Abstract
Detection and isolation of viable alloreactive T cells at the single-cell level requires a cell surface marker induced specifically upon T cell receptor activation. In this study, a member of the tumour necrosis factor receptor (TNFR)-family, CD137 (4-1BB) was investigated for its potential to identify the total pool of circulating alloreactive T cells. Optimal conditions for sensitive and specific detection of allogeneic-induced CD137 expression on circulating T cells were established. Thereafter, CD137+ alloreactive T cells were phenotypically and functionally characterized by multi-parameter flow cytometry. Alloantigen-induced CD137 expression identified both alloreactive CD8+ T cells (mean ± standard error of the mean: 0·21 ± 0·07%) and alloreactive CD4+ T cells (0·21 ± 0·05%). CD137+ alloreactive T cells were detected in different T cell subsets, including naive T cells, but were found preferentially in CD28+ T cells and not in the terminally differentiated T cell subset. Upon allogeneic (re-)stimulation, the cytokine-producing as well as proliferative capacity of T cells resided mainly within the CD137-expressing fraction. About 10% of the CD137+ alloreactive T cells produced any combination of interferon (IFN)-γ, interleukin (IL)-2 and TNF-α. Polyfunctional alloreactive T cells, defined by multiple cytokine expression, were observed infrequently. In conclusion, activation-induced CD137 expression is a fast assay allowing for detection and functional analysis of the total alloreactive T cell compartment at the single-cell level by multi-parameter flow cytometry.
Keywords: alloreactivity, CD137, CD4+ T cells, CD8+ T cells, multi-parameter analysis
Introduction
Alloreactive T cells are important mediators of rejection or tolerance of the transplanted organ [1]. Research on circulating alloreactive T cells has evolved during recent years from measuring proliferating cells or their cytokine production in a mixed lymphocyte reaction to analyses at the single T cell level. Limiting dilution assays have enabled quantification of alloreactive CD4+ [2] and CD8+ [3] T cell precursor frequencies [4]. The disadvantages of these assays include its high labour-intensive character and complexity with respect to data analysis. The newer techniques have characterized T cell responses at the single-cell level by enzyme-linked immunospot (ELISPOT) [5,6] assay, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled T cell flow cytometric analysis of proliferation [7,8] or cytokine flow cytometry [9,10]. ELISPOT has been used successfully to identify alloreactive cytokine-producing T cells with a lower limit of detection of 1:10·000 in the peripheral blood monuclear cell (PBMC) population [11]. However, this assay is limited, as predominantly memory T cells [12] are detected and alloreactive T cells cannot be characterized in more detail.
Multi-parameter flow cytometry has the potential for in-depth phenotypic analysis of alloreactive cytokine-producing T cells. However, this method has gained little interest, as the very low frequency of cytokine+ alloreactive T cells requires a skilled flow cytometry measurement resulting in low background signals [13]. If this is not applied it may lead to the erroneous conclusion that cytokine+ alloreactive T cells cannot be detected in the peripheral blood [14]. Similar to the ELISPOT assay, alloreactive T cells not producing the analysed cytokine go undetected, resulting in an underestimation of alloreactive T cell precursor frequencies [15].
Identification of antigen-reactive T cells by activation-induction expression of co-stimulatory molecules such as CD154 and CD137 has recently enabled identification and isolation of virus-reactive T cells on the single-cell level [16–19]. We have adapted the CD154 live assay [16,17] and were able to identify alloreactive CD4+ T cells with great sensitivity and specificity in combination with phenotypic and functional analysis on the single-cell level [13]. The short-term culture period allows for a fast assay without significant changes in the phenotype of the T cell subsets.
However, CD154 is not expressed by alloreactive CD8+ T cells [13] and therefore the assay cannot be used to identify the total pool of alloreactive T cells. Cell surface expression of CD137, a member of the tumour necrosis factor receptor (TNFR) family, has been used successfully to identify antigen-reactive cells in both the CD4+ and CD8+ T cell compartments [18,20–22].
In this study, a high-sensitivity multi-parameter flow cytometric fast assay was used to evaluate activation-induced CD137 expression as a tool for detection of alloreactive T cells in different T cell subsets, in combination with their cytokine secretion profile.
Material and methods
Blood samples
Human leucocyte antigen (HLA)-typed buffy coats were purchased from Sanquin blood bank, Rotterdam, the Netherlands. These buffy coats were obtained from healthy human donors upon receiving written informed consent with regard to scientific use. The current study did not require approval from an ethical committee according to the Dutch Medical Research Involving Human Subjects Act (WMO). PBMCs were isolated as described previously [23] and resuspended in RPMI-1640 containing glutamax (GibcoBRL, Paisley, Scotland) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated pooled human serum, referred to further as standard culture medium. HLA typing was performed by serological and DNA-based techniques according to international [American Society for Histocompatibility and Immunogenetics/European Federation for Immunogenetics (ASHI/EFI)] standards. Combinations of HLA-mismatched PBMCs for both HLA I and II alleles were used for the experiments. In addition, for analysis of the polyfunctional nature of cytomegalovirus-specific T cells (in comparison to that of alloreactive T cells), we used buffy coats from cytomegalovirus (CMV) seropositive-typed blood bank donors.
Alloantigen-specific stimulation
A two-way mixed lymphocyte reaction (MLR) was performed by culturing PBMCs of two individuals at a 1 (2 × 106):1 (2 × 106) ratio using polypropylene tubes (BD Pharmingen, Erembodegem, Belgium). Prior to stimulation the PBMCs, used as stimulator, were labelled with CFSE (Molecular Probes®, Leiden, the Netherlands) to discriminate between stimulator and responder PBMCs during fluorescence activated cell sorter (FACS) analysis. As a control for background CD137 expression, cells were stimulated with autologous CFSE-labelled PBMCs. The use of co-stimulation was first evaluated by performing an MLR in the presence of 1 μg/ml anti-CD28 (Serotec, Kidlington, Oxford, UK) and 1 μg/ml anti-CD49d (BD Pharmingen) to allow for detection of alloreactive T cells with a high activation threshold. For experiments in which the CD137 signal was dissected into T cell subsets, as well as cells expressing CD28 or not, co-stimulation consisted of only anti-CD49d (1 μg/ml).
Cell-surface expression of CD137 or CD154 on alloantigen-stimulated T cells
Alloreactive responder (CFSE−) T cells were characterized by staining the cell surface with AmCyan-labelled anti-CD3, Pacific blue (PacBl)-labelled anti-CD4, allophycocyanin-cyanin 7 (APC-Cy7)-labelled anti-CD8, phycoerythrin (PE)-labelled CD45RO (all from BD Pharmingen) and fluorescein isothiocyanate (FITC; R&D Systems Europe Ltd, Abingdon, UK) or phycoerythrin (PE)-Cy7-labelled CCR7 (BD Pharmingen) and APC-labelled anti-CD137 (clone 4B4-1; BD Pharmingen) or APC-labelled anti-CD154 (clone TRAP1; BD Pharmingen). In addition, the peridinin chlorophyll protein (PerCP) channel was used as a dump channel to exclude unwanted cells (DUMP+ cells) from the analysis by using antibodies directed against CD14, CD16 and CD19 and by inclusion of a viability marker [using either 7-aminoactinomycin D (7-AAD) from BD Pharmingen or the Live/Dead® Fixable Dead cell stain kits from Molecular Probes, Invitrogen, Ltd, Paisley, UK]. In addition, a comparison between alloreactive CD137-expressing CD4+ T cells and CD154-expressing CD4+ T cells was made as we [13] and others [16,17] have previously described CD154 as a sensitive marker for detection of antigen-reactive CD4+ T cells. The CD154 assay has been described in detail previously [13,24].
Percentages of alloreactive CD137+ or CD154+ T cells were determined by analysing the samples on the fluorescence-activated cell sorter (FACS) Canto II (BD Pharmingen) using FACSDiva software version 6·1.2. We theoretically set our limit of detection of signal-positive T cells at 0·001% and aimed at 100 events within this positive gate requiring at least 0·5–1 × 106 viable CD3+ T cells to be acquired. The CD137 signal was dissected into defined T cell subsets; naive T cells, (CD45RO–CCR7+), central memory T cells (CD45RO+CCR7+), effector memory T cells (CD45RO+CCR7−) and the terminally differentiated effector memory T cells (CD45RO−CCR7−). The loss of cell surface expression of CD28 is considered as a marker of CD8+ and CD4+ T cell differentiation and was included in the analysis by staining the cell surface with PE-labelled anti-CD28 (BD Pharmingen) [25,26]. Only the CFSE−DUMP− (= responder) T cells were included for analysis of CD137+ T cells. Alloreactive CD137-expressing T cells were corrected for the CD137 signal observed in T cells following co-culture with autologous CFSE-labelled PBMCs (background signal).
Cytokine-producing alloantigen-stimulated T cells
Responder PBMCs were stimulated with CFSE-labelled stimulator (allogeneic) PBMCs at a 1 (5 × 106):1 (5 × 106) ratio for 24 h, of which the last 12 h were in presence of Brefeldin A (Golgiplug; BD Pharmingen) and monensin (Golgistop; BD Pharmingen). Co-culture with autologous CFSE-labelled PBMCs and stimulation with the combination of phorbol myristate acetate (PMA) (50 ng/ml; Sigma Aldrich, St Louis, MO, USA) and ionomycin (1 μg/ml; Sigma Aldrich) were included to determine the background and maximal capacity of T cells to produce cytokines, respectively. In addition, we included stimulation with CMV-pp65 peptide mixture (1 μg/ml; PepTivator-CMV pp65; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) as a positive control in the analysis of polyfunctional (antigen-reactive T cells producing more than one cytokine) CD137+CD4+ and CD8+ T cells. The cell surface was stained to identify CD3+CD4+ and CD3+CD8+ T lymphocytes within the DUMP– cell fraction. Following fixation and permeabilization CD137 and cytokines were stained intracellularly with APC-labelled anti-CD137 and PECy7-labelled anti-interferon (IFN)-γ combined with either PE-labelled anti-interleukin (IL)-2 or PE-labelled anti-TNF-α. Staining for three cytokines intracellularly required a depletion of T cells of stimulator PBMCs (>98% depleted for CD3) using CD3 microbeads (Miltenyi Biotec), according to the manufacturer's instructions, and the following antibodies were used to stain for cytokines: PECy7-labelled anti-IFN-γ combined with FITC-labelled anti-IL-2 and PE-labelled anti-TNF-α. Preliminary experiments showed that intracellular staining of CD137 gave similar results to cell surface staining. At least 0·5–1 × 106 viable CD3+ T cells were acquired on the FACSCanto II. Alloreactive cytokine-producing T cells were corrected for the cytokine signal observed following autologous stimulation. In additional experiments, the alloreactive CD137-expressing cytokine+ T lymphocytes were classified further within T cell subsets using antibodies specific for CD45RO, CCR7 and CD28. Samples were measured on the FACSCanto II (BD Pharmingen) and analysed using FACSDiva software version 6·1.2 (BD Pharmingen).
Isolation of alloantigen-specific CD137+ T cells
Alloantigen-specific CD137+ T cells were induced using the live assay in the presence of co-stimulatory antibodies. Briefly, 5 × 107 (2·5 × 107 of responder PBMCs and 2·5 × 107 of CFSE-labelled stimulator PBMCs) were incubated in a six-well plate for 24 h in the presence of the combination of anti-CD28 and anti-CD49d (both at a final concentration of 1 μg/ml). Responder T lymphocytes (CFSE−) were dissected subsequently in CD137-expressing (CD137-enriched) T cells and those lacking CD137 (CD137-depleted) and isolated using FACS sorting. To demonstrate that CD137-expressing T lymphocytes identified all alloreactive T lymphocytes, we chose to obtain T lymphocytes that were maximally depleted for CD137 (<1% contaminating CD137+ T cells). Proliferation of the different fractions to allogeneic restimulation (as described below) was used as functional read-out.
Cell tracking dye (yellow=PKH-67/red=PKH26) (PKH)-labelling and proliferation of CD137-enriched and -depleted T cell fractions
The different responder T cell fractions (CD137-enriched and -depleted, respectively), obtained as described above from a 24-h two-way MLR (primary MLR), were labelled with PKH-26 according to the manufacturer's instructions (Sigma Aldrich, Zwijndrecht, the Netherlands) and used at a concentration of 5 × 104/well in standard culture medium. These responders were restimulated in triplicate using irradiated (40 Gy) allogeneic and autologous CD3-depleted PBMCs (at a concentration of 5 × 104/well) or anti-CD3 and anti-CD28-coated beads (1:1 ratio; Dynabeads® human T-activator CD3/CD28; Invitrogen Dynal As, Oslo, Norway) as negative and positive controls, respectively. Following 3 and 6 days of stimulation, the cells were harvested and stained using monoclonal antibodies directed against CD3, CD4 and CD8 and dead cells were excluded using 7-AAD. Percentages of dividing T cells were compared for the different responder T cell fractions by analysis of samples on the FACSCanto II (BD Pharmingen) and using FACSDiva software version 6·1.2 (BD Pharmingen) or ModFit LT software (Verity Software House Inc., Topsham, ME, USA).
Statistical analysis
For comparisons between groups, the t-test, Mann–Whitney U-test, one-way analysis of variance (anova) or Kruskal–Wallis test were used, as appropriate. Post-hoc analysis was performed using Bonferroni's test for multiple comparisons. Two-sided P-values at α < 0·05 were considered statistically significant.
Results
Alloreactive CD137-positive CD8+ T cells are present predominantly within the CD28+ T cell fraction
Optimal conditions for identification of CD137-expressing alloreactive CD8+ T cells were studied after 24 h of stimulation, as we and others observed maximal CD137 expression at this time-point [21,22]. Events were acquired within a preset lymphocyte gate (Fig. 1a). CFSE-labelling of stimulator PBMCs allowed for the discrimination of stimulator (CFSE+) and responder (CFSE–) PBMCs. In addition, any unwanted cells and events were excluded from the analysis using a DUMP-channel (see Methods section). Responder (defined as: CFSE–DUMP−) CD8+ T cells were selected for further analysis (Fig. 1a).
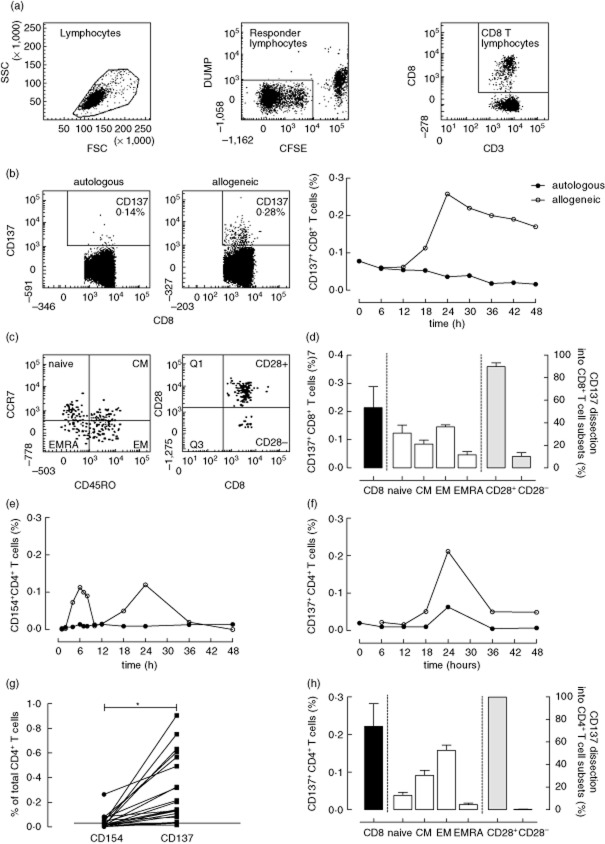
Fig. 1.
CD137 expression on CD8+ and CD4+ T cells upon alloantigen stimulation. A typical example of the gating strategy applied for analysis of alloreactive CD137-expressing CD8+ T cells is given in (a). Briefly, lymphocytes were gated and subsequently carboxyfluorescein diacetate succinimidyl ester (CFSE)−DUMP− T cells were identified as the responder T lymphocytes; finally, CD8+ T cells were selected. Percentages of CD137-expressing CD8+ T cells upon autologous (b, left plot) or allogeneic (b, right plot) stimulation are depicted in a dot-plot with CD8 on the x-axis and CD137 on the y-axis. A representative example (one of three) of the kinetics of the CD137 signal is depicted with respect to the autologous signal (closed circles) and the allogeneic signal (open circle). A typical flow cytometric example of dissection of the maximal allogeneic CD137 signal at 24 h into subsets using CD45RO and CCR7 and into those expressing CD28 (CD28+) versus those that are not (CD28−) is depicted in (c). Naive T cells are CD45RO−CCR7+, central memory (CM) CD45RO+CCR7+, effector memory (EM) CD45RO+CCR7− and terminally differentiated effector memory (EMRA) are CD45RO−CCR7−. In addition, mean ± standard error of the mean (s.e.m.) (n = 3) values of the dissection of CD137-expressing alloreactive CD8+ T cells (black bars, plotted on the left y-axis) into T cell subsets (white bars, plotted on the right y-axis) and those expressing CD28 or not (grey bars, plotted on the right y-axis) are given in (d). The use of the CD137 live assay to identify alloreactive CD4+ T cells was also evaluated. For this purpose, a similar approach was taken as for the CD8+ T cells. The kinetics of CD154 (e)- or CD137 (f)-expressing CD4+ T cells upon autologous (closed circles) as well as allogeneic (open circles) stimulation was studied. Next, we compared pairwise the net frequency of alloreactive CD4+ T cells (allogeneic signal corrected for the autologous signal) using the CD154 (circles) and CD137 (squares) live assay, respectively (g). In addition, mean ± s.e.m. (n = 10) values of the dissection of CD137-expressing alloreactive CD4+ T cells (black bars, plotted on the left y-axis) into T cell subsets (white bars, plotted on the right y-axis) and those expressing CD28 or not (grey bars, plotted on the right y-axis) are given in (h) (graph).
A highly variable spontaneous background of CD137+CD8+ T cells (median 0·15%, range 0·02–0·62%) was observed. This background CD137 signal was similar for T cells in whole blood, freshly isolated PBMCs, or thawed and overnight recovered PBMCs (n = 5 for every condition, data not shown) and did not increase significantly upon autologous stimulation. Addition of co-stimulatory antibodies increased the frequency of CD137-expressing CD8+ T cells upon allogeneic stimulation without affecting (autologous) background.
Kinetic analysis in the presence of co-stimulation showed that alloreactive CD137-expressing CD8+ T cells were barely detectable at 6 h but peaked at 24 h (Fig. 1b). The median net frequency of CD137+ alloreactive CD8+ T cells at 24 h was 0·05% (range 0·0–1·38%). The alloreactive CD137 signal was detectable both in memory and naive (approximately 30%) CD8+ T cells (Fig. 1c,d). Almost all alloreactive CD137+CD8+ T cells expressed CD28 (Fig. 1c,d), identifying the dominant presence of alloreactive T cells within less differentiated memory T cells.
Alloreactive CD4+ T cells identified by anti-CD137 staining compared to the CD154 fast assay
In contrast to the low autologous CD154 signal (<0·01%) within CD4+ T cells, a highly variable (autologous) background (median 0·05% ranging from 0·01 to 0·21%) was observed with respect to CD137-expressing CD4+ T cells. In general, this background was substantially lower for CD4+ compared to CD8+ T cells. Addition of co-stimulation increased the frequency of CD137+CD4+ T cells in a similar fashion, as described previously, for CD154+CD4+ T cells [13].
In contrast to the biphasic pattern of alloreactive CD154+CD4+ T cells (Fig. 1e), peaking at 6 and 24 h, maximal numbers of CD137+CD4+ T cells were observed at 24 h (Fig. 1f). CD137 expression was present on a significantly larger population (P < 0·001) of alloreactive CD4+ T cells compared to CD154 (0·07 ± 0·02% versus 0·21 ± 0·05%, Fig. 1g).
In addition, CD137+CD4+ T cells hardly co-expressed CD154 24 h after allogeneic stimulation, which is similar to that upon stimulation by CMV peptides (i.e. the fraction of CD137+CD154+ of total CD137+CD4+ T cells varied between 5–10%; data not shown). The alloreactive CD137+CD4+ T cells were present in both the naive and memory T cell fraction (Fig. 1h), although CD137+CD4+ T cells were found predominantly in CD28+ and memory T cells (Fig. 1h).
Cytokine expression and polyfunctionality of alloreactive CD137+ T cells
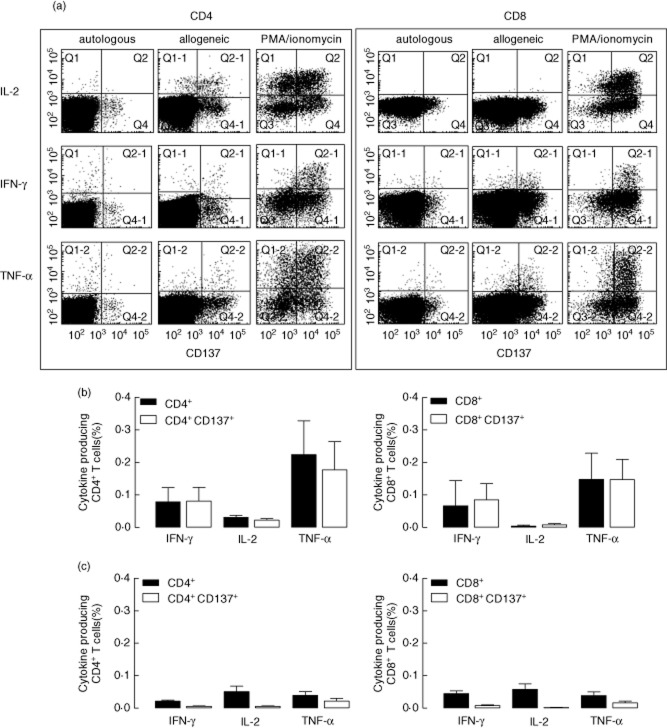
Figure 2a shows a typical flow cytometric example of cytokine-producing CD137+CD4+ (left panel) and CD8+ (right panel) T cells following autologous, allogeneic or polyclonal stimulation. The percentage of alloreactive cytokine+ CD137+ T cells was, on average, similar to the total net alloreactive cytokine-producing CD4+ (Fig. 2b, left graph) and CD8+ (Fig. 2b, right graph) T cells, indicating that all cytokine+ alloreactive T cells express CD137. However, the autologous background signal in CD137+cytokine+ T cells was substantially lower (0·001–0·02%, Fig. 2c, open bars) than the background signal for cytokine+ T cells (0·02–0·06%, Fig. 2c, closed bars). Depending on the cytokine and T cell analysed, this resulted in an average signal (allogeneic) to noise (autologous) ratio of 10 (range 3–16) for cytokine+ CD137+ T cells compared to 2–3 for alloreactive CD137+ T cells. Only a relatively small fraction (2–10%) of CD137-expressing alloreactive CD4+ or CD8+ T cells were cytokine-positive, depending on the cytokine measured.
Fig. 2.
Cytokine-producing T cells upon alloantigen stimulation and CD137 expression. A typical flow cytometric example of the analysis of autologous, allogeneic and polyclonal induction of cytokine producing CD4+ (left panel) and CD8+ (right panel) T cells is given in (a), with CD137 expression on the x-axis and different cytokines on the y-axis. Mean ± standard error of the mean (s.e.m.) (n = 8) cytokine-producing cells upon allogeneic stimulation (corrected for autologous background) are displayed as percentage of total CD4+ (b, left graph, closed bars) and CD8+ (b, right graph, closed bars) T cells, respectively, or as a percentage of CD4+CD137+ (b, left graph, open bars) and CD8+CD137+ (b, right graph, open bars), respectively. Background cytokine-producing CD4+ (c, left graph) or CD8+ (c, right graph) T cells induced by autologous stimulation are depicted as percentage (mean ± s.e.m.) of total CD4+ or CD8+ T cells (closed bars) or as a percentage of CD137+CD4+ or CD8+ T cells (open bars).
Polyfunctional antigen-reactive T lymphocytes expressing multiple cytokines (e.g. IL-2, IFN-γ and TNF-α) upon stimulation are considered to be instrumental for strong cellular immune responses [27]. A typical flow cytometric example (for CD4+ T cells) depicts the method (Boolean gating strategy) used to analyse polyfunctional antigen-reactive T cells (Fig. 3a). Very few alloreactive polyfunctional CD137+CD4+ T cells were observed in contrast to stimulation with CMV-pp65 or PMA-ionomycin, where the fraction of double and triple cytokine-producing cells is significantly higher (P < 0·05) (Fig. 3b). A minority of alloreactive CD8+ T cells was polyfunctional, but the relative contribution of these cells to the total number of cytokine+ cells was similar to CMV-pp65 antigen-specific T cells (Fig. 3c).
Fig. 3.
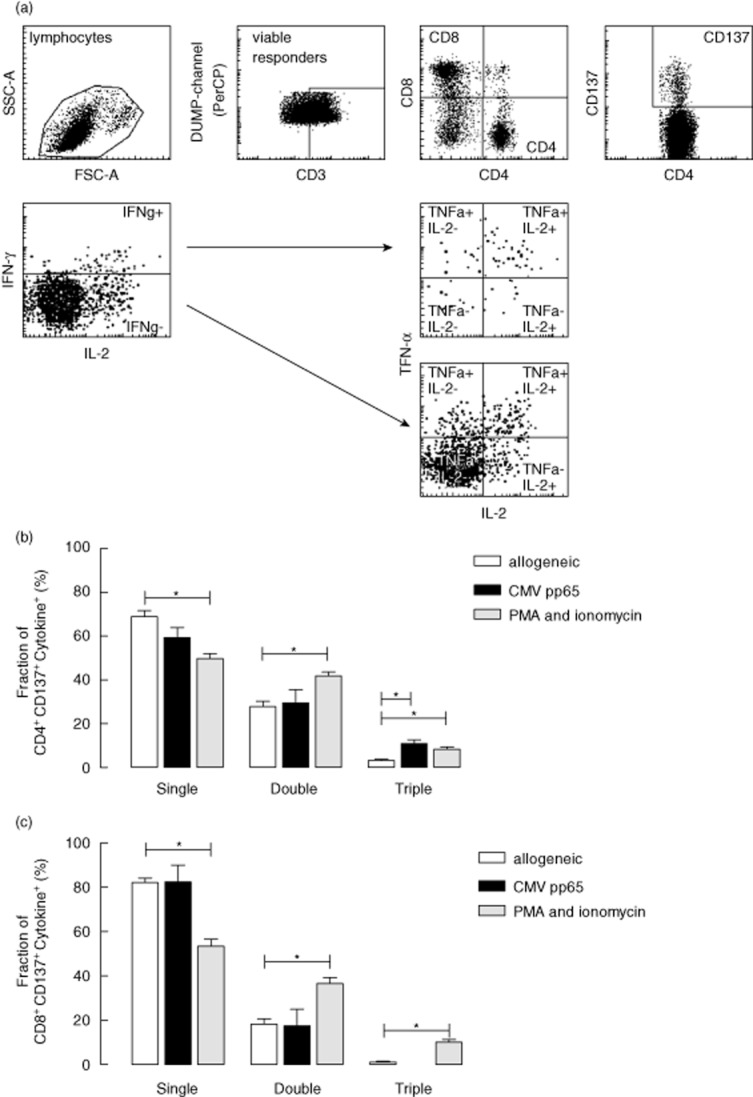
Polyfunctionality of alloreactive CD137-expressing T cells. In addition, we also applied a multi-parameter approach to the functional analysis of alloreactive CD137-expressing CD4+ (b) and CD8+ (c) T cells. A typical flow cytometric analysis for polyfunctional CD137-expressing CD4+ T cells is given in (a) (showing the Boolean gating strategy applied). The Boolean gating strategy is as follows: lymphocytes are gated and then plotted in a dot-plot to discriminate between stimulator peripheral blood mononuclear cells (PBMCs) (that are depleted of T cells) and responder T cells. Next, the CD4+ (or CD8+) T cells are gated and the CD137-expressing cells identified. The CD137-expressing T cells are dissected subsequently into two subsets, with respect to the presence or absence of cytokine ‘1'-producing cells [e.g. interferon (IFN)-γ]. Each of these subsets are plotted subsequently to visualize frequencies of cytokine ‘2'- and ‘3'-producing cells [e.g. interleukin (IL)-2 and tumour necrosis factor (TNF)-α]. Cytokine profiles were generated by calculating the frequencies of the eight different possible combinations. In order to determine the contribution of either single, double or triple cytokine-producing T cells to the total cytokine producing capacity of CD137-expressing T cells, the sum of single, double and triple cytokine-producing T cells was calculated and set at 100% and subsequently the fraction of either single, double or triple cytokine-producing T cells determined. On the y-axis, the fraction of the total cytokine+ CD137-expressing CD4+ (b) and CD8+ (c) T cells, which is set at 100%, producing one, two or three cytokines upon stimulation is depicted. The open bars represent mean ± standard error of the mean (s.e.m.) of 18 independent experiments of allogeneic stimulation of PBMCs, the closed bars represent the mean ± s.e.m. of three independent experiments of cytomegalovirus (CMV)-pp65 stimulation and the grey bars resemble the mean ± s.e.m. of 18 independent experiments of stimulation with the combination of phorbol-12-myristate-13-acetate (PMA) and ionomycin.
Alloreactive CD137+ cytokine+ cells in T cell subsets
The autologous background for cytokine-producing CD137+CD4+ or CD8+ T cells, by zooming into T cell subsets, decreased to 0·000–0·003% within the different T cell subsets (data not shown). Figure 4a,d shows representative dot-plots of IFN-γ- and IL-2-positive alloreactive CD137+CD4+ and CD8+ T cells, respectively. The total IL-2 (left panel, Fig. 4b,e, respectively)- and IFN-γ (right panel, Fig. 4b,e, respectively)-producing CD137-expressing T cells were dissected into T cell subsets, respectively. Most of the IL-2-producing CD137-expressing CD4+ T cells had a naive (approximately 50%) or central memory (approximately 40%) phenotype (Fig. 4b, left panel, left graph) and were predominantly CD28+ (approximately 97%; Fig. 4b, left panel, right graph). IFN-γ-producing CD137-expressing CD4+ T cells were mainly of the central memory phenotype (approximately 66%, Fig. 4b, right panel, left graph) and more than 80% expressed CD28 (Fig. 4b, right panel, right graph). These data are summarized in Fig. 4c (left graph: IL-2 and right graph: IFN-γ).
Fig. 4.
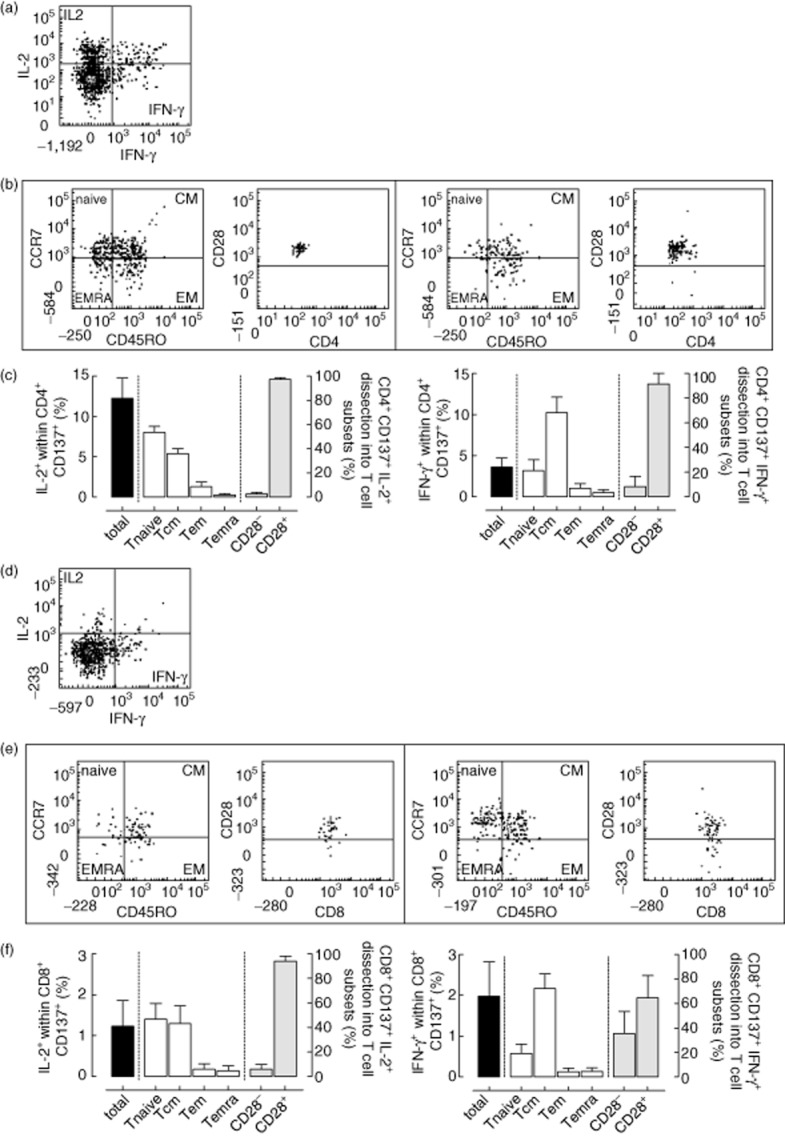
Dissection of alloreactive CD137-expressing and cytokine-producing T cells in subsets and T cells expressing or lacking CD28. A typical flow cytometric example of CD137+CD4+interleukin (IL)-2+ and/or interferon (IFN)-γ+ alloreactive T cells is given in (a) and the dissection of IL-2 (b, left panel)- and IFN-γ (b, right panel)-producing CD137+CD4+ T cells into T cell subsets and cells expressing CD28 (CD28+) or lacking CD28 (CD28−) are given in (b). The different T cell subsets include: naive (CD45RO−CCR7+), central memory (Tcm; CD45RO+CCR7+), effector memory (Tem; CD45RO+CCR7−) and terminally differentiated effector memory (Temra; CD45RO−CCR7−). In (c), the mean ± standard error of the mean (s.e.m.) (n = 6) values of the dissection of IL-2 (left panel)- and IFN-γ (right panel)-producing CD137-expressing alloreactive CD4+ T cells (black bars, displayed on the left y-axis) into T cell subsets (white bars, displayed on the right y-axis) and those expressing CD28 or not (grey bars, displayed on the right y-axis) are given. A similar approach was taken and display is given for cytokine+ CD137-expressing CD8+ T cells (d–f).
IL-2-producing CD8+CD137+ alloreactive T cells were found predominantly within the naive T cell subset and T cells expressing CD28 (Fig. 4e, left panel). IFN-γ-producing alloreactive CD8+CD137+ T cells were found predominantly in memory T cells, predominantly of the central memory phenotype (Fig. 4e, right panel). These data are summarized in Fig. 4f (left graph: IL-2 and right graph: IFN-γ).
CD137 expression identifies the total alloreactive proliferating T cell pool
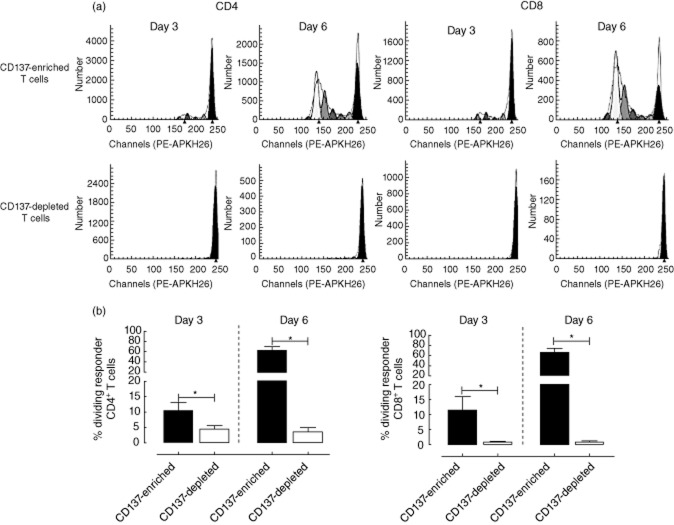
Alloreactive T cells are defined historically as T cells proliferating in response to allogeneic antigen-presenting cells. To determine whether activation-induced CD137 expression was able to identify the total proliferating alloreactive T cell pool, we isolated the living allogeneic CD137-expressing responder T cells generated in an HLA-mismatched MLR using cell sorting and labelled them with PKH-26. Following a 3- and 6-day restimulation with irradiated allogeneic CD3-depleted PBMCs, the proliferative capacity resided predominantly within the CD137-expressing CD4+ as well as CD8+ T cells (Fig. 5a, showing a representative example of the analysis using ModFit LT software). Significantly higher (P < 0·05) percentages of dividing CD4+ (day 3: 10·31 ± 2·65% versus 4·23 ± 1·38%; day 6: 62·02 ± 7·34% versus 3·52 ± 1·27%) as well as CD8+ T cells (day 3: 11·53 ± 4·37% versus 0·78 ± 0·36%; day 6: 66·03 ± 8·44% versus 0·86 ± 0·21%) were located within the CD137-enriched fraction when compared to the CD137-depleted one (Fig. 5b, CD4: left graph and CD8: right graph).
Fig. 5.
Proliferative capacity upon alloantigen restimulation. In order to analyse where the alloreactive proliferative capacity is located, 5 × 107 [2·5 × 107 of responder stimulator peripheral blood mononuclear cells (PBMCs) and 2·5 × 107 of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled stimulator PBMCs] were incubated in a six-well plate for 24 h in the presence of a combination of anti-CD28 and anti-CD49d (both at a final concentration of 1 μg/ml). CFSE-labelling was used to discriminate between stimulator and responder cells upon 24-h stimulation. Responder T lymphocytes (CFSE−) were dissected subsequently in CD137-expressing (CD137-enriched) T cells and those lacking CD137 (CD137-depleted) and isolated using fluorescence activated cell sorting (FACS). These fractions were cell tracking dye (yellow=PKH-67/red=PKH26) (PKH-26)-labelled and proliferation to allogeneic restimulation [second mixed lymphocyte reaction (MLR)] was analysed following 3 and 6 days of restimulation. A typical example of the analysis of proliferation using ModFit LT software is given in (a). Percentages (n = 3; mean ± standard error of the mean) of dividing CD4+ as well as CD8+ T cells following a 3- and 6-day second MLR within the CD137-enriched (closed bars) and -depleted (open bars) T cell fractions are depicted in (b). *P-value < 0·05 using Wilcoxon's signed-rank test.
Discussion
This study shows that expression of CD137 at the cell surface or intracellularly can be used to identify the total pool of alloreactive T cells. There are some obvious advantages with the use of activation-induced CD137 to detect alloreactive T cells compared to other assays that are used for detection of T cell alloreactivity. First, it offers the possibility to identify alloreactive CD8+ and CD4+ T cells simultaneously on the single-cell level within a relatively short period of time (i.e. 24 h) with high sensitivity and specificity. Furthermore, as the cells are still viable, in the case of the cell surface-based method for staining CD137 it remains possible to isolate and propagate them when needed for further research. Interestingly, using this advantage of the fast live assay, we have demonstrated the proliferative capacity to allogeneic restimulation to reside predominantly within CD137-expressing alloreactive CD4+ as well as CD8+ T cells. Compared to our previously described CD154 live cell assay the CD137 assay detected an even higher frequency of alloreactive T cells in the CD4+ T cell compartment. Moreover, combining CD137 expression with multiple parameters enabled an in-depth phenotypical analysis or analysis of functional properties, i.e. enumeration of cytokine-producing cells. This leads to novel findings, such as remarkably few alloreactive T cells in the more differentiated CD28− memory T cells, such as the CD8+ terminally differentiated effector memory (TEMRA) subpopulation. This could explain our previous observation that an increase in this cell population is associated with a decreased risk for acute rejection after kidney transplantation [28].
In addition, CD137 is expressed by alloreactive T cells with both a naive or memory phenotype within the CD4+ and CD8+ T cells and can therefore be used for identification of the total alloreactive T cell compartment. Naive [18] and central memory T cells [29] produce no to low amounts of effector molecules and are easily missed if detection of alloreactive T cells is based on cell proliferation or IFN-γ positivity only. With respect to alloreactive naive and central memory cytokine+ CD4+ as well as CD8+ T cells, our data are confirmed by a paper by Melenhorst et al. [30], who described the alloreactive T cells to be both of the naive and antigen-experienced (i.e. memory) phenotype. The presence of alloreactive memory is hypothesized to be caused by virus-specific T cells that cross-react with mismatched HLA-peptides. In contrast to this paper, where sorted naive as well as memory T cells were primed using HLA-mismatched stimulator cells for several days, and subsequently cytokine-producing capacity to allogeneic restimulation was examined, we demonstrated alloreactivity to be present in naive and memory T cell subsets within total PBMC upon a primary allogeneic stimulation combined with co-stimulatory antibodies.
A disadvantage of the CD137 assay is the variable spontaneous background signal in CD4+ and CD8+ T cells. The CD154 live assay does not have this disadvantage, and CD154 expression on freshly isolated PBMCs does not usually exceed 0·01% of CD4+ T cells. Given the relatively high frequency of alloreactive T cells it was still possible to use the CD137 assay, although in some cases a low signal to noise ratio of approximately 2:3 was observed.
However, if we progressively combined intracellular CD137 expression with more parameters (e.g. cytokine staining and markers for T cell subsets), the background signal of multi-parameter-positive CD137+ alloreactive T cells dropped to very low levels (<0·001%), increasing the signal to noise ratios significantly. This finding is in accordance with results published by others, and reflects the decrease in chance for combinations of a-specific staining when multiple antibodies are used [27]. However, flow cytometry with a combination of six or more parameters in search of low frequencies of antigen-reactive T cells is technically challenging, and requires a skilled operator.
The frequency of cytokine+ alloreactive T cells was similar to the frequency of CD137+ cytokine+ alloreactive T cells, implying that virtually all cytokine-producing cells express CD137. However, the majority of alloreactive CD137-expressing CD4+ as well as CD8+ T cells did not produce cytokines, in accordance with our observations for alloreactive CD154-expressing CD4+ T cells [13]. This was observed for a variety of T helper type 2 (Th2) and Th1 cytokines that we tested (unpublished observations and [13]). A similar observation, although to a lesser extent, has been made for CD137+ virus-specific CD4+ and CD8+ T cells, indicating that even after a strong antigenic stimulus, a substantial part of antigen-reactive T cells are non-cytokine producers [22]. This low frequency of cytokine-expressing alloreactive T cells found with flow cytometry is also observed with the ELISPOT assay [31,32].
At present it is not clear why these cells do not secrete cytokines, but they might represent the antigen-reactive T cells with a low T cell receptor avidity. Cytokine non-secreting cells are not anergic and proliferate in the presence of IL-2 [13], which probably explains why the alloreactive T cell precursor frequency determined by proliferation assays is substantially higher [33–36] than the results obtained with IFN-γ ELISPOT [11] or direct cytokine staining by flow cytometry, as shown in this study. The clinical importance of CD137+ cytokine+ or cytokine− alloreactive T cells might differ depending on the type of transplantation. For instance, when we tested the alloreactive T cell signal in HLA-matched (or HLA-identical) combinations we found alloreactive CD4+ T cells in a very low frequency, probably because of indirect alloreactivity [13]. These alloreactive CD4+ T cells, which do not express cytokines, might be important for the risk of graft-versus-host disease after bone marrow transplantation [37–39], but probably have little impact on the risk for acute rejection after kidney transplantation [40]. Currently, we are investigating how alloreactive CD137+ T cells develop following kidney transplantation, and whether or not the presence of certain subsets of alloreactive T cells are related specifically to the risk for acute rejection after kidney transplantation. In addition, their presence and function long after transplantation is of considerable interest.
In conclusion, the CD137 multi-parameter flow cytometry fast assay allows for phenotypic and functional determination of alloreactive precursor frequencies of both CD4+ and CD8+ T cells with high sensitivity and specificity.
Disclosure
All authors declare no competing interests. This study was funded by the Malpighi Foundation.
References
- 1.Reinsmoen NL. Cellular methods used to evaluate the immune response in transplantation. Tissue Antigens. 2002;59:241–250. doi: 10.1034/j.1399-0039.2002.590401.x. [DOI] [PubMed] [Google Scholar]
- 2.Orosz CG, Adams PW, Ferguson RM. Frequency of human alloantigen-reactive T lymphocytes. II. Method for limiting dilution analysis of alloantigen-reactive helper T cells in human peripheral blood. Transplantation. 1987;43:718–724. [PubMed] [Google Scholar]
- 3.Kaminski E, Hows J, Goldman J, Batchelor R. Optimising a limiting dilution culture system for quantitating frequencies of alloreactive cytotoxic T lymphocyte precursors. Cell Immunol. 1991;137:88–95. doi: 10.1016/0008-8749(91)90059-k. [DOI] [PubMed] [Google Scholar]
- 4.Sharrock CE, Kaminski E, Man S. Limiting dilution analysis of human T cells: a useful clinical tool. Immunol Today. 1990;11:281–286. doi: 10.1016/0167-5699(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Fuentes MP, Warrens AN, Lechler RI. Immunologic monitoring. Immunol Rev. 2003;196:247–264. doi: 10.1046/j.1600-065x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 6.Volk HD, Kern F. Insights into the specificity and function of (allo)antigen-reactive T cells. Am J Transplant. 2001;1:109–114. [PubMed] [Google Scholar]
- 7.Nikolaeva N, Uss E, van Leeuwen EM, van Lier RA, ten Berge IJ. Differentiation of human alloreactive CD4+ and CD8+ T cells in vitro. Transplantation. 2004;78:815–824. doi: 10.1097/01.tp.0000133308.60226.fa. [DOI] [PubMed] [Google Scholar]
- 8.Tapirdamaz O, Mancham S, van der Laan LJ, et al. Detailed kinetics of the direct allo-response in human liver transplant recipients: new insights from an optimized assay. PLoS ONE. 2010;5:e14452. doi: 10.1371/journal.pone.0014452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura LE, Walker JM, Maecker HT. Optimization of whole blood antigen-specific cytokine assays for CD4(+) T cells. Cytometry. 2000;40:60–68. doi: 10.1002/(sici)1097-0320(20000501)40:1<60::aid-cyto8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Methods. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 11.Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65:906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 13.Litjens NH, van de Wetering J, van Besouw NM, Betjes MG. The human alloreactive CD4+ T-cell repertoire is biased to a Th17 response and the frequency is inversely related to the number of HLA class II mismatches. Blood. 2009;114:3947–3955. doi: 10.1182/blood-2009-03-211896. [DOI] [PubMed] [Google Scholar]
- 14.Rentenaar RJ, Vosters JL, van Diepen FN, Remmerswaal EB, van Lier RA, ten Berge IJ. Differentiation of human alloreactive CD8(+) T cells in vitro. Immunology. 2002;105:278–285. doi: 10.1046/j.0019-2805.2002.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 17.Frentsch M, Arbach O, Kirchhoff D, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 18.Wolfl M, Kuball J, Ho WY, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandvliet ML, van Liempt E, Jedema I, et al. Simultaneous isolation of CD8(+) and CD4(+) T cells specific for multiple viruses for broad antiviral immune reconstitution after allogeneic stem cell transplantation. J Immunother. 2011;34:307–319. doi: 10.1097/CJI.0b013e318213cb90. [DOI] [PubMed] [Google Scholar]
- 20.Wehler TC, Nonn M, Brandt B, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109:365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 21.Wolfl M, Kuball J, Eyrich M, Schlegel PG, Greenberg PD. Use of CD137 to study the full repertoire of CD8+ T cells without the need to know epitope specificities. Cytometry A. 2008;73:1043–1049. doi: 10.1002/cyto.a.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehler TC, Karg M, Distler E, et al. Rapid identification and sorting of viable virus-reactive CD4(+) and CD8(+) T cells based on antigen-triggered CD137 expression. J Immunol Methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19:1483–1490. doi: 10.1681/ASN.2007090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litjens NH, Boer K, Betjes MG. Identification of circulating human antigen-reactive CD4+ FOXP3+ natural regulatory T cells. J Immunol. 2012;188:1083–1090. doi: 10.4049/jimmunol.1101974. [DOI] [PubMed] [Google Scholar]
- 25.Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80:208–217. doi: 10.1038/ki.2011.110. [DOI] [PubMed] [Google Scholar]
- 26.Litjens NH, de Wit EA, Betjes MG. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing. 2011;8:2. doi: 10.1186/1742-4933-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa SCD, Lu FX, Yu J, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 28.Betjes MG, Meijers RW, de Wit EA, Weimar W, Litjens NH. Terminally differentiated CD8+ temra cells are associated with the risk for acute kidney allograft rejection. Transplantation. 2012;94:63–69. doi: 10.1097/TP.0b013e31825306ff. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 30.Melenhorst JJ, Scheinberg P, Williams A, et al. Alloreactivity across HLA barriers is mediated by both naive and antigen-experienced T cells. Biol Blood Marrow Transplant. 2011;17:800–809. doi: 10.1016/j.bbmt.2010.12.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Besouw NM, Vaessen LM, Zuijderwijk JM, et al. The frequency of interferon-γ-producing cells reflects alloreactivity against minor histocompatibility antigens. Transplantation. 2003;75:1400–1404. doi: 10.1097/01.TP.0000064376.78084.50. [DOI] [PubMed] [Google Scholar]
- 32.Reinsmoen NL, Cornett KM, Kloehn R, et al. Pretransplant donor-specific and non-specific immune parameters associated with early acute rejection. Transplantation. 2008;85:462–470. doi: 10.1097/TP.0b013e3181612ead. [DOI] [PubMed] [Google Scholar]
- 33.Atkins RC, Ford WL. Early cellular events in a systemic graft-vs.-host reaction. I. The migration of responding and nonresponding donor lymphocytes. J Exp Med. 1975;141:664–680. doi: 10.1084/jem.141.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford WL, Atkins RC. The proportion of lymphocytes capable of recognizing strong transplantation antigens in vivo. Adv Exp Med Biol. 1973;29:255–262. doi: 10.1007/978-1-4615-9017-0_37. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P, Bevan MJ. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977;29:1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- 36.Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 37.Broen K, van der Waart AB, Greupink-Draaisma A, et al. Polymorphisms in CCR6 are associated with chronic graft-versus-host disease and invasive fungal disease in matched-related hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1443–1449. doi: 10.1016/j.bbmt.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Ratajczak P, Janin A, Peffault de Latour R, et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010;116:1165–1171. doi: 10.1182/blood-2009-12-255810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vokaer B, Van Rompaey N, Lemaitre PH, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- 40.Gerrits JH, van de Wetering J, Postma S, et al. Stable T-cell reactivity after successful tapering of azathioprine in HLA-identical living-related kidney transplant recipients despite minor histocompatibility antigen mismatches. Nephrol Dial Transplant. 2007;22:353–361. doi: 10.1093/ndt/gfl678. [DOI] [PubMed] [Google Scholar]