Abstract
Adult stem cell therapies have provided success for more than 50 years, through reconstitution of the hematopoietic system using bone marrow, umbilical cord blood, and mobilized peripheral blood transplantation. Mesenchymal stem cell (MSC)-mediated therapy is a fast-growing field that has proven safe and effective in the treatment of various degenerative diseases and tissue injuries. Since the first derivation of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), there has been impressive progress toward developing safe clinical applications from PSCs. Recent successes in transgene-free iPSC reprogramming have brought attention to the potential of clinical applications of these pluripotent cells, but key hurdles must be overcome, which are discussed in this review. Looking to the future, it could be advantageous to derive MSC from iPSC or human ESC in cases where genetic engineering is needed, since in the PSCs, clones with “safe harbor” vector integration could be selected, expanded, and differentiated. Here, we describe the status of the progress of the use of MSC and PSCs in clinical trials and analyze the challenges that should be overcome before iPSC-derived MSC therapy can be used widely in the clinic.
Keywords: Mesenchymal stem cells, Induced pluripotent stem cells, Cellular therapy, Clinical trials, Regenerative medicine
Introduction
An emerging approach to treat disorders requiring the replacement of injured or dying cells is to replace those cells with healthy ones generated from stem cells, which have the potential to differentiate into multiple mature cell types. In particular, adult stem cell-based therapies have been successful for several decades, with the first hematopoietic stem cell (HSC) transplantation occurring over 50 years ago [1]. Recent discoveries based on embryonic stem cells (ESCs) [2] and induced pluripotent stem cells (iPSCs) [3] escalate the hope for future regenerative medicine applications, with one human ESC-based therapy already being tested in a first-in-man Phase I clinical trial. In spite of the great potential, there are technical challenges to be overcome before PSCs can be applied to clinical applications in a broader fashion.
In this review, we highlight the potential of iPSC-derived MSCs and several other iPSC derivatives currently developed as stem cell therapy candidates and provide an evaluation of challenges to overcome potential barriers toward clinical usage of PSC-based products.
Mesenchymal Stem Cells
For decades, cell therapies using adult stem cells have rescued thousands of patients from induced or genetic disorders [1]. Bone marrow (BM)-derived HSC therapy was first delivered to patients in 1956, following extensive testing in a canine model [4], afterward becoming a standard clinical procedure, particularly as a treatment for leukemia and lymphoma (reviewed in ref. [5]). MSCs were first described by Friedenstein and colleagues as an adherent fibroblast-like subset of the BM microenvironment called the “marrow stromal cells,” which was capable of supporting hematopoiesis. Later, these fibroblast-like cells were found to have adult stem cell properties as they could differentiate into cartilage, bone, fat, and tendon [1]. MSCs have been evaluated for regenerative medicine applications either through direct differentiation into these tissues or indirectly through protein or cytokine secretion and immune suppression [1, 6–9]. MSCs are a promising tool for cell therapies because they are easily accessible from various tissue sources such as BM, fat, umbilical cord, and others, easily isolated, show robust in vitro expansion to clinical scale and allow for cryostorage with minimal loss of stem cell characteristics. MSCs have demonstrated systemic migration capabilities after i.v. transplantation, in particular to areas of hypoxia or tissue damage [10]. Even systemic administrations of allogeneic MSCs do not cause any adverse effects, in part due to immune-modulatory effects [11, 12]. MSCs have been considered safe as they do not show tumor formation after transplantation [13] and have been widely tested and proven efficacious in preclinical and clinical studies for cardiovascular [14] and neurodegenerative [15] diseases, graft-versus-host disease (GvHD) [11], and autoimmune disease. Because of the ability of MSCs to differentiate to osteoblasts, Caplan and colleagues initiated clinical trials for osteogenesis imperfecta using allogeneic BM transplantation with MSC [16]. Le Blanc et al. pioneered a study to investigate immuno-modulatory effects of MSC transplantation therapies for steroid-resistant GvHD [11], and similar methods were applied to other diseases [1]. These early studies established a good clinical record of safety for systemic MSC transplantation.
Several papers have demonstrated that MSCs can be efficiently transduced with retroviral and lentiviral vectors and maintain transgene expression throughout many passages and lineage-specific differentiations, with fewer complications caused by viral integrations [13, 17, 18]. MSCs genetically modified to secrete cytokines and other growth factors have been successfully used in animal models of tissue repair and various other diseases and are therefore poised to be tested in human clinical trials [9]. Clonal analysis of transduced MSCs have shown that MSCs often contain several thousand copies of transgene RNA per cell and can maintain transgene expression for up to 6 months [19]. However, the risk of tumor formation due to insertional mutagenesis by viral vector integrations still raises caution for human clinical applications [20] (discussed in a later section). Identification and utilization of genetically modified MSCs, which have “safe harbor” integrations of the desired transgenes, is restricted due to the limited lifespan of primary MSCs during in vitro expansion. Aging, moreover, significantly reduces the survival and differentiation potential of BM-MSCs [21]. In contrast, using human PSC (hESC or iPSC) to generate MSCs, a vector integration site could be mapped and cells with safe harbor integrations could potentially be expanded nearly indefinitely.
HUMAN PLURIPOTENT STEM CELLS
hESCs have the potential to differentiate into all types of adult human tissues and to grow indefinitely [2]. Since their initial derivation, hESCs have become promising tools for developmental biology and regenerative medicine. However, concerns related to ethical objections regarding the use of human embryos for hESC derivation have dramatically restricted funding of research using these cells and therefore have set back the development of hESCs for clinical trials. Because of their allogeneic nature, immune rejection of cells and tissues derived from hESCs is another potential drawback to their use in transplantation. Immunosuppressive drug regimens, similar to those used for current human tissue and organ transplant procedures, might lessen the severity of the anticipated immune rejection, but at the same time, can also put the tissue recipient at an increased risk of infections. This risk can be lessened by application of human leukocyte antigen–matched tissue, as is currently being practiced in organ transplantation, or could be completely eliminated by the use of the patient’s own tissue. The latter possibility can now be achieved by application of autologous iPSCs, the patient’s own somatic cells, “reprogrammed” to become pluripotent cells [3].
Following groundbreaking work by Yamanaka and colleagues demonstrating that mouse fibroblasts could be converted into iPSCs by retroviral delivery of four transcription factors (Oct4, Sox2, Klf4, and Myc), other groups reported that terminally differentiated human somatic cells could be reprogrammed into a pluripotent state using retroviral or lentiviral vectors transferring the same four transcription factors. In many ways, iPSCs are similar to hESCs, in their morphology, gene expression, in vitro differentiation potential, and teratoma formation. However, inherent “epigenetic memory” of the starting cells may influence specific differentiation and in vivo functionality of tissues derived from such reprogrammed cells. More research in this area is needed to determine the best starting somatic cell for iPSC generation that allows for reproducible differentiation into different types of functional tissues for human clinical applications. iPSCs hold great potential for regenerative medicine, as can already be demonstrated in mouse models of Parkinson’s disease [22] and sickle cell anemia [23]. Disease-specific iPSC lines for modeling “diseases in a dish,” screening new drug compounds, and developing new therapies have been used successfully [24–26] (detailed review in Shinya Yamanaka and Masato Nakagawa paper in this issue). However, clinical applications of iPSCs have been criticized because of the possibility to form tumors by integrated oncogenes, c-Myc in particular [27], by insertional mutagenesis that has the potential to cause cancers [28] or disrupt tumor suppressor genes [20], and recently, for epigenetic memories and genomic aberrations in the reprogrammed cells [29]. Therefore, to manufacture iPSCs for clinical applications, several precautions need to be taken, as discussed in detail in the following section.
As BM-MSCs can easily be harvested from adult sources and cultured in vitro, many preclinical and clinical studies have used BM-MSCs [1]. Although easy access to BM-MSCs is recognized as a great advantage, extended in vitro culture reduces the differentiation potential of MSCs, which limits their therapeutic efficacy [30]. To overcome this shortfall, MSCs derived from iPSCs may therefore be considered for human cell and gene therapy applications as iPSCs have the potential to be expanded indefinitely without senescence (Fig. 1). Several laboratories, including ours, have already shown that MSCs derived from hESC have the same in vitro and in vivo characteristics as MSCs derived from adult sources [31, 32]. Our group reported that hESC-derived MSCs were karyotipically stable, had the same cell surface phenotype as MSCs isolated from adult BM, and could home similarly to areas of hypoxic injury in a hindlimb ischemia model [32]. Lian et al. [33] showed that MSCs derived from human iPSCs can be generated in clonal expansion cultures and can be differentiated into osteoblasts, adipocytes, and chondrocytes and promote vascular and muscle regeneration. This team also described a greater regenerative potential of MSCs derived from iPSCs, which may be attributed to superior survival and engraftment after transplantation, because of higher telomerase activity and less senescence as compared to BM-MSCs. In these studies, iPSC- or hESC-derived MSCs were comparable to BM-MSCs in surface marker expression, differentiation potential, and in in vivo regenerative potential in the hind limb ischemia mouse model. Future studies should examine the efficiency of MSC derivation based on different clinically relevant protocols or cell sources, with term follow-up of in vivo safety and efficacy studies.
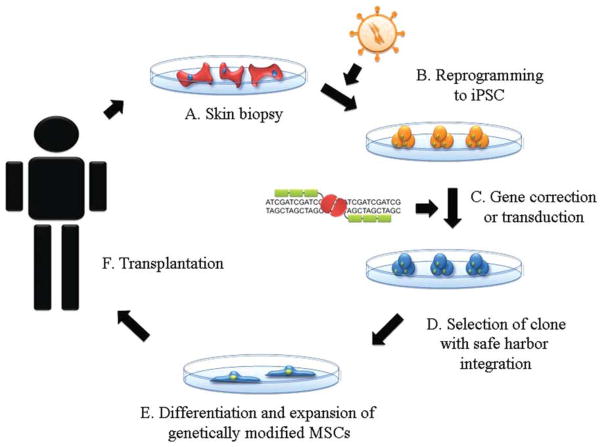
Figure 1.
Schematic diagram of iPSC therapy. (A): Fibroblasts from skin biopsy are cultured from patients. (B): Patient-specific cells can be reprogrammed by viral delivery of induction factors or nonintegrating methods. (C): Gene correction can be accomplished by vector-mediated gene transfer or gene exchange by homologous recombination. (D): Gene-corrected iPSCs can be screened by sequencing to find a clone with proper gene correction or integration into a safe harbor site. (E): Gene-modified iPSCs can be differentiated into MSCs and expanded. (F): MSCs with integration into the controlled site can be tested, expanded, and purified in a good manufacturing practice facility and could then be transplanted to the patient, following appropriate clearance by all regulatory agencies. Abbreviations: iPSC, induced pluripotent stem cell; MSCs, mesenchymal stem cells.
Genetically manipulated MSCs may also serve as cellular therapeutics since MSCs can be used as targeted drug delivery vehicles (detailed review in ref. [9]). For instance, MSCs could be transduced with a transgene expressing vascular endothelial cell growth factor (VEGF) to stimulate revascularization in ischemic heart and peripheral limb tissue [34]. Previous direct injection of VEGF protein and gene therapy vectors carrying VEGF showed promise in Phase I–II clinical trials but did not achieve significance in Phase III trials [34]. MSCs, however, migrate to ischemic areas, remain there for an extended period of time, as has been demonstrated in pre-clinical animal models [9, 35], and could continuously deliver VEGF. This could become a cellular therapy using highly tested allogeneic, transduced MSCs. These MSCs could be generated from iPSCs that were created in an integration-free system and transduced with a VEGF vector; these could be selected for safe harbor integrations of the transgene to exclude the possibility of tumor formation due to insertional mutagenesis. Other diseases such as Huntington’s disease or other neurodegenerative disorders, could also be targeted with this type of approach, using highly characterized iPSC-derived MSC batches engineered to produce neurotrophic factors [15].
REGULATORY ISSUES FOR FUTURE SAFE THERAPIES FROM HUMAN PSCs
The US Food and Drug Administration (FDA) regulates the clinical application of cell and gene therapy. The final cellular product administered into a patient must meet important safety and efficacy criteria, such as identity, purity, potency and clinical safety, and efficacy [36]. Besides criteria that all cellular products must meet, such as sterility, viability and freedom from endotoxin, particular concerns for stem cells are (1) characterization of the product, including in vitro and in vivo potency, (2) freedom from cell differentiation to undesired cell types, (3) in vivo cell migration/trafficking to non-target site(s), (4) potential uncontrolled cell proliferation or tumorigenicity, (5) immunogenicity, (6) graft-versus-host effects, (7) interactions with devices, other tissues or drugs in vivo, and (8) for gene-modified cells, potential uncontrolled biological activity of the transgene, alteration of expression of the non-transgenes, and insertional mutagenesis. In this section, we discuss individual areas of consideration for iPSC-derived cellular products in the path to the clinic.
Development of Clinically Relevant iPSCs and Their Derivatives
At this point, clinically applicable iPSC do not yet exist, but are under development. For iPSC-based therapies, several categories must be considered to meet regulatory requirements. One of the most important goals for the manufacturing of a safe stem cell product is the prevention of tumor formation after transplantation. Tumors could be generated in iPSC-mediated clinical applications by insertional mutagenesis caused by transgenes used for reprogramming [37], by enhancer effects caused by particular viral sequences found in retroviral or lentiviral vectors [28], and by disruptions of essential genes caused by integrated vector cassettes [20].
Teratomas could be caused by undifferentiated cells contaminating the differentiated final product. Integrated c-Myc delivered by a retroviral vector has been shown to cause tumor formation in 40% of mice due to the reactivation of silenced genes [37]. In adult stem cell therapies, genetically modified cells can carry the risk of tumor generation. An HSC gene therapy clinical trial to treat X-linked severe combined immunodeficiency disease (X-SCID) using a retrovirus caused 4 out of 11 children to develop leukemia [28, 38] and 1 out of 10 Wiskott–Aldrich syndrome gene therapy clinical trial patients developed an acute lymphocytic leukemia [39] due to transgene integrations in the proximity of the LMO2 proto-oncogene promoter. Numerous other stem cell gene therapy clinical trials using retro- or lentiviral vectors that were not carrying a growth factor receptor gene, however, have avoided this outcome [40]. Another concern may be cell transformation caused by gene disruption. An HSC therapy paper claimed that integrated lentiviral vector had disrupted a tumor-suppressor gene leading to premature termination of endogenous genes that could cause tumor formation [20]. This effect could be monitored in in vitro cell immortalization assays and by serial transplantation experiments in vivo [13, 41].
MSCs derived from iPSCs with safe harbor therapeutic gene integrations, or gene corrections by homologous recombination, could significantly reduce the chance of tumor formation as these cells can be screened to avoid gene disruptions or oncogene activation. iPSC colonies can be specifically selected for proper gene insertion, can be highly tested, and can then be expanded at large scale for master cell bank generation prior to directed differentiation to MSCs or other lineages. Gene-modified iPSC-derived MSCs could be used for safe administration of a therapeutic gene product to specific sites of injury or inflammation, as MSCs are known to migrate to such areas in vivo [9, 15, 42].
Improving reprogramming technology for safe iPSC derivation is important for human therapeutic applications, and permanent transgene integrations for reprogramming should be avoided. Recent papers have described many approaches to accomplish this, such as adenoviral vector transductions, DNA plasmid vector transfections, Cre-LoxP excision of reprogramming vector cassettes transferred by a lentiviral vector, transposons, episomal Epstein-Barr virus, mRNA transfections, and protein transfections [43]. All of these methods avoided transgene integration or persistence, and tumor formation in chimeric mice could not be observed (detailed review in Gustavo Mostosavsky paper in this issue). Additionally, small molecule-mediated reprogramming has become interesting for clinically relevant iPSC generation [44]. A small molecule approach could be simpler and may not be associated with the same side effects as an RNA approach. However, such approaches are currently rather inefficient in the generation of iPSCs and are under further development.
Epigenetic Memory and Genetic Aberrations
Another important concern for cellular therapies is whether the transplanted cells may become unstable or could be transformed into tumors. A number of studies have demonstrated that iPSCs contain abnormalities at the genetic and epigenetic level and that these defects are often related to oncogenic pathways [29, 45–47]. The epigenetic memory of iPSCs with its incomplete epigenetic reorganization and skewed differentiation potential also raises the question of whether such cells may actually be suitable for therapeutic applications (detailed review in Ren-He Xu paper, Juan Carlos Izpisua Belmonte paper, Hans Schoeler, and Jared Sterneckert paper in this issue). These issues will be addressed in iPSC derived cellular therapies currently under development.
Cell Culture Conditions
Even though iPSCs can be reprogrammed by integration-free methods, there are still a number of concerns to be addressed before any of these methods can be applied to generate a clinical grade cellular product. Current FDA regulations mandate the derivation and manufacture of cell and gene therapy products to be compliant with current Good Tissue Practice (cGTP) and Good Manufacturing Practice (cGMP) regulations, which include collecting, storing, and recovery of patient samples, derivation, culturing and differentiation of tissues, screening, testing, validating of products and procedures, packaging, labeling, and distribution of final products [36]. However, a Phase I clinical trial applying hESC-derived neuronal tissues for the treatment of spinal cord injury was recently approved by the FDA. The hESCs were not derived under GMP conditions and had been cultured on mouse feeder cells; however, they were highly tested for communicable xenogeneic diseases [48]. Nonetheless, it will be in the best interest of the laboratory manufacturing an iPSC- or hESC-derived cellular product to be in compliance with cGTP and cGMP regulations, otherwise the product will not be able to progress to Phase II or III clinical trials, and will have to be rederived. Additionally, to generate a safe and clinically acceptable iPSC-derived product, xeno-free cell culture conditions should be used to minimize the risk of transmitting disease or causing human immune reactions [36]. In the past, hPSCs have been derived and cultured using media containing animal-based serum replacements and a mouse embryonic fibroblast feeder layer. Martin et al. [49] found that both xenogeneic serum replacement and feeder cells are sources of nonhuman sialic acid Neu5Gc, which causes immunological reactions involving human antibodies. Therefore, human clinical applications of iPSCs should use cultures with either human feeder cells or a feeder-free system applying a chemically defined matrix.
Consistency of iPSC Derivation and Differentiation
Standard Operating Procedures (SOPs) are mandated in a GMP environment. These SOPs guarantee that a safe and efficacious cellular product will be manufactured in a reproducible manner. Although there are several clinically applicable reprogramming technologies, the consistency of iPSC-derived products is still a concern. SOPs cannot eliminate variations in cell reprogramming, expansion and differentiation efficiencies, but rather, will have to adapt to these properties inherent in iPSCs and iPSC-derived products. In all likelihood, well growing colonies and differentiated tissues will have to be selected under GMP conditions, and appropriate tests will have to be performed to assure their safety and efficacy in the planned human clinical application [29, 47, 50]. Single cell clonal expansion of human iPSCs have shown low survival rates compared to mouse counterparts [51]; therefore, developing reliable and reproducible standard protocols to differentiate and select iPSC-derived cellular products is a pressing issue.
There are indications that due to epigenetic memory or incomplete reprogramming, some iPSC lines favor specific differentiation pathways [52]. Differentiated cells can be thought of as a heterogeneous population of desired, differentiated cells mixed with undesired, undifferentiated cells, in spite of the application of efficient direct-differentiation methods [53]. To eliminate undifferentiated PSCs within the population of differentiated cells, several techniques have to be assessed. Cell sorting using a clinical grade flow cytometric cell sorter under GMP conditions can be one of the solutions. Introduction of a suicide gene only expressed in undifferentiated cells and antibodies directed against stem cell-specific surface markers could be used to selectively kill or capture and remove undifferentiated PSCs.
Safety and Efficacy
Safety of a cellular product remains the most important criterion for human applications. Therefore, toxicology studies must be performed on the proposed final product. Such studies must be evaluated in acute and chronic in vivo models and must encompass the examination of major organs, neighboring tissues, blood chemistry, and blood cell counts after the transplantation into the in vivo models [36].
Efficacy and functional consistency of an iPSC-derived cellular product is also important. Specific efficacy and potency tests need to be developed for each product. Such tests could be in vitro or in vivo tests, testing a specific function of the final product. The in vivo functionality and efficacy of the cellular product could be evaluated in a transplantation model and could be correlated with the in vitro assay. If the assays show consistent correlation, then the in vitro assay could later be used as a surrogate marker for the in vivo assay. The same model could possibly be applied to the measurement of cell surface markers associated with established efficacy in an in vivo model. In later phase clinical trials, the efficacy assay is mandated by the FDA [36].
Currently, it is not clear whether fully differentiated cells or progenitor cells would be more suitable for engraftment and functionality. Recently, a fetal neural stem cell transplantation paper raised the issue of tumorigenicity. Fetal neural stem cell injections into an ataxia telangiectasia patient caused tumor formation 4 years after transplantation. Tissue analysis confirmed that those tumors originated from donor tissues [54]. Fully differentiated cellular products have low potential to form tumors. However, they also have low engraftment efficiency and poor durability in most cases. Therefore, systematic in vivo studies applied to the state of differentiation of specific iPSC-derived cellular products should be conducted before human applications are considered.
There is no standard protocol prescribing the number of cells to be transplanted for maximum effectiveness. Any such number will have to be derived for the particular cellular product and the tissue to be treated. However, given the low engraftment and survival rates of transplanted cells, in the past, large numbers of cells have been transferred into patients. Delivery of cells to certain anatomic locations may require novel procedures or novel delivery devices, and care needs to be taken not to disrupt the local environment (detailed review in ref. [8]). If injected intravenously in very high numbers, cells run the risk of clogging blood vessels and potentially causing pulmonary emboli or infarctions. Therefore, an initial safe dose regimen and dose escalations, based on animal data, must be used with careful consideration of the route of administration and the dose schedule.
In early phase MSC preclinical and clinical trials, safety of transplanted MSC was well documented in animal models and in human trials, but in vivo efficacy was controversial in humans [1]. While some MSC trials have shown clear efficacy, others have not achieved significant outcomes [55–59]. Mixed results from early MSC clinical trials may be due the lack of fundamental MSC biology and low engraftment efficiency, which can lead to low therapeutic efficacy [7, 8]. Recently, several preclinical studies have shown enhanced efficacy of MSC therapies by overexpression of trophic factors, preconditioning with hypoxic environment, and surface antigen modifications [60, 61]. Among others, our laboratory demonstrated that hypoxic preconditioned MSCs prior to transplantation improves their tissue regenerative potential in mice with hindlimb ischemia [60]. The recent work by Sackstein and colleagues improved homing efficiency of BM-MSCs into the bone; they chemically modified the MSC surface antigen CD44 and intravenously injected such manipulated MSCs into nonobese diabetic/SCID mice. Transplanted MSCs were found to have homed into the bone and showed sustained survival as compared to unmanipulated MSCs [62]. Further long-term in vivo studies must be conducted to assure that such surface antigen modifications are safe and can increase the homing and therapeutic efficacy of transplanted MSCs, derived from both adult tissues and from pluripotent sources.
Conclusion
hPSCs have enormous potential for regenerative medicine. Adult stem cell (HSC)-based therapies have demonstrated safety and efficacy for several decades, and adult MSC therapies are showing efficacy in some trials, with mixed results such as only transient effects in others due to poor cellular retention or other factors that need to be further optimized. Creation of large batches of iPSC-derived MSCs would allow full in vivo testing in preclinical studies. Although iPSCs might offer great hope for stem cell therapies, there are important safety issues to be considered before these cells are suitable for clinical trials. However, the potential future ability to generate stem cell lines matched to a particular patient, and to perform homologous gene correction or targeted transgene insertion into a safe harbor site in the genome prior to further expansion and differentiation offers amazing potential for future regenerative medicine therapies and is the goal of major research efforts worldwide.
Acknowledgments
We thank members of the Nolta lab for critical reading of the manuscript and the California Institute for Regenerative Medicine (CIRM), National Institutes of Health (NIH), and philanthropic donors for supporting our research. We apologize to our colleagues whose work could not be cited due to the space limitation. Y.J. and J.A.N. are supported by CIRM grants TR1-01257 and TR2-01787 and NIH grants 5RC1AG036022 and RO1 HL073256. Y.J. is a former scholar of the Howard Hughes Medical Institute med into grad initiative. G.B. is performing GMP-level iPSC derivation funded by CIRM Prime Award No. DRI-Q14S4, Stanford University Subaward No. 25628820-46710 to the University of California Davis GMP facility.
Footnotes
Author contribution: Y.J., G.B., and J.A.N.: conception and design and manuscript writing.
Disclosure of potential conflicts of interest is found at the end of this article.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED, Ashley CA, Lochte HL, Jr, et al. Homografts of bone marrow in dogs after lethal total-body radiation. Blood. 1959;14:720–736. [PubMed] [Google Scholar]
- 5.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Meyerrose T, Olson S, Pontow S, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 13.Bauer G, Dao MA, Case SS, et al. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308–1315. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripa RS, Haack-Sorensen M, Wang Y, et al. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the stem cells in myocardial infarction (STEMMI) trial. Circulation. 2007;116(11 suppl):I24–I30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 15.Joyce N, Annett G, Wirthlin L, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 17.Brouard N, Chapel A, Thierry D, et al. Transplantation of gene-modified human bone marrow stromal cells into mouse-human bone chimeras. J Hematother Stem Cell Res. 2000;9:175–181. doi: 10.1089/152581600319388. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Lu S, Batchu R, et al. Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther. 1999;6:1611–1616. doi: 10.1038/sj.gt.3300973. [DOI] [PubMed] [Google Scholar]
- 19.Mosca JD, Hendricks JK, Buyaner D, et al. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000;379(Suppl):S71–S90. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 20.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 24.Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambal A, Mitchell G, Cary W, et al. Generation of HIV-1 resistant and functional macrophages from hematopoietic stem cell-derived induced pluripotent stem cells. Mol Ther. 2011;19:584–593. doi: 10.1038/mt.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 28.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science (New York, NY) 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 29.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsara O, Mahaira LG, Iliopoulou EG, et al. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2011;20:1549–1561. doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 31.Hematti P. Human embryonic stem cell-derived mesenchymal progenitors: An overview. Methods Mol Biol (Clifton, NJ) 2011;690:163–174. doi: 10.1007/978-1-60761-962-8_11. [DOI] [PubMed] [Google Scholar]
- 32.Gruenloh W, Kambal A, Sondergaard C, et al. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2011;17:1517–1525. doi: 10.1089/ten.tea.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 34.Fierro F, Kalomoiris S, Sondergaard C, et al. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011 doi: 10.1002/stem.720. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyerrose TE, Roberts M, Ohlemiller KK, et al. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 38.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boztug K, Schmidt M, Schwarzer A, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 41.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 42.Gruenloh W, Kambal A, Sondergaard C, et al. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2011;17:1517–1525. doi: 10.1089/ten.tea.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: Reprogramming a la carte. Nat Rev Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 44.Zhu S, Li W, Zhou H, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 46.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Strauss S. Geron trial resumes, but standards for stem cell trials remain elusive. Nat Biotechnol. 2010;28:989–990. doi: 10.1038/nbt1010-989. [DOI] [PubMed] [Google Scholar]
- 49.Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 50.Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen DE, Melton D. Turning straw into gold: Directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- 54.Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menasche P. Cardiac cell therapy: Lessons from clinical trials. J Mol Cell Cardiol. 2011;50:258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Si YL, Zhao YL, Hao HJ, et al. MSCs: Biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10:93–103. doi: 10.1016/j.arr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Tolar J, Villeneuve P, Keating A. Mesenchymal stromal cells for graft-versus-host disease. Hum Gene Ther. 2011;22:257–262. doi: 10.1089/hum.2011.1104. [DOI] [PubMed] [Google Scholar]
- 59.Tolar J, Le Blanc K, Keating A, et al. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1555. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner J, Kean T, Young R, et al. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]