-presenting cells (APC) regulate virus persistence [2,10–13]. In fact, as part of the hypersplenic syndrome, the spleen is often removed in patients with HCV-mediated liver cirrhosis and portal hypertension with thrombocytopenia because of their association with thrombocytopenia and the subsequent risk of further reductions in platelet counts following anti-viral therapies; the risk associated with spleen removal includes an increased risk of infection [14–16]. Our laboratory has demonstrated recently that splenectomy in HCV patients is followed by an increase of interferon (IFN)-γ production and a reduction of programmed death 1 (PD-1) expression by CD4+ T cells in peripheral blood of patients with HCV-related cirrhosis [17]. To extend these observations, we report herein the phenotype of exhausted CD8+ T cells in the spleen of patients with HCV-related cirrhosis undergoing splenectomy. Our data have significant implications for understanding the cellular alterations that occur in HCV-related cirrhosis and the subsequent loss of spleen.
Materials and methods
Subjects
Sixteen patients with HCV-related liver cirrhosis undergoing splenectomy for severe thrombocytopenia were studied. In all patients, the spleen was removed because of severe thrombocytopenia that was a contraindication for interferon (IFN)-α therapy. In addition, there were nine patients with HCV-related liver cirrhosis who underwent liver transplantation. Liver, spleen and peripheral blood mononuclear cells were isolated from patients. All subjects gave their written informed consent and experimental protocols were conducted under the Guidelines of the Research Ethics Committee of Kyushu University.
Isolation of mononuclear cells and CD8+ T cells
Peripheral blood mononuclear cells (PBMC) were separated from heparinized fresh blood by gradient centrifugation on Ficoll-Isopaque, while spleen mononuclear cells (SMC) and liver mononuclear cells (LMC) were isolated from fresh explanted tissues using established protocols [18]. Briefly, spleen tissues were digested mechanically and dissociated cells filtered through 100-μm nylon mesh, while liver specimens were first digested with 1 mg/ml of collagenase type IV (Sigma-Aldrich, Tokyo, Japan) and filtered through 100-μm nylon mesh. Digested spleen and liver cells were separated by Ficoll-Isopaque gradient centrifugation to obtain SMC and LMC. CD8+ T cells were negatively isolated using magnetic beads (CD8 isolation kit II; Miltenyi Biotec, Auburn, CA, USA) from PBMC, SMC and LMC; >95% viability by trypan blue dye exclusion and >90% purity by flow cytometry were considered as acceptable. All cells were washed and cryopreserved in fetal cow serum containing 10% dimethylsulphoxide (DMSO) and stored in liquid nitrogen until used.
Mononuclear cell immunophenotyping
PBMC, LMC and SMC (1 × 106) were stained for cell surface antigen expression at 4°C in the dark for 30 min, washed twice in 2 ml phosphate-buffered saline containing 1% bovine serum albumin and 0·01% sodium azide, and fixed in 500 μl of 1% paraformaldehyde. Cells were stained for CD8, CD14, PD-1, PD-L1, PD-L2 (BD Biosciences, San Diego, CA, USA), CD45RA, CCR7, CD11c (e-Biosciences, San Diego, CA, USA), T cell immunoglobulin and mucin domain-containing protein-3 (Tim-3) (R&D Systems, Minneapolis, MN, USA) and galectin-9 (Biolegend, San Diego, CA, USA). Of note, we stained peripheral blood, liver and spleen antigen-presenting cells (APC) identified as CD14+ (i.e. monocytes) or CD11c+ (i.e. myeloid dendritic cells) for the PD-1 ligands L1 and L2 and the Tim-3 ligand galectin-9 [19,20]. CD8+ T cells in PBMC, LMC and SMC were arrayed as naive T cells (CCR7+CD45RA+), central memory T cells (CCR7+CD45RA−), effector memory T cells (CCR7−CD45RA−) and terminal differentiated effector memory T cells that re-expressed CD45RA–EMRA (CCR7−CD45RA+) [21].
CD8+ T cell cytokine staining and tetramers
For intracellular cytokine staining of CD8+ T cells, fresh PBMC, LMC and SMC were cultured in vitro for 6 h in plates precoated with anti-CD3 (10 μg/ml; R&D Systems) and anti-CD28 (5 μg/ml; R&D systems) monoclonal antibodies. Cells were washed once with fluorescence activated cell sorter (FACS) buffer and stained with T cell markers at 4°C in the dark for 30 min and then fixed and permeabilized with the Cytofix/Cytoperm Kit (BD Biosciences), washed twice with permeabilization buffer, and stained using anti-IFN-γ (BD Biosciences). Multi-parameter flow cytometry was performed using a FACSCaliber Flow Cytometer (BD Biosciences) equipped with FlowJo software (Tree Star, Ashland, OR, USA).
Fluorochrome-labelled HLA-A0201 tetramers for CD8+ T cell staining [Medical and Biological Laboratories (MBL), Nagoya, Japan] included HCV NS3 1073 (CINGVCWTV), NS3 1406 (KLVALGINAV) and NS5B 2594 (ALYDVVSKL), while HLA-A2402 tetramers included HCV E2 717 (EYVLLLFLL), NS3 1292 (TYSTYGKFL) and NS5B 2870 (CYSIEPLDL). After incubation with human Fc receptor blocking reagent (MBL) at room temperature for 5 min, cryopreserved mononuclear cells (1 × 106) were stained for tetramers at 4°C in the dark for 30 min, and stained for CD8, PD-1 and Tim-3.
Statistical analysis
All continuous variables were expressed as mean ± standard deviation (s.d.) and compared between groups by Student's t-test. All analyses were two-tailed and P-values < 0·05 were considered statistically significant.
Results
Subjects
The characteristics of patients undergoing splenectomy for portal hypertension or during liver transplantation are summarized in Table 1. As expected, patients undergoing splenectomy during the course of liver transplantation had signs of more advanced disease represented by higher bilirubin levels, lower prothrombin activity and Child Pugh class C. Conversely, patients undergoing splenectomy for portal hypertension had significantly lower platelet counts.
Table 1.
Clinical characteristics of patients with hepatitis C virus (HCV)-related liver cirrhosis undergoing splenectomy for portal hypertension and thrombocytopenia or during liver transplantation
| Portal hypertension (n = 16) | Liver transplantation (n = 9) | |
|---|---|---|
| Age (years) | 61 ± 8·3 | 60 ± 6·3 |
| Male sex (%) | 8 (50%) | 4 (44%) |
| Platelet count (/mm3) | 5000 ± 1900 | 7300 ± 1900* |
| ALT (IU/l) | 53 ± 30 | 53 ± 54 |
| Total bilirubin (mg/dl) | 1·3 ± 0·4 | 3·1 ± 2·3* |
| Albumin (g/dl) | 3·5 ± 0·3 | 2·8 ± 0·7* |
| Prothrombin activity (%) | 74 ± 10 | 58 ± 16* |
| Child–Pugh class A–B | 16 (100%) | 3 (33%)* |
Variables are expressed as mean ± standard deviation.
P < 0·05. ALT: alanine aminotransferase.
Exhaustion markers in CD8+ T cells and APC in different organs
CD8+ T cells were positive for exhaustion markers (i.e. expressing both PD-1 and Tim-3) more frequently in the spleen (8·8 ± 5·8%) and the liver (17·2 ± 15·3%) compared to the peripheral blood (3·9 ± 5·0%, P < 0·01 versus the liver and the spleen) of patients with HCV-related liver cirrhosis (Fig. 1a). Upon stimulation with anti-CD3/CD28, IFN-γ expression was lower in spleen- and liver-derived cells (12·2 ± 6·3 and 10·1 ± 5·8%, respectively) compared to peripheral blood-derived cells (19·6 ± 9·2%; P < 0·05, for both comparisons) (Fig. 1a).
Fig. 1.
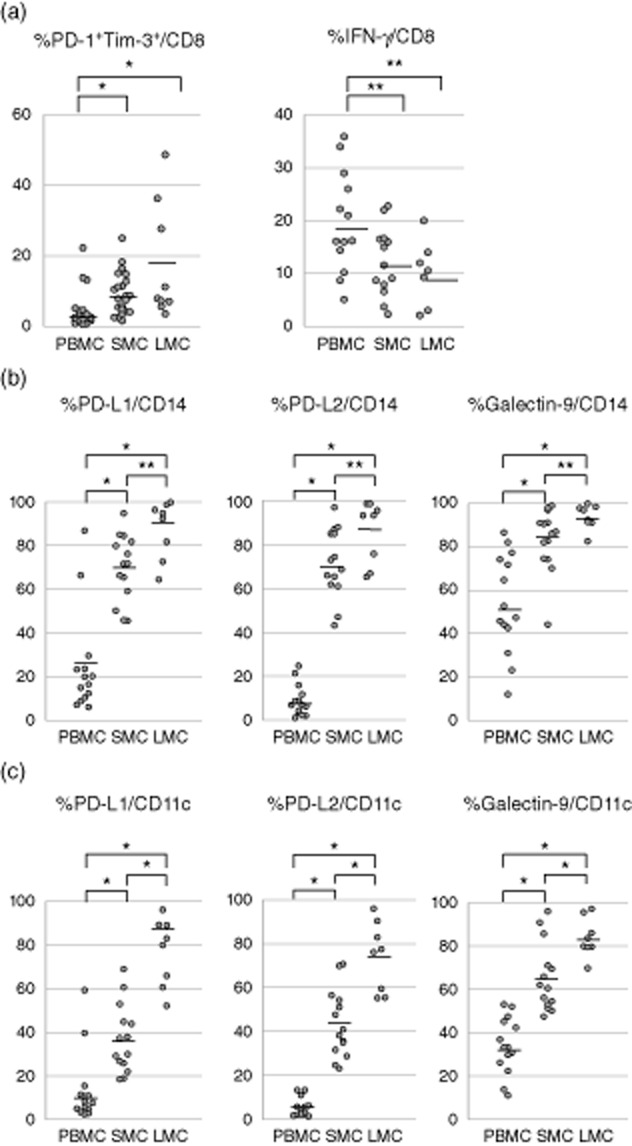
Phenotype of CD8+ T cells and antigen-presenting cells (APC) in different tissues from patients with hepatitis C virus (HCV)-related cirrhosis. (a) Expression of exhaustion markers programmed death 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein-3 (Tim-3) on CD8+ T cells from spleen, liver and peripheral blood, and interferon (IFN)-γ production from CD8+ T cells upon CD3 and CD28 stimulation. The frequency of dual PD-1+ and Tim-3+ (i.e. exhausted) T cells in the spleen and the liver are significantly higher compared to the peripheral blood, while the IFN-γ production from CD8+ T cells in the spleen and the liver are decreased. *P < 0·01 and **P < 0·05. (b) Expression of the PD-L1, PD-L2 and galectin-9 ligands on CD14+ monocytes from different organs. The frequency of PD-L1+, PD-L2+ and galectin-9+ cells in the spleen and the liver is higher compared to the peripheral blood. (c) Expression of the PD-L1, PD-L2 and galectin-9 ligands on CD11c+ dendritic cells from different organs. The frequency of PD-L1+, PD-L2+ and galectin-9+ cells in the spleen and the liver is higher compared to the peripheral blood. *P < 0·01 and **P < 0·05.
The frequency of CD14+ monocytes expressing PD-1 ligands (PD-L1, PD-L2) and Tim-3 ligand (galectin-9) was higher in the spleen (PD-L1; 69·9 ± 14·8%, PD-L2; 71·5 ± 15·1%, galectin-9; 83·4 ± 13·9%) and liver (PD-L1; 87·7 ± 12·3%, PD-L2; 85·8 ± 13·3%, galectin-9; 93·4 ± 5·3%) compared to peripheral blood (PD-L1; 24·7 ± 6·3%, PD-L2; 8·9 ± 7·1%, galectin-9; 54·0 ± 22·1%, P < 0·01 for all comparisons) (Fig. 1b). Similar differences were observed in ligand expression on CD11c+ dendritic cells from the spleen (PD-L1; 37·0 ± 15·1%, PD-L2; 43·5 ± 14·8%, galectin-9; 65·4 ± 15·1%), liver (PD-L1; 77·7 ± 14·6%, PD-L2; 74·6 ± 14·8%, galectin-9; 82·7 ± 8·4%) and peripheral blood (PD-L1; 13·5 ± 15·6%, PD-L2; 5·4 ± 4·1%, galectin-9; 34·2 ± 12·6%; P < 0·01 for all comparisons) (Fig. 1c).
CD8+ T cell differentiation markers
The frequency of naive T cells in peripheral blood (22·8 ± 15·7%) and spleen (16·9 ± 16·1%) was significantly higher compared to the liver (2·4 ± 2·4%; P < 0·05 for LMC versus SMC, P < 0·01 for LMC versus PBMC) (Fig. 2b). Exhausted effector memory CD8+ T cells identified by PD-1+Tim-3+ co-expression were represented significantly more in spleen (7·5 ± 7·3%) and liver (10·8 ± 7·9%) compared to peripheral blood (2·7 ± 2·9%; P < 0·05 for both comparisons), and the same tendency was observed for central memory cells (liver; 5·8 ± 5·5%, spleen; 2·5 ± 2·5%, peripheral blood; 0·6 ± 0·8%). For both EMRA and naive T cells, the frequency of exhausted cells was similar in the three tissues (Fig. 3).
Fig. 2.
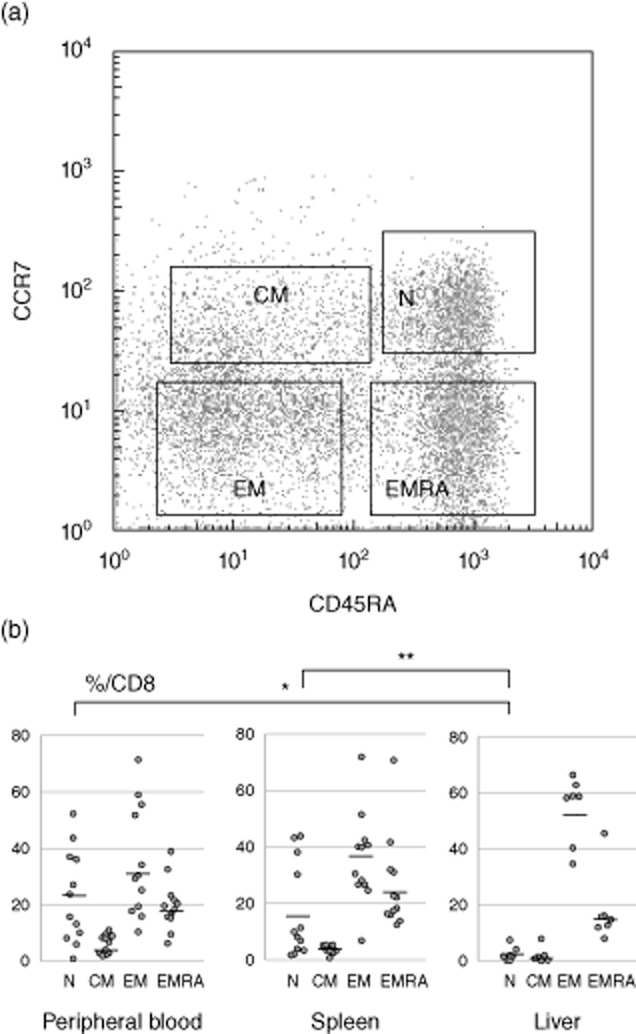
Differentiation of CD8+ T cells in different organs. (a) CD8+ T cells are classified as naive (N: CCR7+CD45RA+), central memory (CM: CCR7+CD45RA−), effector memory (EM: CCR7− CD45RA−) and terminal effectors with CD45 RA-positive (EMRA: CCR7− CD45RA+). (b) The frequency of naive T cells in the peripheral blood and the spleen are higher in comparison with the liver. *P < 0·01 and **P < 0·05.
Fig. 3.
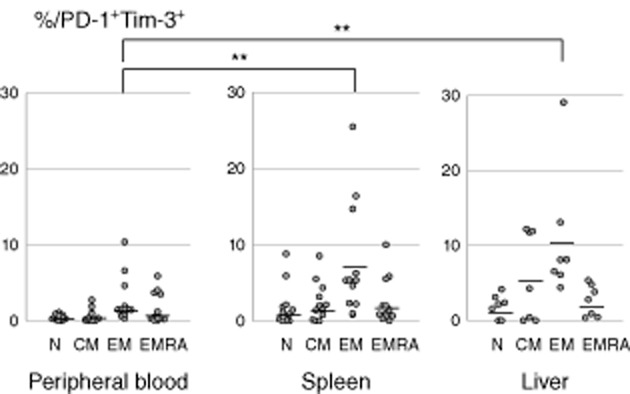
Exhaustion and differentiation markers in CD8+ T cells from different organs. The frequency of dual programmed death 1 (PD-1)+ and T cell immunoglobulin and mucin domain-containing protein-3 (Tim-3)+ effector memory T cells in the spleen and liver are higher in comparison with the peripheral blood. **P < 0·05.
HCV-specific spleen CD8+ T cells
HCV-specific tetramer positive T cells were represented more significantly in the spleen (0·60 ± 0·15%) compared to the peripheral blood (0·20 ± 0·11%, P < 0·05) and the latter tissue also had lower expression of exhaustion markers (82·5 ± 9·5 versus 58·3 ± 21·6% in peripheral blood; P < 0·05) (Fig. 4).
Fig. 4.
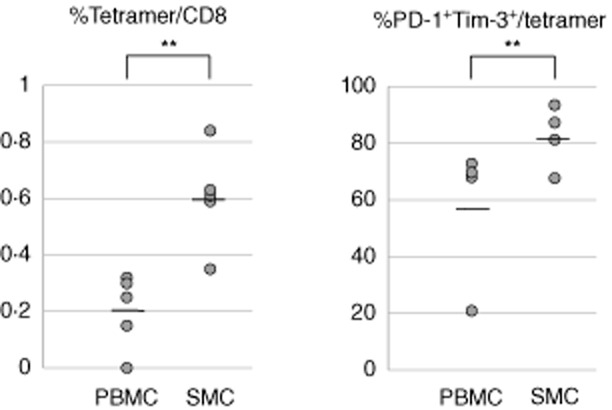
Hepatitis C virus (HCV)-specific T cells from different organs are studied with human leucocyte antigen (HLA) class I tetramers. (a) HCV-specific CD8+ T cells are enriched in the spleen compared to peripheral blood. (b) Dual programmed death 1 (PD-1)+ and T cell immunoglobulin and mucin domain-containing protein-3 (Tim-3) expression was increased in the spleen compared to peripheral blood. **P < 0·05.
Effect of splenectomy on CD8+ T cells and APC
Following splenectomy, the frequency of exhausted peripheral blood CD8+ T cells was reduced significantly (2·6 ± 1·5 versus 1·7 ± 1·2%; P < 0·05), while the IFN-γ production was increased (15·7 ± 7·8 versus 20·9 ± 9·3%; P < 0·05) (Fig. 5a). Similarly, splenectomy was associated with a reduced expression of PD-L2 (2·5 ± 2·2 versus 7·4 ± 5·4% before; P < 0·05) and galectin-9 (29·7 ± 21·2 versus 60·5 ± 20·1% before; P < 0·01) on CD14+ monocytes (Fig. 5b) and CD11c+ dendritic cells (PD-L2: 1·6 ± 0·8 versus 4·3 ± 2·8% before; P < 0·05; galectin-9: 20·7 ± 14·4 versus 39·9 ± 12·2% before; P < 0·01) (Fig. 5c).
Fig. 5.
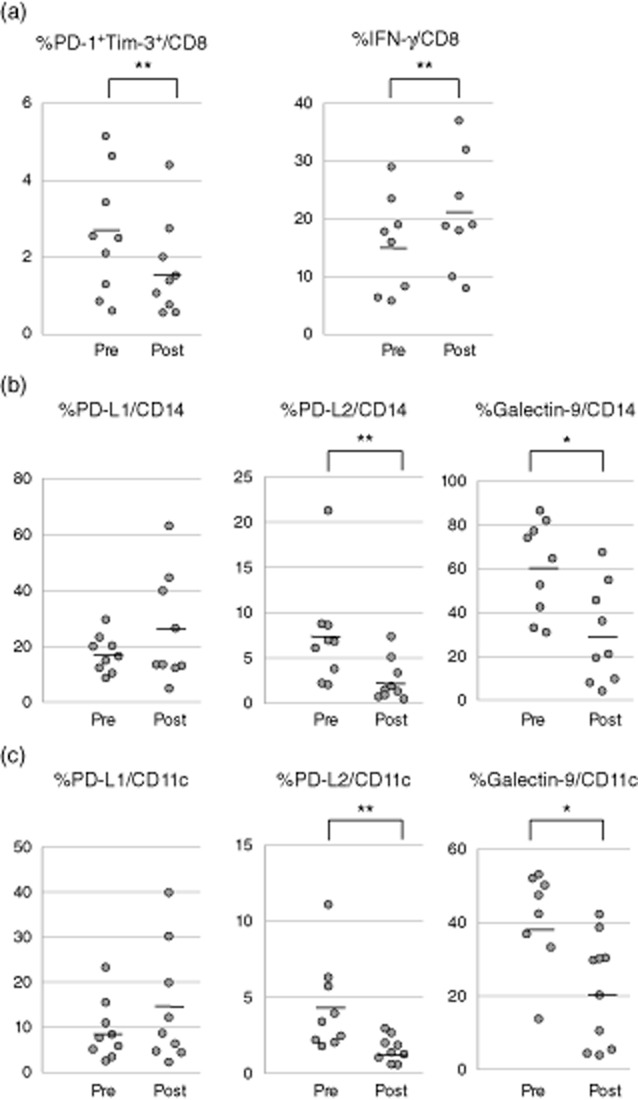
CD8+ T cell exhaustion and function markers are studied before and after splenectomy. (a) Dual programmed death 1 (PD-1)+ and T cell immunoglobulin and mucin domain-containing protein-3 (Tim-3) expression is decreased following splenectomy, while interferon (IFN)-γ production is increased compared to pre-splenectomy. (b) The frequency of the ligands PD-L2 and galectin-9-expressing monocytes (CD14+) is decreased following splenectomy compared to pre-splenectomy. (c) The frequency of PD-L2 and galectin-9 on dendritic cells (CD11c+) is decreased following splenectomy. *P < 0·01 and **P < 0·05.
Discussion
The factors impairing HCV clearance and allowing chronic infection remain largely enigmatic, despite enormous research efforts to dissect potential therapeutic targets. Indeed, virus-mediated T cell exhaustion limits T cell function, thus promoting chronic disease, and we report for the first time that spleen effector memory T cells manifest significant exhaustion while spleen APC over-express inhibitory receptor ligands when compared to peripheral blood in patients with HCV-related cirrhosis. Further, splenectomy leads to a reduction of exhaustion markers and an increase of IFN-γ production along with a reduced APC expression of inhibitory receptor ligands.
There is a relative paucity of data on the issue of T cell exhaustion, and the majority of studies focus on patients with viral infections. There are significant data on the clinical significance of CD8+ cells and their subsets, including the related issue of epitope spreading [22–30]. T cell exhaustion has been characterized as over-expression of several inhibitory receptors, including PD-1 [2,31] and Tim-3 [11,12]. The expression of both Tim-3 and PD-1 on CD8+ T cells is thus the established marker for exhaustion and may contribute to the perpetuation of HCV infection [13]. During HCV chronic hepatitis, splenomegaly occurs following portal hypertension [7], and splenectomy may reduce portal hypertension and increase the number of white blood cells and platelets [14,32], along with an established risk of overwhelming post-splenectomy infections (OPSI) by encapsulated bacteria such as Streptococcus pneumonia [15]. Most recently, new therapeutic approaches limited the need for surgery in these cases, as represented by the use of eltrombopag before anti-viral induction [33], and make the present study design unlikely to be recapitulated in the future. We performed a detailed investigation of T cell phenotypes in the spleen, liver and peripheral blood of patients with HCV-related cirrhosis and portal hypertension, and clarified that spleen T cell phenotypes are not so different despite the observation that peripheral naive T cells are decreased and peripheral effector memory T cells are increased when compared to healthy subjects [34].
The surgical removal of lymphoid compartments, as in the case of tonsillectomy, improves autoimmune disease based on T cell changes [35], but it remains to be determined whether lymphoid compartments regulate T cell exhaustion, and only a few studies have investigated spleen CD8+ T cells in HCV-related liver cirrhosis. In HCV chronic infection, exhaustion markers are expressed highly in liver HCV-specific CD8+ T cells [2] and we have reported previously that spleen CD4+ T cells become exhausted and functionally impaired [17]. HCV-specific T cells within the liver over-express PD-1 [2] and we report herein a similar observation in the spleen. Further, effector and memory T cells heterogeneity includes separate models of precursors, decreasing potential, signal strength and asymmetric cell fate [36], but we could not identify differences in exhaustion markers. Indeed, we report that PD-1 and Tim-3 double-positive naive T cells are found in the spleen and liver but not in peripheral blood, thus suggesting that this specific homing may contribute to chronic infection establishment. Indeed, HCV antigens are over-expressed in liver, and thus our data that exhausted T cells are expressed more frequently in liver and spleen, compared to peripheral blood, is consistent with a local immune response. Future studies should focus on whether such CD8+ T cells are viral specific.
We should also note that we studied APC ligands interacting with exhaustion markers on T cells to regulate the T cell response [19,20], as represented by the effects of both PD-1 and Tim-3 ligands. Myeloid dendritic (CD11c+) cells in the peripheral blood from patients with chronic HCV infection over-express the PD-1 ligands (PD-L1, PD-L2) and induce the proliferation of regulatory T cells [37,38], while the Tim-3 ligand galectin-9 is well represented in the serum and liver (particularly Kupffer cells) during chronic HCV infection [39]. We report that both myeloid dendritic cells and monocytes in the spleen express all three ligands significantly more compared to peripheral blood and hypothesize that this may contribute to CD8+ T cell dysfunction in the spleen. We are particularly intrigued by the possibility that antibodies against PD-1 and Tim-3 ligands may restore the in-vitro cytotoxicity of virus antigen-specific T cells [2,13], thus counteracting exhaustion, but the anti-viral efficacy of this approach remains inconclusive [40]. In a similar fashion, transcription factors such as T-bet or eomesdermin (Eomes) [41] may control T cell exhaustion, and the resulting poor effector function and gene therapy may target this pathway [42,43] or the cytokine signalling 3 suppressor (SOCS3) through interleukin (IL)-7 [44].
Lastly, we investigated whether or not T cell exhaustion could be modified by splenectomy performed to allow anti-viral treatment by increasing the platelet count [14]. Of note, T cell function improves following splenectomy, as represented by the decrease in CD8+ T cell exhaustion markers and APC PD-1 and Tim-3 ligands in peripheral blood and we speculate that splenectomy may reduce the efflux of exhaustion ligands to T cells. In conclusion, this is the first study aimed at identifying markers of T cell exhaustion in the spleen of patients with HCV-related cirrhosis and portal hypertension and our data suggest cumulatively that the spleen may act as a rheostat for modulating this phenomenon impairing T cell functions. Based on the consistent lines of evidence reported, we suggest that this pathway constitutes an optimal therapeutic target in chronic HCV infection, particularly at advanced stages.
Disclosure
None.
References
- 1.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. doi: 10.1053/j.gastro.2008.02.033. 37 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- 4.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 5.Giannini E, Borro P, Botta F, et al. Serum thrombopoietin levels are linked to liver function in untreated patients with hepatitis C virus-related chronic hepatitis. J Hepatol. 2002;37:572–577. doi: 10.1016/s0168-8278(02)00274-x. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 7.Weksler BB. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007;26(Suppl. 1):13–19. doi: 10.1111/j.1365-2036.2007.03512.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 9.Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 10.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 11.Golden-Mason L, Palmer BE, Kassam N, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahan RH, Golden-Mason L, Nishimura MI, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akahoshi T, Tomikawa M, Korenaga D, Ikejiri K, Saku M, Takenaka K. Laparoscopic splenectomy with peginterferon and ribavirin therapy for patients with hepatitis C virus cirrhosis and hypersplenism. Surg Endosc. 2010;24:680–685. doi: 10.1007/s00464-009-0653-6. [DOI] [PubMed] [Google Scholar]
- 15.Cameron PU, Jones P, Gorniak M, et al. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PLoS ONE. 2011;6:e23164. doi: 10.1371/journal.pone.0023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120–126. doi: 10.1016/j.gassur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto N, Shimoda S, Kawanaka H, et al. Modulation of CD4(+) T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol. 2011;165:243–250. doi: 10.1111/j.1365-2249.2011.04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamihira T, Shimoda S, Nakamura M, et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology. 2005;41:151–159. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 19.Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 20.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6:e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg RA, Via CS. T cells, murine chronic graft-versus-host disease and autoimmunity. J Autoimmun. 2012;39:240–247. doi: 10.1016/j.jaut.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS ONE. 2013;8:e60186. doi: 10.1371/journal.pone.0060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LR, Wohlleber D, Reisinger F, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- 25.Mangalam AK, Luckey D, Giri S, et al. Two discreet subsets of CD8 T cells modulate PLP(91-110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. J Autoimmun. 2012;38:344–353. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39:347–353. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian L, De Hertogh G, Fedeli M, et al. Loss of T cell microRNA provides systemic protection against autoimmune pathology in mice. J Autoimmun. 2012;38:39–48. doi: 10.1016/j.jaut.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Toth I, Le AQ, Hartjen P, et al. Decreased frequency of CD73+CD8+ T cells of HIV-infected patients correlates with immune activation and T cell exhaustion. J Leukoc Biol. 2013 doi: 10.1189/jlb.0113018. doi: 10.1189/jlb.0113018. [DOI] [PubMed] [Google Scholar]
- 29.Unger WW, Velthuis J, Abreu JR, et al. Discovery of low-affinity preproinsulin epitopes and detection of autoreactive CD8 T-cells using combinatorial MHC multimers. J Autoimmun. 2011;37:151–159. doi: 10.1016/j.jaut.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 30.West EE, Jin HT, Rasheed AU, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 32.Akahoshi T, Tomikawa M, Kawanaka H, et al. Laparoscopic splenectomy with interferon therapy in 100 hepatitis-C-virus-cirrhotic patients with hypersplenism and thrombocytopenia. J Gastroenterol Hepatol. 2012;27:286–290. doi: 10.1111/j.1440-1746.2011.06870.x. [DOI] [PubMed] [Google Scholar]
- 33.McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–2236. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 34.Sathaliyawala T, Kubota M, Yudanin N, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, et al. Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. J Immunol. 2012;188:5160–5165. doi: 10.4049/jimmunol.1102834. [DOI] [PubMed] [Google Scholar]
- 36.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolganiuc A, Paek E, Kodys K, Thomas J, Szabo G. Myeloid dendritic cells of patients with chronic HCV infection induce proliferation of regulatory T lymphocytes. Gastroenterology. 2008;135:2119–2127. doi: 10.1053/j.gastro.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 38.Jeong HY, Lee YJ, Seo SK, et al. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J Leukoc Biol. 2008;83:755–764. doi: 10.1189/jlb.0307168. [DOI] [PubMed] [Google Scholar]
- 39.Mengshol JA, Golden-Mason L, Arikawa T, et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seigel B, Bengsch B, Lohmann V, Bartenschlager R, Blum HE, Thimme R. Factors that determine the antiviral efficacy of HCV-specific CD8(+) T cells ex vivo. Gastroenterology. 2013;144:426–436. doi: 10.1053/j.gastro.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrini M, Calzascia T, Toe JG, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]


