Abstract
Acupuncture modulates brain activity at the limbic–paralimbic–neocortical network (LPNN) and the default mode network (DMN). Since these brain networks show gender differences when mediating emotional and cognitive tasks, we thus hypothesize that women and men may also respond differently to acupuncture procedure at these brain regions. In order to test this hypothesis, we retrieved the data of 38 subjects, 19 females and 19 males, who had brain fMRI during acupuncture from previous studies and reanalyzed them based on sex status. Deactivation at the LPNN/DMN during needle manipulation of acupuncture was more extensive in females than in males, particularly in the posterior cingulate (BA31), precuneus (BA7m) and angular gyrus (BA39). The functional correlations between the right BA31 and pregenual cingulate (BA32), hippocampus or contralateral BA31 were significantly stronger in females than in males. The angular gyrus (BA39) was functionally correlated with BA31 in females; in contrast, it was anticorrelated with BA31 in males. Soreness, a major component of the psychophysical responses to needle manipulation, deqi, was correlated in intensity with deactivation of the angular gyrus in females; no such relationships were observed in males. In contrast to lesser deactivation at the LPNN/DMN networks, needle manipulation during acupuncture induced greater activation at the secondary somatosensory cortex and stronger functional connectivity with the anterior-middle cingulate (BA32/24) in males than in females. Our study suggests that brains with sex dimorphism may process the acupuncture stimulation differently between women and men.
Keywords: Acupuncture, Brain activity, Sex, Gender
1. Introduction
Acupuncture, an ancient Chinese needle treatment, is used to relieve the clinical symptoms of pain, mood, and autonomic related disorders (Lundeberg et al., 2007). During the acupuncture procedure, needle is inserted at acupoints and then rotated manually. This manipulation of the needle after insertion induces deqi, a composite of unique sensations such as soreness, aching, pressure and heaviness, which is essential to the efficacy of acupuncture according to traditional Chinese medicine (Kong et al., 2007a,b). It is proposed that pronounced action on the limbic system of the brain during the needle manipulation may underlie the beneficial effects of acupuncture on the clinical symptoms (Hui et al., 2007).
Using functional magnetic resonance imaging (fMRI), we and others have demonstrated that acupuncture produces deactivation of the limbic–paralimbic–neocortical network (LPNN) including the amygdala, hippocampus, septal nuclei, cingulate gyrus, precuneus and angular gyrus coupled with activation of the sensorimotor network (SMN) and a few paralimbic structures (Hui et al., 2005; Kong et al., 2007a,b; Bai et al., 2007; Wang et al., 2007; Dhond et al., 2008; Fang et al., 2008; Qin et al., 2008). These limbic and limbic-associated structures have been shown to play a primary role in regulating emotion (Joseph, 1992; LeDoux, 1998). Furthermore, we have observed a marked similarity of the hemodynamic response to acupuncture with that of the default mode network (DMN) to attention tasks such as the cognitive examination (Buckner and Vincent, 2007; Raichle et al., 2001). The DMN structures modulated by acupuncture include 1) the frontal pole (FP) and the anterior cingulate in the medial prefrontal cortex (mPFC); 2) the hippocampus in the medial temporal lobe (MTL) and 3) the posterior cingulate (BA31), retrosplenial cortex (RSC) and precuneus (PCN) in the medial parietal cortex (MPC) (Bai et al., 2007; Dhond et al., 2008; Fang et al., 2008; Hui et al., 2005; Qin et al., 2008; Wang et al., 2007).
While sex is a biological term used to describe female vs. male, gender is a social term used to describe the identification of female vs. male. Although the majority of the studies do not measure sex hormones, except in some unusual situations, gender and sex are identical for the human subjects in the research studies. Studies have shown that the LPNN and DMN are sexually differentiated (Joseph, 2000), and sex dimorphisms exhibit significantly at the amygdala, hippocampus and neo-cortex (Juraska, 1991; Cahill, 2006). Functionally, gender differences in the brain are observed in the limbic system or the DMN when conducting emotional or cognitive tasks (Gur et al., 2000; Cahill et al., 2004; Goldstein et al., 2005). Though women seek complementary treatment modalities including acupuncture more frequently than men (Ben-Arye et al., 2009), the majority of studies evaluating acupuncture effects combine genders. Lund and Lundeberg (2008) have noticed that pain shows gender variations that might influence acupuncture treatment. Brain fMRI shows the gender differences when experiencing calibrated pain (Kong et al., 2010a,b) or during resting state (Kong et al., 2010a,b). Because gender differences are shown in the LPNN and the DMN to mediate the tasks of emotion, cognition and pain and that these neural networks are also affected by acupuncture, we hypothesize that women and men may have different brain activation/deactivation patterns at the LPNN and the DMN in response to acupuncture procedure. In this study, using fMRI, we compared neural responses of the task-negative LPNN/DMN as well as the task-positive SMN in response to needle manipulation of acupuncture between two genders.
2. Results
2.1. Subjects
Data on 38 subjects (19 females and 19 males) who had brain fMRI during acupuncture at matched acupoints were used for this study. All the subjects reported deqi sensations with a minimum total score of 3, and none reported sharp pain during needle manipulation. Females were comparable with males in age (Mean±SE: 28.7±1.8 vs. 29.4±2.0) and ethnicity (percentage of Caucasian: 68% vs. 74%) (Table 1). There was no statistical difference in the total score of deqi between two genders (Table 1). The status of feeling anxious during the procedure between the two sex groups was also similar.
Table 1.
Sample characteristics by subjects' sex.
| Female n = 19 | Male n = 19 | P value | |
|---|---|---|---|
| Age, year, mean±SE | 28.7±1.8 | 29.4±2.0 | 0.79 |
| Caucasians, n/total (%) | 13/19 (68%) | 14/19 (74%) | 0.59 |
| Total deqi score, mean±SE | 10.0±1.4 | 8.0±l.l | 0.27 |
| Aching score, mean±SE | 1.9±0.4 | 1.6±0.3 | 0.55 |
| Dull pain score, mean±SE | 1.0±0.3 | 0.8±0.2 | 0.50 |
| Soreness score, mean±SE | 1.2±0.2 | 0.7±0.2 | 0.10 |
| Feeling anxious, n/total (%) | 5/16 (31%) | 4/16 (25%) | 0.69 |
Deqi is the unique sensation of acupuncture which includes aching, dull pain and soreness. Mean±SE with T-test or n/total (%) with Chi Square test are presented. P values for statistical significance are shown.
2.2. The BOLD deactivation/activation patterns induced by acupuncture needle manipulation between females and males
Consistent with our previous study (Hui et al., 2005; Napadow et al., 2007), needle manipulation during acupuncture induced clusters of the deactivated regions, especially at the pregenual cingulate (BA32/24), subgenual area (SG25), temporal pole, medial temporal lobe, posterior cingulate (BA31), precuneus and the visual cortex (BA18/19), in both females and males (Fig. 1). However, the procedure induced more extensive decreases in signal intensity in the regions that are known to be core regions of the DMN, especially marked in the posterior cingulate (BA31) and precuneus, in females than in males (P<0.0001) (Fig. 2A). Using peak signal changes, the left angular gyrus (BA39) showed deactivation in females but activation in males during acupuncture needle manipulation (Fig. 1). The differences in signal intensities at the area of the angular gyrus on both left and right sides were more deactivated in females than in males (P<0.0001) (Fig. 2B).
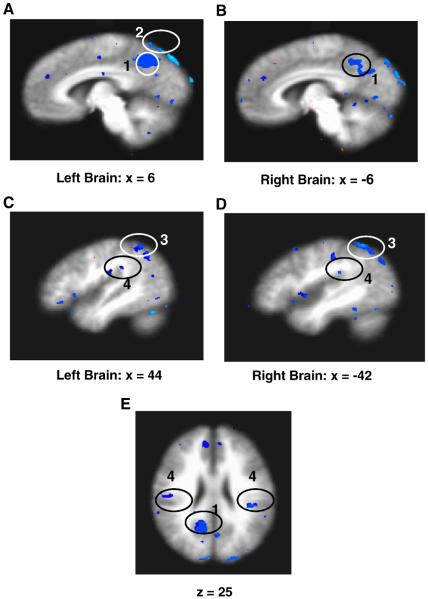
Fig. 1.
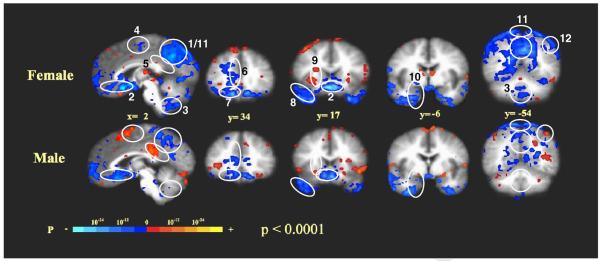
fMRI signal changes during acupuncture in females and males: right paramidsagittal section, x=2 and coronal brain sections from anterior to posterior, y=34 to −54 are shown. The identified brain regions are the following: 1) posterior cingulate (BA31); 2) subgenual area (SG25), subgenual cingulate (BA32/24) and ventromedial prefrontal cortex (BA11); 3) cerebellar vermis; 4) sensorimotor area (SMA, BA6); 5) posterior cingulate (BA23 dorsal); 6) pregenual cingulate (BA32/24); 7) orbitofrontal cortex; 8) temporal pole; 9) anterior insula; 10) medial temporal lobe (amygdala, hippocampus, parahippocampus); 11) precuneus and 12) angular gyrus.
Fig. 2.
Comparisons of fMRI signal changes at the representative brain regions during acupuncture between females vs. males: female vs. male deactivation/actiona signals (bars) and the comparisons between two genders (p<0.0001) by imagings (circles) are illustrated. A) Paramidsagittal sections, x=6 for left brain (L) and B) x=−6 for right brain (R), the identified brain regions (1) posterior cingulate (BA31) and (2) precuneus (PCN) and their average signal changes are shown. Paramidsagittal sections, C) x=44 and D) x=−42, are illustrated. Both L and R angular gyrus (3) and their signal changes are shown. (4) illustrates secondary somatosensory cortex, SII (BA40). E) Axial brain section, z=25, with identified regions of SII (BA40) and BA31, and signal changes of SII between female and male are shown.
Activation of the brain during acupuncture procedure was more limited in distribution, occurring primarily in the somatosensory and association cortices, the insula and the anterior middle cingulate in both genders (Fig. 1). In contrast to having weaker deactivation of the task-positive LPNN/DMN networks, males had stronger activation at the sensorimotor brain regions during acupuncture than females, as shown by the peak signal changes in the supplementary motor area (SMA), contralateral secondary somatosensory cortex (SII), posterior cingulate (BA23 dorsal), posterior middle cingulate (BA24 dorsal) and middle temporal gyrus (BA22) (Fig. 1). For differences of regional signal change, females had less activation at left contralateral SII and deactivation at right SII as opposed to male subjects who had activation at both sides (P<0.0001) (Fig. 2C); no significant difference was observed between genders in other sensorimotor, insula and cerebellar vermis regions (data not shown).
We further chose one acupoint, LV3, which showed the strongest brain response to the stimulation of acupuncture among the three acupoints we used, and compared the average signal changes, instead of the peak changes, at the representative brain structures between two genders (Fig. 3). The acupuncture procedure at LV3 induced extensive deactivation in the regions of the DMN, especially at the left (p<0.0001) and right (p<0.05) posterior cingulate (BA31), the left precuneus (p<0.001) and the right angular gyrus (p<0.05), in females but activation in these regions except the right precuneus in males. Again, females had less activation at bilateral SII than male subjects.
Fig. 3.
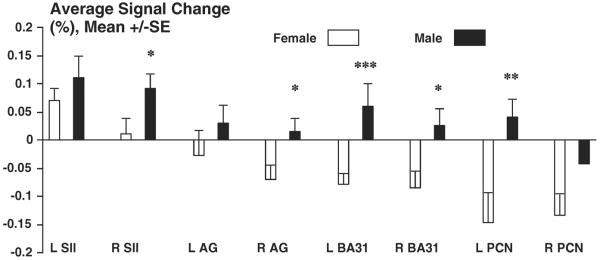
Comparisons of average signal changes at different brain structures during acupuncture on LV3 between females vs. males: To use one acupoint, LV3, female vs. male deactivation/actiona signals (bars) and the comparisons between two genders are illustrated. The identified bilateral brain regions, posterior cingulate (BA31), precuneus (PCN), angular gyrus and secondary somatosensory cortex, SII (BA40) between female and male are shown. *p<0.05; **p<0.001; ***p<0.0001.
2.3. Functional connectivity of LPNN/DMN network during acupuncture needle manipulation between females and males
Fair et al. have studied the data from blocked or mixed blocked/event-related fMRI designs for resting-state functional connectivity analyses (Biswal et al., 1995; Fair et al., 2007; Fox et al., 2005; Vincent et al., 2007), and found that despite having quantitative differences, residuals derived from event-related design data are qualitatively similar to “continuous” resting-state data. We thus used right BA31 as a seed to conduct seed-based cross-correlation analysis (CCA) in both females and males (Fig. 4A) to study the functional connectivity in the task-negative (deactivation) during acupuncture (Biswal et al., 1995; Fair et al., 2007; Fox et al., 2005; Vincent et al., 2007) although covarying activity might also contribute to this. The choice of right BA31 as a seed was based on that the ipsilateral right but not oppositional left posterior cingulate (BA31) showed significant deactivation during the needle manipulation of acupuncture (Figs. 1 and 2), suggesting a different neural pathway for DMN from the one for sensation in the brain. The correlation maps were derived by Z scores and correlation coefficient factor, r values, at structures, and the differences in the correlations between females and males were demonstrated by t-test. In the LPNN/DMN networks coherent activities were significantly stronger in females than in males. With the right posterior cingulate (BA31) as a seed, the females vs. male correlation metrics at several core regions were as follows: 1) right pregenual cingulate (BA32) (r=+0.995 and Z=+5.31 vs. r=+0.164 and Z=+0.17), 2) right hippocampus (r=+0.994 and Z=+4.13 vs. r=−0.628 and Z=−0.74), 3) left posterior cingulate (BA31) (r=+0.996 and Z=+4.23 vs. r=−0.606 and Z=−0.70) and 4) right angular gyrus (r=+0.999 and Z=+5.44 vs. r=−0.940 and Z=−1.78) (Fig. 4A and Table 2). In fact, there was anticorrelation between the right posterior cingulate (BA31) and the right angular gyrus, and there were almost no correlations between BA31 and the other brain structures in the LPNN/DMN networks in males (Table 2). With the use of the pregenual cingulate (BA32) or subgenual area (SG25) as a reference region, coherent activity was found in the posterior cingulate (BA31) with similar gender difference (data not shown).
Fig. 4.
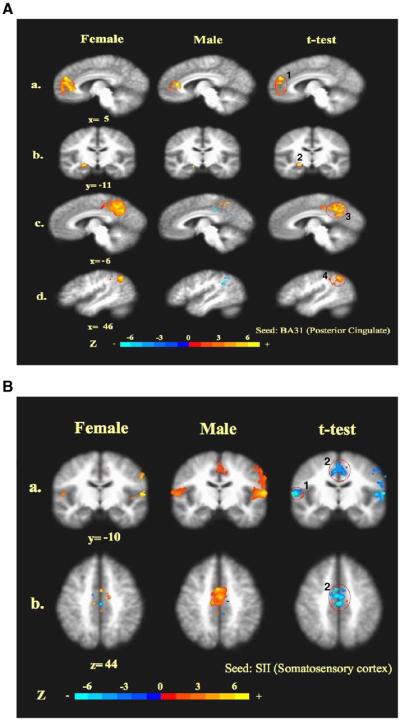
Cross-correlation analysis (CCA) of brain networks during acupuncture: the correlations between the seed (masked) and the other brain regions in both female and male brains are shown, and the orange colors represent positive correlation vs. the blue colors which represent negative correlations. T-test for the gender comparisons is also shown, and the labeled orange colors in the t-test pictures of 5A represent statistically significant difference (the deactivation of female was more significant compared with male) vs. blue colors in the t-test pictures of 5B which represent a statistically significant difference (the activation of male was more significant compared with female) on the voxel numbers induced by acupuncture. 5A illustrates the function connectivities in the task-negative network. Seed: right posterior cingulate (BA31), 4.5 mm radius around peak voxel (x, y, z) 9, −46, 39 for females and 14, −43, 48 for males. a) y=−5: correlation between BA31 and the pregenual cingulate (BA32) (1); b) y=−11: correlation between BA31 and hippocampus (2); c) x=6: correlation between right BA31 and the contralateral posterior cingulate (BA31) (3); d) x=46: correlation between BA31 and the angular gyrus (BA39) (4). 5B illustrates task-positive network. Seed: left (contralateral) secondary somatosensory cortex, SII (BA40), 4.5 mm radius around peak voxel (x, y, z) −58, −21, 23 for females and −58, −18, −16 for males. a) y=−10: correlations between left SII and right SII. (1) or the anterior middle cingulate (BA32/24) (2); b) z=44: correlation between left SII and the anterior middle cingulate (BA32/24).
Table 2.
Cross-correlation analysis (CCA) during acupuncture in females vs. males.
| Structures (Brodmann Area) | Female |
Male |
||
|---|---|---|---|---|
| Correlation | Z score | Correlation | Z score | |
| R posterior cingulate (BA31) as the seed-correlated | ||||
| With R pregenual cingulate (BA32) | r=+0.995 | +5.31 | r=+0.164 | +0.17 |
| With R hippocampus | r=+0.994 | +4.13 | r=−0.628 | −0.74 |
| With L posterior cingulate (BA31)a | r=+0.996 | +4.23 | r=−0.606 | −0.70 |
| With R angular gyrus (BA39) | r=+0.999 | +5.44 | r=−0.940 | −1.78 |
| L SII (BA40) as the seed-correlated | ||||
| With R SII (BA40) | r=+0.746 | +0.96 | r=+0.994 | +4.02 |
| With L anterior-middle cingulate (BA32/24) | r=+0.697 | +0.60 | r=+0.995 | +4.16 |
Right (R) or left (L) brain structures with Brodmann Area (BA) are shown. Correlation coefficient factor, r, and statistical Z scores are indicated.
Seed 1: right posterior cingulate (BA31), 4.5 mm radius around peak voxel (x, y, z) 9, −46, 39 for females and 14, −43, 48 for males.
Seed 2: contralateral (left) secondary somatosensory cortex, SII (BA40), 4.5 mm radius around peak voxel (x, y, z) −58, −21, 23 for females and −58, −18, −16 for males.
In contrast to stronger inter-regional correlations in the LPNN/DMN networks in females than males, males showed much stronger correlations than females in the sensorimotor, the task-positive, network induced by needle manipulation during acupuncture (Fig. 4B and Table 2). Since it is well known that the sensation region of the brain is on the opposite side from the stimulation, we thus chose to use the left secondary somatosensory cortex (SII, BA40) as a seed. The correlation between SII on the left and right hemispheres (females vs. males: r=+0.746 and Z=+0.96 vs. r=+0.994 and Z=+4.02) and the correlation between the left SII and the right middle cingulate (BA32/24) (females vs. males: r=+0.697 and Z=+0.60 vs. r=+0.995 and Z=+4.16) during acupuncture were compared between two genders. Females showed limited correlation of the left SII with the contralateral SII and almost no correlation with the middle cingulate (BA32/24) at the activation sensorimotor network. Although both SII and the posterior middle cingulate (BA23 dorsal) were activated by needle manipulation (Fig. 2), the functional connectivity between these two regions was not found in either gender.
2.4. The relationship between psychophysical response and brain activities induced by needle manipulation
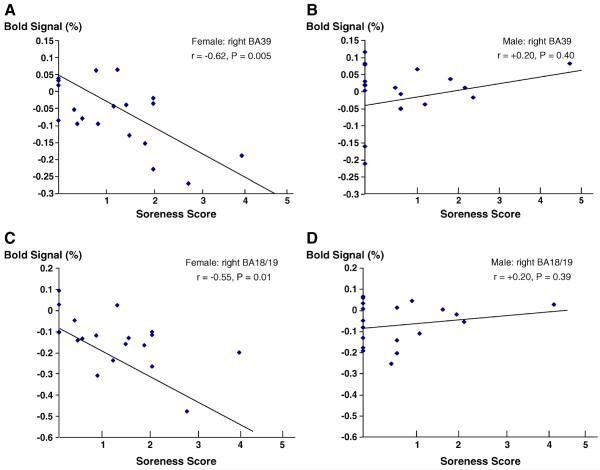
Although there being no statistical differences between females and males in psychophysical responses, total deqi score, to acupuncture needle stimulation (Table 1), we found that the severity of soreness, a major component of deqi, was significantly correlated with deactivation at the right angular gyrus (r=−0.62, p=0.005) (Fig. 5A) and the right visual cortex (BA18/19) (r=−0.55, p=0.01) (Fig. 5C) in females. In contrast, soreness was not correlated with brain activities at either the angular gyrus (Fig. 5B: r=+0.20, p=0.40) or the visual cortex (BA18/19) (Fig. 5D: r=+0.20, p=0.39) in males. Most female subjects (14/19 subjects) had significant deactivation at the right angular gyrus; only a few male subjects (5/19 subjects) showed slight deactivation at this region (Figs. 5A and B). Soreness was not correlated with the neural activities by needle manipulation at somatosensory cortex and other brain structures (data not shown). Although general trends were observed, the correlations between brain activities at the angular gyrus or visual cortex (BA18/19) and other types of psychophysical responses such as dull pain or aching in deqi sensations did not reach statistical significance (data not shown).
Fig. 5.
Gender differences in correlations between average BOLD signals in the brain regions and soreness induced by acupuncture: the severity of soreness induced by acupuncture (x-axis) and its correlation with the average BOLD signal change (%) (y-axis) in females (A and C) vs. males (B and D) are shown for each individual subject. Negative values below zero represent deactivation; positive values above zero represent activation at the brain regions. A and B: the angular gyrus (BA39); C and D: the visual cortex (BA18/19). Correlation coefficient factors, r, and p values for statistical significance are indicated.
3. Discussion
Female and male brains are similar in many aspects but different in others, which could lead to similar but also sex different neural responses depending on environmental stimuli (Witelson, 1991; Cahill, 2006). Pain shows gender variations that might influence acupuncture treatment (Lund and Lundeberg, 2008). This study revealed gender differences in the level of response and the preferential spatial distribution in each task-negative or task-positive network during needle manipulation of acupuncture (Table 2; Figs. 1, 2 and 3) despite the similarity in the general pattern of hemodynamic response (Hui et al., 2005; Bai et al., 2007; Fang et al., 2008). Brain activities induced by needle stimulation during acupuncture shown in this study can be interpreted as deactivation/activation during an attention demanding task, e.g. a sensory stimulation relevant to the resting state. Interestingly, needle manipulation during acupuncture had effects with gender differences not only on the brain regions related to sensation but also on the networks related to cognition and emotion.
Needle manipulation during acupuncture induced significantly stronger deactivation of the LPNN/DMN system especially at the posterior cingulate (BA31), precuneus and angular gyrus in females but stronger activation at the sensorimotor network (SII) in males (Figs. 1, 2 and 3). The brain shows gender differences early in development (Joseph, 2000; Cosgrove et al., 2007), and the differences continue to be subtle but significant as the brain matures (Kovalev et al., 2003; Cahill, 2006). Gender differences are observed in endogenous opioids (Dahan et al., 2008), dopaminergic, serotonergic, and gamma-aminobutyrinergic markers (Kovalev et al., 2003) indicate that male and female brains are neurochemically distinct, and all these neurotransmitters are shown to be modulated by acupuncture (Han et al., 1980; Chen et al., 2008; Yang et al., 2008). Therefore, the gender different patterns induced by needle manipulation during acupuncture are likely to reveal the sex dimorphisms of brain, which is also observed in the cognitive or emotional tasks.
Acupuncture induced deactivation of core structures at the three hubs of the DMN: 1) the medial prefrontal lobe; 2) the amygdala, hippocampus and parahippocampus at the medial temporal lobe and 3) the posterior cingulated (BA31), precuneus and retrosplenial cortex at the medial parietal cortex (Fig. 1). Greater deactivation in females than in males was especially significant at BA31 and precuneus (Fig. 2). At LV3 acupoint, the average responses were deactivations in females vs. activations in males at the DMN regions (Fig. 3). The functional connectivities between BA31 and the pregenual cingulate (PCG) or hippocampus were much stronger in females than males (Fig. 4A and Table 2). All these brain structures influenced by the needle manipulation mediate cognitive and emotional tasks with gender differences. For example, BA31 and precuneus are the brain structures involved in cognitive decline and the development of Alzheimer's disease (AD) (Miller et al., 2008), and being a female is a risk factor to develop AD (Sotiropoulos et al., 2008). In the resting state, women have relatively higher glucose metabolism than men at the posterior cingulate (BA31) of the DMN (Gur et al., 1995). Animal studies demonstrate that sex influences the role of the hippocampus in memory and learning (Shors et al., 2001), and in responding to chronic stress (McEwen, 2002). Women exhibit greater activation in the middle, inferior, and orbito-frontal cortices than men shown by fMRI when conducting tasks of auditory verbal working memory (Goldstein et al., 2005). Men and women have different activated patterns in the frontal, mid-temporal and occipitoparietal cortex when performing phonological or spatial or verbal fluency tasks (Gur et al., 2000; Halari et al., 2006).
The angular gyrus, a limbic-associated structure, revealed substantial gender differences during acupuncture, a new finding with potential significance. The angular gyrus was deactivated and had positive correlation with the posterior cingulate (BA31) in females; however, it was activated and had anticorrelation with BA31 in males (Figs. 2 and 4A). Traditionally female social activities such as child rearing may have contributed to the functional evolution of the angular gyrus (Joseph, 2000). Imaging studies show that gender differences for antisocial, violent, and psychopathic behavior may in part be attributable to differences in the dorsal and ventral prefrontal cortex, amygdala, and angular gyrus (Raine et al., 1997; Miczek et al., 2007; Proverbio et al., 2008). When mediating cognitive tasks, the angular gyrus is activated in men but not so in women (Halari et al., 2006). The angular gyrus is functionally connected with amygdala when responding to emotional face expression (Iidaka et al., 2001). Gender differences also exist in the size and activities of amygdala (Goldstein et al., 2001; Seifritz et al., 2003; Hamann et al., 2004; Cahill et al., 2004). The amygdala and hypothalamus demonstrated deactivation during acupuncture, but did not show significant gender differences in this study (Fig. 2).
Although deqi, induced by needle manipulation, is essential for clinical efficacy of acupuncture according to traditional Chinese medicine, there is no universally agreed metric for deqi (You, 1992). While it is possible that the anticorrelated areas were artificially introduced by global signal regression in the seed-based correlation analyses (Murphy et al., 2009), it is noteworthy that the severity of soreness in deqi was not correlated with the activation of the somatosensory cortex but rather with the deactivation of the brain networks controlling emotion and cognition, further suggesting another major pathway for the mediation of acupuncture action. Additionally, the scores of soreness, a major component of deqi sensations, were correlated with deactivation at the angular gyrus and visual cortex (BA18/19) only in females; but no such correlations were observed in males (Fig. 5). One possibility is that females and males process pain or other sensations by acupuncture differently in the brain, thus women seek acupuncture treatment more frequently than men (Ben-Arye et al., 2009). Another possibility is that deqi during acupuncture is not completely parallel with the brain activities. Women and men have different activation at the brain regions including BA31 when viewing painful stimuli (Proverbio et al., 2009). Gender differences in the brain activities when conducting cognitive or emotional tasks were observed at the DMN and limbic regions even when the behavioral outcomes of the tasks between females and males do not show differences in the scores of cognitive tasks (Grabowski et al., 2003; Piefke and Fink, 2005).
Since this study was not originally designed for gender differences in acupuncture, there are limitations. Considering the effects of circulating sex hormones accounting for partly sex difference, one limitation was the lack of information on the menstrual circle phase of the females. Although potential subjects with known depression or anxiety were excluded from the study, we did not conduct a formal psychiatric examination to evaluate any unreported psychiatric disorder. Psychological tests are probably needed to examine the baseline mental status of the subjects and to correlate with the brain function. We cannot completely rule out the possibility that men might have been less likely than women to report sharp pain during acupuncture, which would enhance activation and attenuate deactivation of the brain systems in males (Hui et al., 2007). However, women and men did not show differences on reporting dull pain and aching (Table 1), arguing against that men only underreported sharp pain in our study. This study was to examine the sex differences of brain responding to a part of the acupuncture procedure, e.g. sensory stimulation by needle manipulation. Since needle manipulation evoking deqi sensation is an important factor for the efficacy of acupuncture, this study suggests that the gender effect probably exists in acupuncture treatment and needs to be considered when conducting an acupuncture study.
4. Conclusion
To the best of our knowledge, this is the first report on the sex differences of the brain responding to the acupuncture stimulation as shown by fMRI. Significant gender differences were observed at both the LPNN/DMN networks and the sensation network in the brain. Since the modulation of these brain structures with acupuncture may be involved when used to treat clinical symptoms, our study suggests that the efficacy or action of acupuncture treatment might be different between women and men.
5. Experimental procedures
5.1. Subjects
The data from 38 subjects participating in a previous study were used (Hui et al., 2007) for this study analysis based on gender status. The subjects were right handed, generally healthy and screened to exclude those with neurological, psychiatric and medical disorders, and contraindications for exposure to high magnetic field. Experiments were conducted with the written consent of each subject and approved by the Massachusetts General Hospital Institutional Review Board.
5.2. Acupuncture
A single licensed acupuncturist in clinical practice for over 25 years administered the acupuncture for all subjects (Hui et al., 2005; Napadow et al., 2007). Acupuncture stimulation was administered to classical acupoints on the right, LI4 on the hand, LV3 on the foot or ST36 on the lower leg (Fig. 6A) in randomized order. These three acupoints are commonly used in acupuncture treatment. Stainless steel needles were used for LV3 (0.20 mm diameter) and ST36 (0.22 mm diameter) (KINGLI Medical Appliance Co. Wuxi, China); silver needles (0.23 mm diameter) were used for LI4 (Matsuka, Tokyo, Japan). We used a 0.20 mm diameter for LV3 because it tends to be a more sensitive acupoint compared to ST36, which used a thicker needle with a 0.22 mm diameter. A silver needle was used at LI4, but not at other acupoints, because this acupoint was close to the fMRI scan. Since silver needles are soft, we used a little thicker needle (0.23 mm). Subjects were told that “we will be having various kinds of stimulation” and were not informed if we were doing tactile or acupuncture. This manuscript only used the data of acupuncture. They could not see the exact procedure from their supine position in the MRI machine.
Fig. 6.
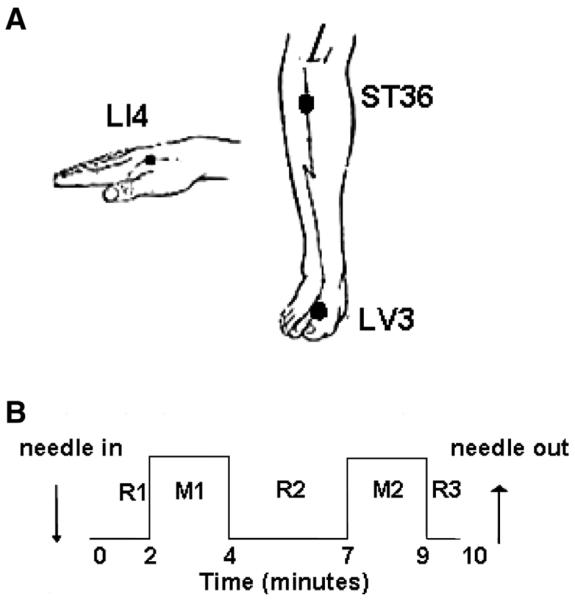
Acupuncture experiment: 1A shows that manual acupuncture was administered to the LI4, ST36, and LV3 acupoints on the right side. 1B shows the paradigm of acupuncture: after remaining in place for 2 min (R1), the needle was rotated with an even motion for 2 min (M1) at the rate of 1 Hz. Needle manipulation was repeated in like manner (M2) after 3 min rest with the needle in place (R2).
5.3. Psychophysical response
Before the fMRI scan started, all subjects were told that at different acupuncture points the procedure may or may not generate different sensations with different intensities. A response to acupuncture during the needle manipulation is characterized by unique sensations including aching, soreness, pressure, heaviness, fullness, warmth, coolness, numbness, tingling and dull pain (Hui et al., 2007). These sensations were scaled from 0 with no sensation to 10 with maximum sensation. All the sensations were asked by a blinded research assistant after each fMRI run. A total score of 3 or higher was defined for the sensations characterized as deqi. Any inadvertent sharp pain lasting more than 1 s was considered noxious stimulation and the concomitant presence of sharp pain with deqi was defined as mixed sensations.
Subjects for this study all reported deqi sensations without any sharp (noxious) pain. Prior to fMRI monitoring, a needle was inserted at an acupoint and a trial of needle manipulation was given followed by asking the subject if any of the deqi sensations were felt and if there was any sharp pain. The acupuncturist was assured that the needle was at the appropriate location and depth before fMRI scanning started. Feeling anxious was a yes/no question after fMRI run.
5.4. Acupuncture with fMRI imaging
Before acupuncture and scanning, the subjects were instructed to keep their eyes closed and to relax during the procedure. An acupuncture needle was inserted at an acupoint before the scan started. During the 10 min scan, the needle was resting for 2 min (R1) and then rotated approximately 180° in each direction with even motion at the rate of 1 Hz for 2 min (M1); after resting in place for 3 min (R2), the identical needle manipulation was repeated (M2) followed by the final resting state (R3) (Fig. 6B). The subject was instructed to raise one finger if any sensation reached the intensity of 7–8 on a scale of 0–10 and 2 fingers in case of any sharp pain. When signaled, the acupuncturist would immediately reduce the magnitude of needle rotation until the subject stopped signaling. Such signals usually lasted for few seconds and appeared in only 11 runs (8 males and 3 females) out of the 152 fMRI runs. At the completion of each scan, the subjects were asked 1) if any of the deqi sensations listed above, or sharp pain, occurred and to rate each sensation on a scale of 0–10; 2) whether they felt anxious by using a yes/no questionnaire; and 3) whether they stayed awake during the procedure.
5.4.1. Image acquisition
Imaging was conducted at the Athinoula A Martinos Center for Biomedical Imaging, Massachusetts General Hospital, on a 1.5 Tesla Siemens Sonata MRI system (Siemens, Erlangen, Germany) equipped for echo planar imaging with a standard quadratic head coil. 38 sagittal slices (3 mm thick with 0.6 mm skip) parallel to the anterior and posterior commissure covering the whole brain were imaged with 4000 ms TR, 30 ms TE, 90° flip angle and 3.13×3.13 mm in-plane spatial resolution. Each functional scan lasted for 10 min. There were 4 dummy scans to allow for equilibration of the MRI signal. A high-resolution 3D MPRAGE sequence was also collected for anatomic localization (Kahn et al., 2008).
5.4.2. Image processing
Primary analysis was carried out using AFNI (Analysis For Neuroimaging (Cox, 1996). The first 15 volumes collected in the first minute of each functional scan were discarded to allow the subject to recover from possible effects of needle insertion and minimize the drift of signals in the baseline prior to active stimulation (Hui et al., 2009). The datasets were transformed to a common three dimensional digital space, Talairach space (Talairach, 1988), normalized to average image intensity and blurred with a Gaussian kernel of full-width half-maximum (FWHM) value of 3 mm to account for any residual differences between runs (Hui et al., 2009). Functional data-sets showing a gross motion exceeding 2 mm on any axis were excluded from further analysis. Duplicate runs of acupuncture on the same acupoint of an individual showing deqi were averaged to provide data for group analysis.
5.5. Statistical analysis
5.5.1. General linear model (GLM) analysis
For each subject, GLM was applied to calculate difference between the acupuncture needle stimulation and the needle resting in place using AFNI's 3dDeconvolve (Cox, 1996). Group analysis was then performed using a random-effects model. To compare the difference between the two genders, a two-sample t-test was performed to compare the fMRI signal changes evoked by acupuncture needle stimulation between the female and male subjects.
A group analysis for each gender (male or female) between the acupuncture needle manipulation and the needle resting in place were calculated separately. 3D % signal change maps were also created so that peak % signal changes and average % signal changes within regions of interest (ROI) could be extracted for each subject and analyzed. Each group p-value map was interpolated to 1 mm using a cubic interpolation algorithm and overlaid onto the group-averaged high-resolution anatomical scan of the subjects; statistical significance of group data was thresholded at p<0.001 (t>3.37) and a minimum cluster size of 3 voxels (total volume 105 mm3) for each gender (Fig. 1). Differences of average % signal changes at ROIs between two gender groups were compared by using t-test and p value<0.0001 was considered statistically significant (Figs. 2 and 3). Spearman's non-parametric correlations were used to examine the relationship between individual psychophysical sensations and the peak % signal changes within ROI for each gender group (Fig. 5).
5.5.2. Seed-based cross-correlational analysis (CCA)
We analyzed the functional connectivity, as measured by coherent BOLD fluctuations over the time course, with the seed-based cross-correlation analysis (CCA) (Biswal et al., 1995; Fair et al., 2007; Fox et al., 2005; Vincent et al., 2007). The method used a sphere of 4.5 mm radius within a primary ROI as a seed and compares it with the time course of every voxel in the brain (Fox et al., 2005; Vincent et al., 2007). We applied a band pass filter 0.003–0.08 Hz onto the preprocessed data to remove cardiac and respiratory signals at frequencies of >0.08 and non-physiological noise at frequencies of <0.003 in order to extract the low frequency BOLD fluctuations described for the core structures (3dFourier, AFNI). The signals found within the ventricles and within the white matter were regressed to remove the global signals which might lead to regionally non-specific correlations (Kahn et al., 2008; Vincent et al., 2007).
The seeds or regions of reference for CCA were chosen from core regions of the intrinsic anticorrelated networks of the human brain. The right posterior cingulate BA31 (ipsilateral to the site of acupuncture stimulation) was used as reference for the task-negative or deactivation network (Fox et al., 2005; Fransson, 2005; Greicius et al., 2003; Kahn et al., 2008; Lowe et al., 1998) and the left secondary somatosensory cortex (contralateral to the site of acupuncture stimulation) for the task-positive or activation network (Fox et al., 2005; Fransson, 2005).
The resultant statistical map of each seed-related peak correlated voxels was normalized to z-values using the Fisher's r-to-z transformation. The z-maps showed values for each voxel that were approximately normally distributed across subjects. The individual z-maps were run through a random-effects t-test analysis (3dttest, AFNI). A t-test thresholded with P<0.001 was run within each gender group to show regions statistically correlated with the seed across all subjects; a separate t-test thresholded with p<0.05 was run to compare the gender between two groups to show regions statistically different between them (Fig. 4). The correlation maps were masked by brain region for display based on the methods previously published (Filipek et al., 1994; Talairach, 1988; Vogt, 2005; Vogt et al., 2003).
Research Highlights
-
▶
Acupuncture modulates brain activity at the limbic–paralimbic–neocortical network (LPNN) and the default mode network (DMN).
-
▶
Deactivation at the LPNN/DMN during needle manipulation of acupuncture was more extensive in females than in males.
-
▶
However, needle manipulation during acupuncture induced greater activation at the secondary somatosensory cortex in males than in females.
-
▶
Soreness during acupuncture was correlated in intensity with deactivation of the angular gyrus in females; no such relationships were observed in males.
-
▶
Our study suggests that brains with sex dimorphism may process the acupuncture stimulation differently between women and men.
Acknowledgments
This work was supported in part by a grant from the NIA, K23AG-022476 for W.Q.Q, and grants from the NIH/National Center for Complementary and Alternative Medicine (R21AT00978) (1-P01-002048-01) for K.K.H, (K01 AT003883) and (R21AT004497) for J.K., the National Center for Research Resources (P41RR14075), the Mental Illness and Neuroscience Discovery Institute and the Brain Project Grant NS 34189.
REFERENCES
- Ben-Arye E, Karkabi S, Shapira C, Schiff E, Lavie O, Keshet Y. Complementary medicine in the primary care setting: results of a survey of gender and cultural patterns in Israel. Gend. Med. 2009;6:384–397. doi: 10.1016/j.genm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth. Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp. Brain Res. 2006;169:1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Hui KK, Nixon EE, Vangel MG, Liu J, Marina O, Napadow V, Hodge SM, Rosen BR, Makris N, Kennedy DN. Characterization of the “deqi” response in acupuncture. BMC Complement. Altern. Med. 2007;7:33. doi: 10.1186/1472-6882-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Marina O, Claunch JD, Nixon EE, Fang J, Liu J, Li M, Napadow V, Vangel M, Makris N, et al. Acupuncture mobilizes the brain's default mode and its anti-correlated network in healthy subjects. Brain Res. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J. Cogn. Neurosci. 2001;13:1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Joseph R. The evolution of sex differences in language, sexuality, and visual–spatial skills. Arch. Sex. Behav. 2000;29:35–66. doi: 10.1023/a:1001834404611. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Huang T, Polich GR, Napadow V, Kathleen H, Mark V, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J. Altern. Complement. Med. 2007a;13:1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Webb JM, Kong JT, Vangel MG, Kwong K. Test–retest study of fMRI signal change evoked by electroacupuncture stimulation. Neuroimage. 2007b;34:1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu PC, LaViolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010a;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain Res. 2010b;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T. Is it all about sex? Acupuncture for the treatment of pain from a biological and gender perspective. Acupunct. Med. 2008;26:33–45. doi: 10.1136/aim.26.1.33. [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Lund I, Naslund J. Acupuncture—self-appraisal and the reward system. Acupunct. Med. 2007;25:87–99. doi: 10.1136/aim.25.3.87. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl Acad. Sci. USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Adorni R. Neural markers of a greater female responsiveness to social stimuli. BMC Neurosci. 2008;9:56. doi: 10.1186/1471-2202-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Adorni R, Zani A, Trestianu L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia. 2009;47:2374–2388. doi: 10.1016/j.neuropsychologia.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OF. Stress and glucocorticoid footprints in the brain—the path from depression to Alzheimer's disease. Neurosci. Biobehav. Rev. 2008;32:1161–1173. doi: 10.1016/j.neubiorev.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Talairach JTP. Co-planar Stereotaxic Atlas of the Human Brain. 1988 [Google Scholar]
- You Z. Preliminary observation on the relation among needling sensation, propagated sensation along meridian (PSM), and acupuncture effect when acupuncture neiguan. Zhen Ci Yan Jiu. 1992;17:75–78. [PubMed] [Google Scholar]