Abstract
In situ photopolymerized semi-interpenetrating networks (sIPNs) composed of poly(ethylene glycol) and gelatin are promising multifunctional matrices for a regenerative medicine approach to dermal wound treatment. In addition to previously demonstrated efficacy in critical defects, sIPNs also function as drug delivery matrices for compounds loaded as either soluble or covalently linked components. Simultaneous release of silver sulfadiazine and bupivacaine from the sIPN would provide multiple-hit management of dermal wounds that minimizes infection, and manages pain along with sIPN absorption of exudates and facilitation of epidermal regrowth. We characterized the release of soluble silver sulfadiazine and bupivacaine and compared it with an established release model. Efficacy of released silver sulfadiazine was confirmed in vitro on Staphylococcus aureus, methicillin resistant S. aureus, and Pseudomonas aeruginosa. Bupivacaine loaded without silver sulfadiazine showed incomplete release, whereas simultaneous loading with silver sulfadiazine facilitated 100% bupivacaine release. Silver sulfadiazine released at 98% without bupivacaine and 96% with bupivacaine. Silver sulfadiazine released onto bacterial cultures inhibited all three strains dose dependently. sIPNs effectively release bupivacaine and silver sulfadiazine while maintaining the antimicrobial activity of silver sulfadiazine. Drug loaded sIPNs have potential to improve wound management by providing multi-drug delivery along with an effective wound treatment.
Quality dermal wound healing proceeds through (i) the removal of nonviable or necrotic tissue, (ii) eradication and prevention of microbial infiltrate, (iii) exudation, and (iv) facilitation of epidermal regrowth.1,2 Phases (ii), (iii), and (iv) are the most dressing dependent, because the process of nonviable tissue removal occurs primarily through debridement and inflammatory activity. Successful wound dressings address these three factors through providing a barrier against pathogenic microbes, and the use of absorbent, biocompatible materials.1,2 However, quality wound treatment does not necessarily target pain management associated with the wound (v). An ideal material for wound management would address all of these issues to achieve successful wound healing as well as improved patient care.
The semi-interpenetrating network (sIPN) composed of in situ photopolymerized poly(ethylene glycol) and gelatin has shown potential as a wound treatment in critical defect and subcutaneous models in vivo.3,4 Also, in situ photopolymerization facilitates conformation of the sIPN matrix to large wound beds with complex contour and crevices. Other efficacious material properties include exudate absorbance, high tensile strength and elasticity, tissue healing integration and adhesion, and favorable tissue healing response.4–6 Adhesion to the wound bed allows the sIPN to remain on the wound until it is naturally sloughed by the healed wound or biodegraded. This sIPN system as well as similar systems such as a poly(ethylene glycol) hydrogels or gelatin hydrogels have been shown to elicit minimal inflammatory cell adhesion and activity in vivo.3,5,7 Furthermore, sIPN functionalized with immobilized, poly(ethylene glycol) conjugated biofunctional molecules, such as the tripeptide, Arg– Gly–Asp has shown to further modulate the host response (Figure 1).4,8 Poly(ethylene glycol) conjugated Arg–Gly–Asp sIPN loaded with keratinocyte growth factor showed increased tissue integration and increased wound contraction along with functional epidermal regrowth in rats.4 Unlike many conventional dressings, the use of the sIPN relieves the need to repeatedly apply treatment throughout the healing time period. These characteristics have allowed sIPNs to succeed at managing issues regarding the healing phases of exudation (iii) and epidermal regrowth (iv), but infection and pain are also an important points of focus.
Figure 1.
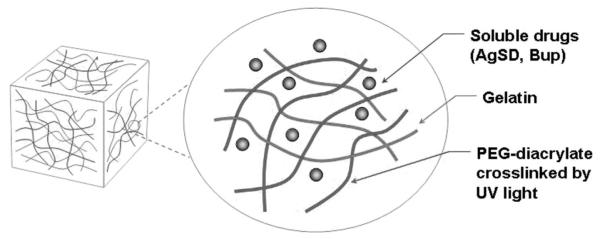
Semi-interpenetrating network (sIPN) drug loading modality schematic for silver sulfadiazine (AgSD) and bupivacaine (Bup).
Drugs can be solubilized in the unpolymerized sIPN solution (Figure 1). Drugs and proteins such as dexamethasone, chlorhexidine digluconate, keratinocyte growth factor, and albumin have been shown to effectively diffuse from the sIPN.3,5 This loading modality may be useful for simultaneously releasing multiple compounds to tackle the challenges of wound healing. Concurrently delivering two drugs to tackle infection (ii) and pain (v) may allow the sIPN to act as a multiple-hit wound treatment for increased dressing efficacy and enhanced patient care.
Infection of large scale burn wounds is a common problem facing wound treatment. Silver-based dressings have become common for addressing this issue. Its popularity stems from a very large antimicrobial profile, including varieties of both gram-positive and gram-negative bacterial strains, as well as several fungal species, with few reports of resistance.9–15 Silver salts are the most common, of which silver sulfadiazine is the most effective and available.16 However, twice daily application of 1% silver sulfadiazine cream limits its clinical utility. Release of silver sulfadiazine from the sIPN may eradicate microbial infiltrate while providing a barrier against new colony infiltration.
Pain management is a critical component of patient care. This is often managed through intravenous and oral analgesics, but with systemic side effects.17,18 Local management of pain may minimize the need for parenteral and oral analgesics to avoid associated side effects. Bupivacaine is a long-acting, local, sodium ion channel inhibitor that is readily used and generally well-tolerated.19 Incorporation of bupivacaine and silver sulfadiazine into the sIPN simultaneously would address most of the primary issues in wound treatment. In this study, we hypothesize that concurrent, multi-drug release from sIPNs is feasible and that characterization of release profiles will provide an understanding of the material structure-function relationship that may lead to enhanced wound healing characteristics through microbial eradication, and pain management.
MATERIALS AND METHODS
Drug-Loaded sIPN Synthesis and Characterization
All starting materials were purchased from Sigma-Aldrich (Milwaukee, WI). Unpolymerized sIPN solutions were prepared with 10% gelatin and 15% poly(ethylene glycol) diacrylate in water at 60°C. 2,2-dimethoxy-2-phenylacetophenone, dissolved in poly(ethylene glycol) diacrylate, was added to this solution immediately before polymerization. sIPN solution was poured into a disc-shaped Teflon mold (r = 7 mm, h = 1 mm) and exposed to UV light (Clearstone Technologies CF1000 with 365 nm LED head) for approximately 3 minutes to polymerize the solution. Drug molecules, silver sulfadiazine and/or bupivacaine, were each dissolved in the sIPN solution at 10 mg/ml before 2,2-dimethoxy-2-phenylacetopheone was added.
Drug Release Assays
The sIPN discs were placed in 5 ml phosphate buffered saline. The entire release buffer was removed at each time point and replaced with fresh buffer. Release buffer containing silver sulfadiazine and/or bupivacaine was collected at 2, 4, 6, 24, and 168 hours. Silver sulfadiazine was assayed using UV/Vis photo-spectroscopy at the maximum absorbance of 297 nm. Maximum absorbance was first established through whole spectrum scans. Bupivacaine required dye conjugation to resolve absorption for the concentrations being assayed.20 Briefly, solutions containing bupivacaine were mixed well with bromothymol blue (0.04% aqueous) in excess, extracted with dichloromethane, dried over magnesium sulfate, filtered to remove drying agent, and dichloromethane was evaporated off. The bupivacaine conjugated with bromothymol blue was resuspended in a known amount of dichloromethane and measured via UV/Vis photo-spectroscopy at 413 nm. Standard solutions of compound in phosphate buffered saline were used to establish Lambert-Beer plots absorptivity coefficients (Table 1). Standards were assayed before and after UV light exposure of same magnitude and duration as needed for sIPN polymerization, and for up to several weeks in solution. These conditions were tested to elucidate the potential impact of UV exposure during in situ photopolymerization, and the solution exposure time associated with the assay. Release buffer solutions were assayed to determine the concentration of a given compound, which was reported as a percentage of the amount loaded. Concentrations were calculated based on the Lambert–Beer plot of drug alone in solution.
Table 1.
Summary of sIPNs compound loading formulations
| Molar Absorption Coefficients (L/mol · cm) |
||
|---|---|---|
| Standard Curve Treatment |
Bupivacaine | Silver Sulfadiazine |
| Drug alone in solution | 454136 | 358112 |
| Drug alone in solution after UV exposure |
490181 (0.701) | 373178 (0.061) |
| Drug alone in solution for 10 d after UV exposure |
473911 (0.681) | 384215 (0.596) |
| Both drugs in solution | 458698 (0.662) | 347764 (0.306) |
sIPNs, semi-interpenetrating networks.
Silver sulfadiazine and/or bupivacaine were loaded as soluble drug compounds. P-value of t-test comparison with drug alone in solution shown in parentheses.
Theoretical Model of Small Molecule Diffusion
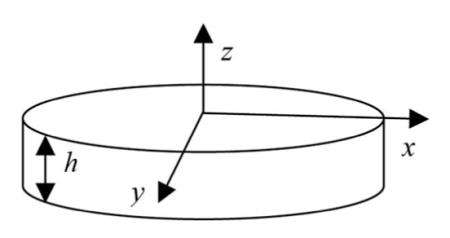
A simple model was developed to describe the diffusion of small drug molecules through the sIPN. sIPN was modeled as a cylinder of thickness h and radius r with loaded drug serving as a transient, finite source. The cylinder can be modeled as a simple disc where z indicates the direction perpendicular to the plane of the disc. The height of the disc is represented by h. The bulk fluid surrounding the sIPN is a constant sink. By assuming that diffusion in the radial direction is negligible (h << r) the mass balance can be represented as
where D1 is the diffusion coefficient of the molecule and C1(z,t) is the concentration of the molecule at a given position along the z axis and time. The boundary conditions for all t ≥ 0 are
At the surface (z = L), the molecule is assumed to be immediately taken away by the bulk fluid, while at the center of the sIPN (z = 0), the net flux of the molecule is 0 as a result of symmetry. An unsteady state exists as a result of the transient, finite sink; therefore, the initial condition (t = 0) was set as −L < z < L, C1 = C1,0, where C1,0 represents the concentration of drug loaded into the sIPN. Through separation of variables and using the above conditions, the analytical solution for C1(z,t) was found to be
which can be re-expressed as:
where m1,0 = C1,0AL is the initial amount loaded at t = 0 and m1,∞ = C1,s AL is the final amount remaining in the sIPN at t → ∞. For this model, m1,∞ = 0.
Bacterial Inhibition
Freshly passaged cultures of Staphylococcus aureus (6538P), methicillin resistant S. aureus (MRSA) (33591), and Pseudomonas aeruginosa (27853) were employed to study inhibition in response to silver sulfadiazine loaded sIPNs and standard silver sulfadiazine solutions. Agar solution was produced using Mueller Hinton agar. Immediately before plating, bacteria suspended in Mueller Hinton broth (0.3 absorbance at 580 nm) were added to the agar solution. This produced an initial seeding density of approximately 107.5 cells/mm2. Ten microliters standard silver sulfadiazine solution were injected into 2 mm diameter wells as a comparison. Silver sulfadiazine loaded sIPNs were placed on the surface of the agars. At the first growth point, silver sulfadiazine loaded sIPNs were placed on agars within an hour of plating. At the second growth point, silver sulfadiazine standards or silver sulfadiazine loaded sIPNs were administered on agars containing confluent cells due to a 24 hour incubation period after plating. Vacant region, or inhibition zone, diameters were measured via caliper (Monostat dial-type vernier caliper, Switzerland) 24 hours after silver sulfadiazine treatment. Silver sulfadiazine was used at ten different concentrations of silver sulfadiazine ranging from 0 mg/ml to 10 mg/ml.
Statistics
All data have been reported as mean ± standard deviation over three independent trials. Absorptivity coefficients from standard solutions were compared by applying paired t-tests over the range of standard concentrations tested to produce Lambert–Beer Law plot (threshold of significant difference P < .05). Non-linear regression was performed on silver sulfa-diazine and bupivacaine release profiles through the application of Eq. (1). Diffusivity, D, was calculated by minimizing the residual sum of squares of the non-linear regression line. Correlation coefficients (r2) between the curve from Eq. (1) fitted with a compound-specific diffusivity and the actual data were reported. Bacterial inhibition dose-response curves were compared using paired t-tests over the range of silver sulfadiazine concentrations tested (threshold of significant difference P < .05).
RESULTS AND DISCUSSION
Bupivacaine and Silver Sulfadiazine Release
Bupivacaine and silver sulfadiazine were loaded into sIPN as soluble components to characterize simultaneous multi-drug release kinetics. Bupivacaine and silver sulfadiazine releases from sIPN into phosphate buffered saline solution were measured for seven days (Figures 2,3). Both bupivacaine and silver sulfadiazine completed the burst phase release between 6 and 24 hours. At 24 hours, cumulative release of bupivacaine loaded concurrently with silver sulfadiazine reached 100 ± 27%. Bupivacaine HCl as an injectable solution is typically given as 2.5 to 5.0 mg/ml for peripheral sympathetic nerve block at approximately 3 hour intervals. One hundred percentage of 10 mg/ml bupivacaine released within 2 to 4 hours exceeded the lower doses that lead to muscle relaxation (7.5 mg/ml), but would not induce other significant toxicity unless 17.5 ml or more of sIPN solution was used. Bupivacaine loaded without silver sulfadiazine reached only 37 ± 8%, most of which occurred within 2 to 4 hours. This is analogous to a 3.7 mg/ml dose. At 24 hours, cumulative release of silver sulfadiazine alone reached 98 ± 7%, while silver sulfadiazine concurrently released with bupivacaine reached 96 ± 1%. With the exception of bupivacaine loaded without silver sulfadiazine, cumulative releases approaching or approximately equal to 100% suggests that radical photopolymerization was not significantly altering the respective drug molecules. When loaded without silver sulfadiazine, a significant percentage of bupivacaine is being sequestered in the sIPN and not released. We hypothesize that bupivacaine is interacting with poly(ethylene glycol) diacrylate before polymerization through Michael-type addition to the acrylate group. When silver sulfadiazine salt is present in solution, the silver ion is able to interact, and possibly coordinate, with the amide nitrogen of bupivacaine and block this addition. Furthermore, sulfadiazine would not be expected to react with the acrylate group because the adjacent sulfonamide group decreases the amine’s nucleophilicity.
Figure 2.
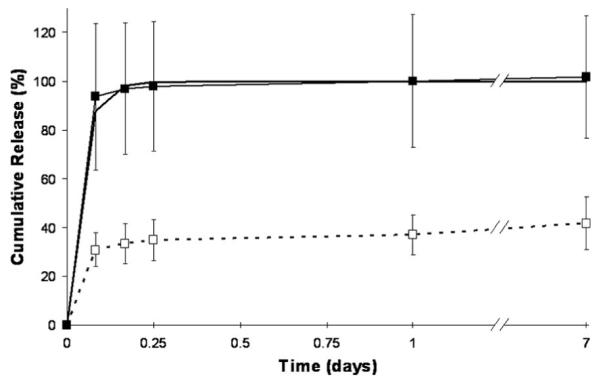
Release profile of soluble loaded bupivacaine, loaded with or without silver sulfadiazine, into a semi-interpenetrating network (sIPN). Theoretical release from diffusion model ( ), bupivacaine alone (
), bupivacaine alone ( ), and bupivacaine loaded concurrently with silver sulfadiazine (
), and bupivacaine loaded concurrently with silver sulfadiazine ( ) are shown. Data plotted as mean ± standard deviation (n = 3).
) are shown. Data plotted as mean ± standard deviation (n = 3).
Figure 3.
Release profile of soluble loaded silver sulfadiazine, loaded with or without bupivacaine, into a semi-interpenetrating network (sIPN). Theoretical release from diffusion model ( ), silver sulfadiazine alone (
), silver sulfadiazine alone ( ) and silver sulfadiazine loaded concurrently with bupivacaine (
) and silver sulfadiazine loaded concurrently with bupivacaine (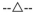 ) are shown. Data plotted as mean ± standard deviation (n = 3).
) are shown. Data plotted as mean ± standard deviation (n = 3).
To model these profiles, several parameters were defined resulting in Eq. (1). This equation gives m1(t), the mass remaining in the sIPN disc at time t, in proportion to m1,0, the initially loaded mass. Plots of 1 – (m1(t)/m1,0), the theoretical fraction of cumulative release, using bupivacaine diffusivity of 3.3 × 10−8 cm2/s and silver sulfadiazine diffusivity of 1.3 × 10−7 cm2/s are shown in Figures 3, 4. The correlation between actual drug release and this theoretical model provides a statistical means to assess the role of diffusive release. The correlation coefficients for release of bupivacaine loaded alone and bupivacaine loaded with silver sulfadiazine were 0.205 and 0.988, respectively, whereas those for silver sulfadiazine loaded alone and silver sulfadiazine loaded with bupivacaine were 0.989 and 0.992, respectively. Therefore, the assumptions mediating small molecule dif fusive release are considered true for all releases except bupivacaine loaded alone due to possible reaction mechanism described above.
Figure 4.
Dose–response curve for silver sulfadiazine inhibition of Pseudomonas aeruginosa, Staphylococcus aureus, and methicillin resistant S. aureus (MRSA). Loading concentrations ranged from 0 to 10 mg/ml. Inhibition zones were measured 24 hours after silver sulfadiazine treatment on freshly plated bacteria (seeding density approximately 107.5 colonies/mm2) and confluent bacteria, respectively. Silver sulfadiazine treatments were given with and without concurrently present bupivacaine (10 mg/ml) as (left column) silver sulfadiazine in water placed within 2 mm wells punched from the agar, or (right column) silver sulfadiazine loaded semi-interpenetrating networks (sIPNs). Soluble silver sulfadiazine loaded without bupivacaine is shown as freshly plated  , and confluent
, and confluent  , whereas soluble silver sulfadiazine loaded concurrently with bupivacaine is shown as freshly plated
, whereas soluble silver sulfadiazine loaded concurrently with bupivacaine is shown as freshly plated  , and confluent
, and confluent  . Inhibition zones are displayed as mean ± standard deviation (n = 3).
. Inhibition zones are displayed as mean ± standard deviation (n = 3).
Wounds sometimes treated with antimicrobials or analgesics are often void of the epidermal layer. Under this situation, the release of bupivacaine from the in situ formed sIPN would be analogous to the local infiltration of injected bupivacaine HCl solution rather than the percutaneous diffusion and absorption of topically applied bupivacaine formulations. Others have shown that the injection of bupivacaine HCl solution can deliver an entire dose instantly, while absorption through the skin can deliver about 75% of the dose in more than 24 hours.21 Although the presence of the epidermal layer reduces the diffusion and absorption of bupivacaine into the dermis, the formation of the epidermal layer could also reduce the need for aggressive pain management. In our study, the in vitro release of loaded bupicaine from the sIPN mostly occurred within 6 hours, well before epidermal formation in most deepithelialized wounds. Hence the diffusion of bupivacaine into the dermis would not likely be limited by epidermal formation in vivo. Under the in vivo condition, factors other than those in the in vitro condition are likely involved in modulating the release and the bioavailability of bupivacaine from the matrix. Some of these factors include: the effect of drug concentration in the tissue immediately adjacent to the matrix, the dynamic process of exudation and interstitial fluid formation, the local and the systemic absorption of the drug, and the absorption through the secondary dressing.
Released Silver Sulfadiazine Efficacy
Drugs released from sIPNs must maintain their efficacy in order to be useful clinically. There lacks an appropriate animal model for testing the analgesic efficacy of locally delivered bupivacaine and the ones available are semi-quantitative. Antibacterial efficacy of silver sulfadiazine was quantified on cultured bacteria. Dose dependent silver sulfadiazine inhibition of cultures containing S. aureus, MRSA, or P. aeuruginosa was determined at two points of bacterial growth (Figure 4). Silver sulfadiazine solutions (Figure 4, left column) and silver sulfadiazine loaded sIPNs (Figure 4, right column) were tested. Silver sulfadiazine loaded sIPNs (with or without concurrently loaded bupivacaine) inhibited freshly plated P. aeruginosa with significantly greater zone diameters over those of S. aureus, or MRSA. S. aureus and MRSA inhibition profiles were similar in response to sIPN treatment except in freshly plated cultures treated with silver sulfadiazine loaded sIPNs without bupivacaine. Silver sulfadiazine loaded sIPNs (with or without bupivacaine) also showed greater zone diameters in all freshly plated cultures than in confluent cultures. This trend was significant in all treatments except P. aeruginosa treated with silver sulfadiazine and bupivacaine loaded sIPN. Silver sulfadiazine loaded sIPN inhibition was not significantly altered by the presence of bupivacaine under any condition. However, S. aureus and MRSA response to silver sulfadiazine loaded sIPNs shows some dependence on concurrent loading of bupivacaine. These data coincide with the literature on silver sulfadiazine inhibition of gram-positive bacteria, which notes that both bacteriostasis and bactericidal activity can be achieved, which we demonstrated through inhibition testing on freshly plated cultures and confluent cultures.22 However, literature on silver sulfadiazine inhibition of P. aeruginosa and other gram-negative bacteria is conflicting. Some investigators have shown that silver sulfadiazine can be bactericidal to gram-negative strains,9,10,12–14 while others have shown some silver sulfadiazine resistance in gram-negative strains.11,12,15 We have shown here that silver sulfadiazine loaded sIPNs can be bactericidal to gram-positive and gram-negative strains. Resistance mechanisms are more prevalent in ionic silver forms. Therefore, the mild resistance to dissolution and ionization observed with silver sulfadiazine salt may have ameliorated resistance in this study.12
CONCLUSIONS
Complete simultaneous release of soluble of silver sulfadiazine and bupivacaine from photopolymerized sIPNs was characterized and the silver sulfadiazine antibacterial efficacy confirmed. Drug properties of silver sulfadiazine and bupivacaine, along with the material properties of sIPNs render silver sulfadiazine and bupivacaine loaded sIPNs a potential matrix for treatment of dermal wounds.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ashley Stajkowski, Dr. David Andes, Karen Marchillo, Tom Stamstad, and Dr. Lee Faucher.
This study was supported by grants from NIH RO1EB6613 and UW-Madison I&EDR.
REFERENCES
- 1.Ayello EA. New evidence for an enduring wound-healing concept: moisture control. J Wound Ostomy Continence Nurs. 2006;33:S1–2. doi: 10.1097/01.won.0000278580.46070.19. [DOI] [PubMed] [Google Scholar]
- 2.Brett DW. A review of moisture-control dressings in wound care. J Wound Ostomy Continence Nurs. 2006;33:S3–8. doi: 10.1097/01.won.0000278581.53694.b6. [DOI] [PubMed] [Google Scholar]
- 3.Stevens KR, Einerson NJ, Burmania JA, Kao WJ. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J Biomater Sci. 2002;13:1353–66. doi: 10.1163/15685620260449741. [DOI] [PubMed] [Google Scholar]
- 4.Waldeck H, Chung AS, Kao WJ. Interpenetrating polymer networks containing gelatin modified with PEGylated RGD and soluble KGF: synthesis, characterization, and application in in vivo critical dermal wound. J Biomed Mater Res A. 2007;82:861–71. doi: 10.1002/jbm.a.31054. [DOI] [PubMed] [Google Scholar]
- 5.Einerson NJ, Stevens KR, Kao WJ. Synthesis and physico-chemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials. 2002;24:509–23. doi: 10.1016/s0142-9612(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 6.Witte RP, Blake AJ, Palmer C, Kao WJ. Analysis of poly(ethylene glycol)-diacrylate macromer polymerization within a multicomponent semi-interpenetrating polymer network system. J Biomed Mater Res A. 2004;71:508–18. doi: 10.1002/jbm.a.30179. [DOI] [PubMed] [Google Scholar]
- 7.Sawhney AS, Pathak CP, Hubbell JA. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials. 1993;14:1008–16. doi: 10.1016/0142-9612(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 8.Phillips JM, Kao WJ. Macrophage adhesion on gelatin-based interpenetrating networks grafted with PEGylated RGD. Tissue Eng. 2005;11:964–73. doi: 10.1089/ten.2005.11.964. [DOI] [PubMed] [Google Scholar]
- 9.Lansdown ABG. Silver 1: its antibacterial properties and mechanism of action. J Wound Care. 2002;11:125–30. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 10.Lowbury EJL. Problems of resistance in open wounds and burns. In: Mouton RP, Brumfitt W, Hamilto-Miller JMT, editors. The rational choice of antibacterial agents. Kluwer Harrap; London: 1975. pp. 18–31. [Google Scholar]
- 11.Silver sulfadiazine. Med Lett Drugs Ther. 1974;16:43–4. [PubMed] [Google Scholar]
- 12.Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006;33:627–34. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 13.Bjarnsholt T, Kirketerp-Moller K, Kristiansen S, et al. Silver against Pseudomonas aeruginosa biofilms. APMIS. 2007;115:921–8. doi: 10.1111/j.1600-0463.2007.apm_646.x. [DOI] [PubMed] [Google Scholar]
- 14.Fox CL, Rappole BW, Stanford W. Control of Pseudomonas infection in burns by silver sulfadiazine. Surg Gynecol Obstet. 1969;128:1021–7. [PubMed] [Google Scholar]
- 15.Modak SM, Fox CLJ. Sulfadiazine silver-resistant Pseudomonas in burns. New topical agents. Arch Surg. 1981;116:854–7. doi: 10.1001/archsurg.1981.01380190006002. [DOI] [PubMed] [Google Scholar]
- 16.Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26:131–8. doi: 10.1016/s0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher G, Rae CP, Kinsella J. Treatment of pain in severe burns. Am J Clin Dermatol. 2000;1:325–9. doi: 10.2165/00128071-200001060-00001. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: a systematic review. J Pain Symptom Manage. 2003;26:1026–48. doi: 10.1016/j.jpainsymman.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Katzung BG. Basic and clinical pharmacology. 9th ed McGraw-Hill; NY: 1997. pp. 418–27. [Google Scholar]
- 20.Dinesh ND, Nagaraja P, Rangappa KS. Sensitive spectrophotometric methods for the analysis of some anesthetic drugs. Indian J Pharm Sci. 2002;64:485–8. [Google Scholar]
- 21.Blanco MD, Bernardo MV, Teijon C, Sastre RL, Teijon RJ. Transdermal application of bupivacaine-loaded poly(acrylamide(A)-co-monomethyl itaconate) hydrogels. Int J Pharm. 2003;255:99–107. doi: 10.1016/s0378-5173(03)00036-x. [DOI] [PubMed] [Google Scholar]
- 22.Hadjipavlou-Litina D, Kontogiorgis C, Pontiki E, Dakanali MA, Katerinopoulos HE. Anti-inflammatory and antioxidant activity of coumarins designed as potential fluorescent zinc sensors. J Enzyme Inhib Med Chem. 2007;22:292–7. doi: 10.1080/14756360601073914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



