SUMMARY
The severity and mortality rates of acute pancreatitis (AP) are significantly elevated in the elderly population. However, due to a lack of appropriate animal models, the underlying mechanisms for this age-dependent vulnerability remain largely unknown. The purpose of this study was to characterize a murine model of AP, which displays age-associated severity, and to use this model to identify pathophysiologies that are distinctive of the aged with AP. AP was induced in young (4–5 months), middle-aged (12–13 months), and aged (23–25 months) C57BL/6 mice by repeated injection of caerulein, a homologue of the gastrointestinal hormone cholecystokinin. Approximately 10% of aged mice died during AP while young and middle-aged mice showed no mortality. Although both young and aged mice exhibited early signs of edema and inflammation in the pancreas, kidney, and lung, young mice showed signs of recovery within 24 h while aged mice exhibited increasingly severe tissue damage and cell death. There was a significant age-dependent increase in pancreatic neutrophil activation and systemic inflammation as assessed by pancreatic myeloperoxidase (MPO) and plasma interleukin-6 (IL-6) concentration, respectively. Importantly, aged but not young mice with AP showed significantly elevated thrombosis in the lung and kidney as well as a marked increase in plasma concentration of plasminogen activator inhibitor-1 (PAI-1), a primary inhibitor of the fibrinolytic system. These results demonstrate that aging is associated with increased severity of AP characterized by augmented and prolonged pancreatic inflammation and the presence of multiple extra-pancreatic sequelae including thrombosis.
Keywords: Aging, Acute Pancreatitis, Systemic Inflammation, Multiple Organ Dysfunction Syndrome, Coagulation
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory disease of the pancreas which is usually accompanied by severe abdominal pain, nausea, and vomiting. While the most frequent causes of AP are gallstones and alcoholism, other factors such as infection, autoimmune response, and hyperlipidemia account for some cases (Tonsi et al. 2009). AP is one of the most common gastrointestinal diseases requiring hospitalization; in the US, over 200,000 patients are admitted to hospitals with AP each year (Russo et al. 2004). In addition, the incidence of AP is markedly increasing worldwide according to studies in England (Goldacre & Roberts 2004), the Netherlands (Eland et al. 2000), Sweden (Lindkvist et al. 2004), and the US (Frey et al. 2006). Although AP is mild and self-limiting in most patients, up to 20% develop severe AP (Banks & Freeman 2006; Frossard et al. 2008) with manifestations of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS). The overall mortality of AP patients is 5%, increasing to between 10 and 25% in patients with severe AP (Tonsi et al. 2009).
AP is particularly life-threatening for elderly people as its incidence and mortality rate increase progressively with advancing age (Andersson et al. 2006; Ellis et al. 2009). The mean age of the first AP attack is in the 6th decade of life (Tonsi et al. 2009). A recent clinical study in China reported that MODS, the main cause of AP-related death (Raraty et al. 2004), is more common in the elderly, and elderly patients (>60 years) with severe AP have significantly higher mortality rates than younger patients (17.0% vs. 5.3%) (Xin et al. 2008). Another recent study in England reported similar findings; while the overall case mortality rate was 5.0%, the mortality rate in young (<40 years) and old (> 80 years) patients was <1% and 12.9%, respectively (Ellis et al. 2009). AP-associated mortality rates are expected to increase with the growing elderly population. Despite recent recognition of this problem, few studies have attempted to understand the mechanisms contributing to the age-associated severity of AP or causes of increased mortality in the aged.
It is widely accepted that unresolved local pancreatic inflammation during the early phase of AP induces the systemic inflammatory response syndrome (SIRS) (Mofidi et al. 2006). SIRS, characterized by a loss of homeostasis, results in progressive whole body inflammation and uncontrolled coagulation which causes disseminated intravascular coagulation (DIC). DIC is a dangerous late complication of SIRS which results in tissue ischemia and MODS, and portends an extremely poor prognosis (Levi et al. 1999). MODS is commonly observed in elderly patients with severe AP (Xin et al. 2008). However, due to a lack of experimental studies with animal models, it remains largely unknown whether increased inflammation, coagulation, and MODS are involved in the aged-associated pathophysiology of AP. In order to reduce morbidity and mortality associated with AP in the elderly, it is important to understand the factors contributing to different pathophysiologies between the young and aged.
In the present study, we demonstrated age-associated pathophysiology including increased mortality, pancreatic inflammation, pancreatic acinar cell death, and systemic inflammation in a murine model of AP. Furthermore, we found that aged mice with AP exhibited increased thrombosis and tissue damage in multiple extra-pancreatic organs.
RESULTS
Age-associated mortality during AP
We first performed a survival study to determine whether mortality from AP increases with age. Survival rates of young (4–5 month-old, n=10), middle-aged (12–13 month-old, n=10), and aged (23–25 month-old, n=10) mice were monitored for 2 weeks after induction of AP by injection with caerulein (50µg/kg, 9 times (9x), i.p.). As presented in Table 1, aged mice showed 10% (1 of 10 mice) mortality while no mortality was seen in young or middle-aged mice; death in the aged occurred within 24 h after the final caerulein injection. To examine the difference in the severity of pancreatic tissue damage, we performed several additional AP experiments (results shown in subsequent figures) in which young, middle-aged, and aged mice were sacrificed 24 h after caerulein injection (9x) for biochemical and histological analyses. Similar to in the survival test, no young or middle-aged mice died in these experiments (within the 24 h time period monitored); however, 7 of 76 (9.2%) aged mice died within 24 h. Some of the aged mice which were sacrificed at the 24 h time point appeared to be distressed, leaving the possibility that a few of them might have died later had they not been sacrificed; in contrast, all young and middle-aged mice showed signs of recovery judged by their activity level, giving the impression that none would have died later had they not been sacrificed at this time point. Taken together, these results (survival test and subsequent experiments) demonstrate that aged mice exhibit a 9.3% increase in mortality during caerulein-induced AP compared to young and middle-aged mice which showed no mortality; the difference in mortality rates between the aged group and the younger groups is statistically significant (p=0.009).
Table 1.
Age-associated mortality during acute pancreatitis
|
Young (4–5 months old) Dead/Total |
Middle-aged (12–13 months old) Dead/Total |
Aged (23–25 months old) Dead/Total |
|
|---|---|---|---|
| Survival test1 | 0/10 | 0/10 | 1/10 |
| 24 h time point study2 | 0/39 | 0/10 | 7/76 |
| Combined | 0/49 (0%) |
0/20 (0%) |
8/86## (9.3%) |
For survival test, all mice were monitored for 2 weeks; one aged mouse died within 24 hours.
For 24 h time point study, all mice were sacrificed for biochemical analysis 24 h after the final injection with caerulein, and mortality during this period of time was recorded.
p<0.01 comparing aged vs. young and middle-aged mice.
Plasma analysis demonstrating age-associated changes in the severity of AP
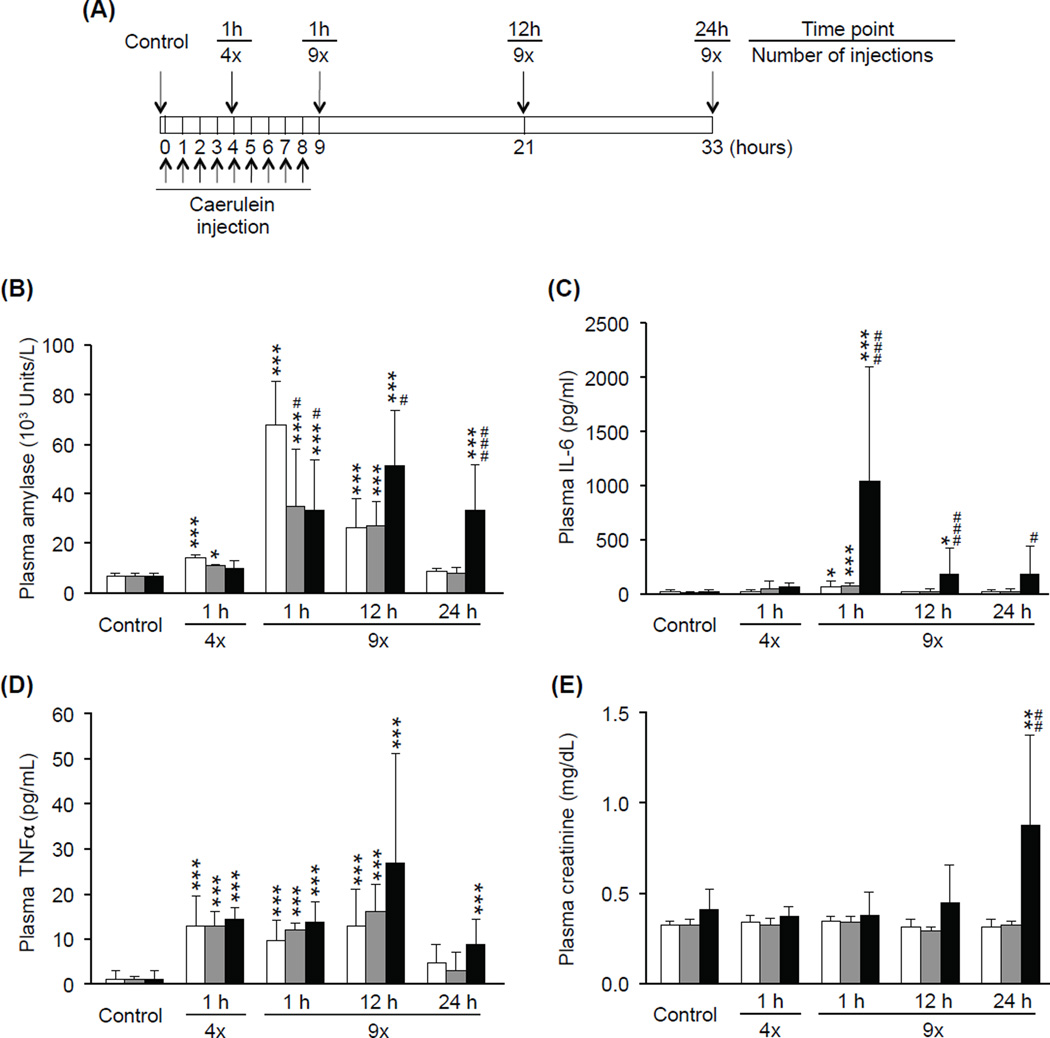
To examine whether the severity of AP increases with age, plasma concentrations of several AP biomarkers were analyzed at multiple time points during the course of AP. Figure 1A depicts a timeline of our model of caerulein-induced AP indicating time points of caerulein injection and sacrifice. Mice were sacrificed either without treatment (Control), 1 h after the 4th caerulein injection, or 1, 12, or 24 h after the final (9th) caerulein injection. Plasma amylase activity is a commonly used marker of pancreatic inflammation (Singh et al. 2007) and used as a standard clinical test for AP diagnosis (Matull et al. 2006). The activity of plasma amylase was significantly elevated in young and middle-aged mice 1 h after the 4th caerulein injection and 1 and 12 h after the 9th caerulein injection. In aged mice, plasma amylase activity did not increase early on, but was significantly elevated 1, 12, and 24 h after the 9th caerulein injection. At the early time points (1 h after 4th injection and 1 h after 9th injection), amylase activity in young mice was higher compared to older groups. At the later time points (12 and 24 h), however, amylase activity in aged mice became significantly higher than both young and middle-aged mice (12 h p=0.049, 0.030; 24 h p<0.001, 0.001) (Fig. 1B). To examine the severity of systemic inflammation during AP, plasma concentration of IL-6, a marker of the severity of AP and SIRS (Saito et al. 2003; Aoun et al. 2009), and TNFα, another major pro-inflammatory cytokine (Malleo et al. 2007), were measured. Plasma IL-6 concentration was significantly increased in young (p=0.011), middle-aged (p<0.001), and aged (p<0.001) mice 1 h after the final (9th) caerulein injection; aged mice showed significantly higher plasma IL-6 concentrations than young and middle-aged mice at this time point (p< 0.001). While plasma IL-6 concentration in young and middle-aged mice was reduced to control levels by 12 h after the final (9th) caerulein injection, IL-6 concentration in aged mice remained high (12h p<0.001, p=0.002; 24h p=0.039, 0.014) (Fig. 1C). Plasma TNFα concentrations were significantly increased in all age groups after caerulein injection, but no significant differences among the age groups were observed (Fig. 1D). Plasma creatinine concentration, a widely used clinical parameter for renal dysfunction (Biczo et al. 2010), was also compared in young, middle-aged, and aged mice after caerulein injection. Plasma creatinine concentration was elevated only in aged mice at the 24 h time point (p=0.008) (Fig. 1E), indicating that acute renal dysfunction during the late stage of AP occurs in an age-dependent manner. Since young and middle-aged mice showed similar responses to AP with respect to mortality and plasma protein analysis, only young and aged mice were used for the remaining experiments.
Figure 1. Plasma analysis demonstrating age-associated changes in the severity of AP.
As shown in the diagram of experimental acute pancreatitis model (A), mice were sacrificed 1h after the 4th injection (4x), or 1, 12, or 24 h after the 9th injection (9x) of caerulein. Control mice were sacrificed without injection. Plasma samples were obtained from these mice and used for analyses of amylase (B), IL-6 (C), TNFα (D) and creatinine (E). White, grey, and black bars indicates young, middle-aged, and aged mice, respectively. Data are mean ± SD; *** p<0.001, ** p<0.01, * p<0.05 compared to control of the same age group; ### p<0.001, ## p<0.01, # p<0.05 compared to the young group, n=5–10 in each group.
Age-associated increase in pancreatic tissue damage during AP
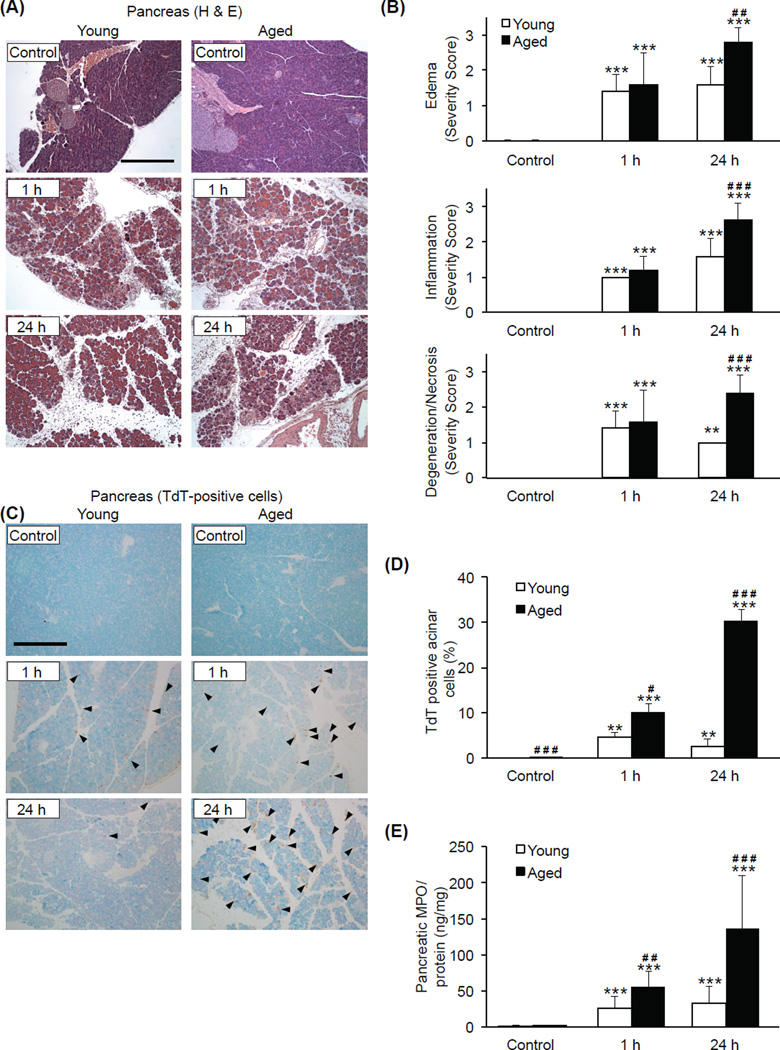
To examine pancreatic tissue damage during AP, histological analysis and scoring was performed on H&E-stained pancreas tissue sections. Tissue sections (n=5 at each time point for each age group) were evaluated microscopically and scored for the severity of edema, inflammation, and degeneration/necrosis. Severe edema, inflammatory cell infiltration, and degenerated/necrotic cells were apparent in the tissue sections from both young and aged mice 1 h after the final (9th) caerulein injection. Histopathological scoring of tissue damage, as noted by edema, inflammation, and degeneration/necrosis, became more severe in aged mice at the 24 h time point (p=0.001, p<0.001, p<0.001) (Fig. 2A and 2B). Pancreatic acinar cell apoptosis, measured by TdT-based analysis of tissue sections, was more pronounced in aged mice compared to young at both 1 and 24 h after the final caerulein injection. In the aged, 9.96 ± 1.96 % and 30.23 ± 2.53 % of cells per field of view showed signs of death at 1 and 24 h, respectively (Fig. 2C and 2D), while signs of cell death were less than 5% in young mice at both time points. Pancreatic myeloperoxidase (MPO) levels, an indicator of neutrophil sequestration (Song et al. 2002), were significantly elevated during AP in both young and aged mice with the levels being 2-fold higher at 1 h (p=0.010) and 4-fold higher at 24 h (p<0.001) in the pancreas of the aged (Fig. 2E). Taken together, these data indicate that the pancreas of aged mice exhibited more severe local inflammatory changes, increased neutrophil sequestration, and abundant cell death compared to that of young mice.
Figure 2. Age-associated increase in pancreatic tissue damage during AP.
Pancreatic tissue sections were obtained from young and aged mice sacrificed 1 or 24 h after the final (9th) caerulein injection. (A) Representative H&E-stained sections (original magnification, 100x, scale bar indicates 200µm). (B) Results of histological scoring. (C) Apoptotic cells indicated by immunohistochemical detection of TdT. Arrowheads represent TdT-positive cells (original magnification, 100x, scale bar indicates 200µm). (D) Acinar cell death expressed as a percentage of total acinar cell mass. (E) Pancreatic MPO levels. *** p<0.001, ** p<0.01 vs. control of the same age group; ### p<0.001, ## p<0.01, # p<0.05 vs. young mice at the same time point. n=5 in each group.
Age-associated severe tissue damage in extra-pancreatic organs during AP
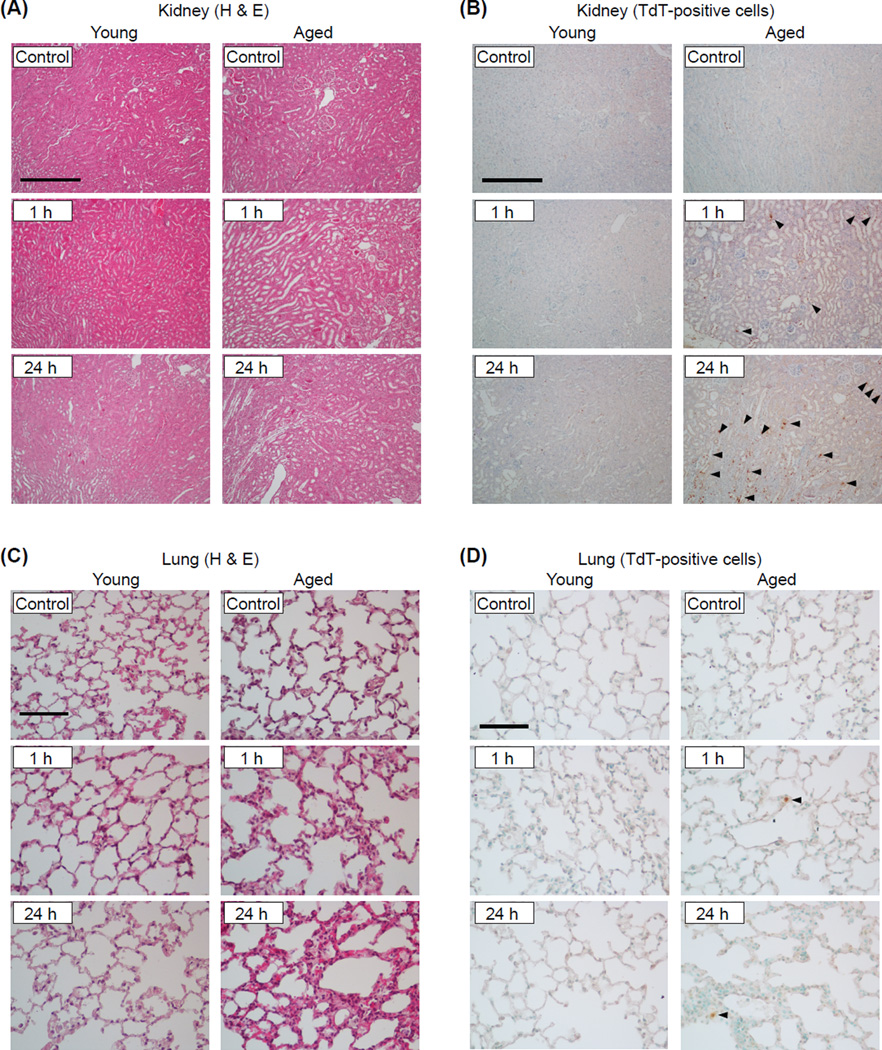
To assess age-associated changes in extra-pancreatic tissue damage during AP, histological analysis of kidney and lung tissue sections was performed. As shown in Figure 3A, the kidney of both young and aged mice exhibited inflammation-related histological changes at 1 h during AP such as tubular dilation and flattening, and loss of brush border. While such changes appear to be resolved by 24 h in the young, kidney pathophysiology further deteriorated in the aged. Renal apoptotic cell death was observed only in aged mice at the 1 and 24 h time points (Fig. 3B). Histological analysis of the lung from aged mice showed interstitial edema, infiltration of inflammatory cells, and thickened alveolar walls 1 h after the final caerulein injection which became more severe by the 24 h time point. The lung from young mice showed minimal inflammatory changes (Fig. 3C). Compared to the kidney, apoptotic cell death was less prominent in the lung during AP although it was clearly specific to aged mice (Fig. 3D). Taken together, these results clearly demonstrate that extra-pancreatic tissue injury during AP is more severe in the aged.
Figure 3. Age-associated severe tissue damage in the kidney and lung during AP.
Kidney and lung tissue sections were obtained from young and aged mice sacrificed 1 or 24 h after the final (9th) caerulein injection. The sections were either stained with H&E or processed for immunohistochemical detection of TdT-positive apoptotic cells. Representative H&E-stained kidney sections (A) and TdT-positive cells (indicated by arrowheads) in the kidney (B) (original magnification, 100x, scale bar indicates 200µm). Representative H&E-stained lung sections (C) and TdT-positive (indicated by arrowheads) in the lungs (D) (original magnification, 400x, scale bar indicates 50µm). n=5 in each group.
Age-dependent coagulation during AP
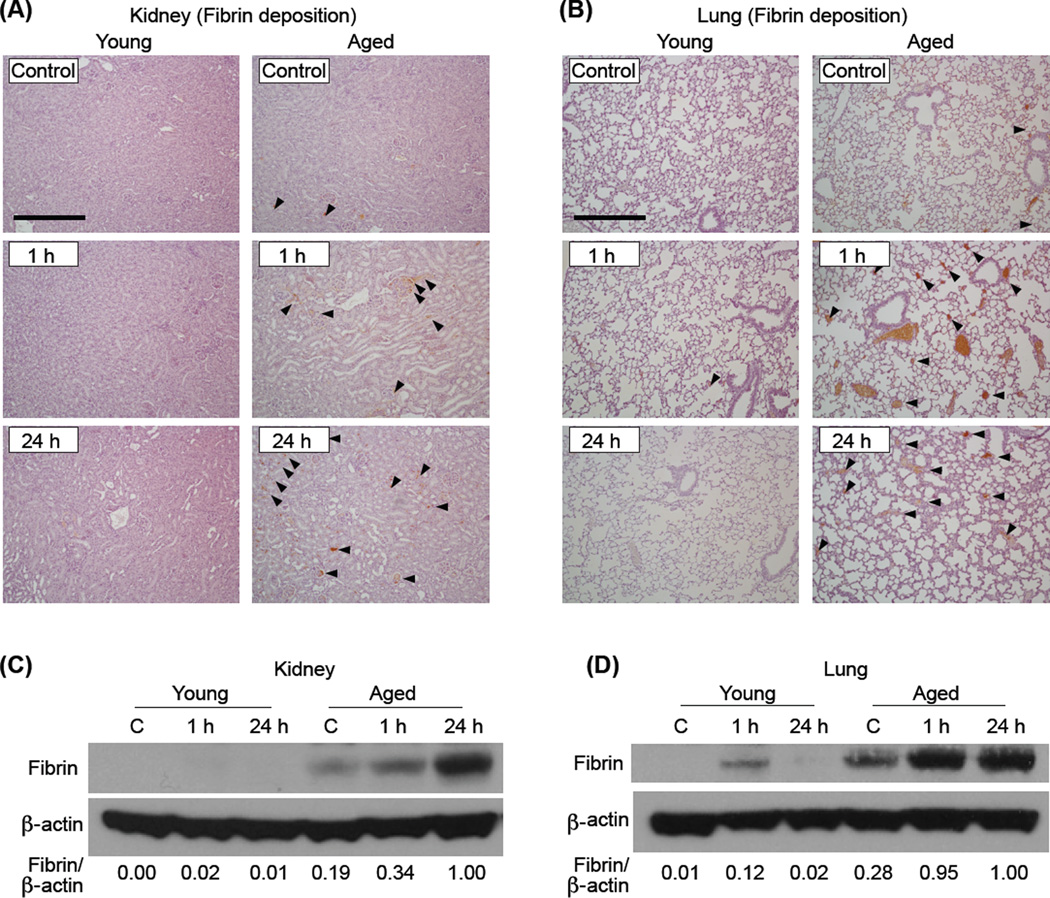
Fibrin formation in mouse tissues was assessed to examine age-associated changes in microvascular coagulation during AP. Age-dependent fibrin formation during AP was assessed by immunohistochemical analysis using a fibrin-specific monoclonal antibody that does not react with fibrinogen, a circulating precursor of fibrin (Fig. 4A and B). Aged, but not young, mice showed significant AP-induced fibrin deposition within the microvasculature of the kidney and lung, a characteristic feature of DIC which occurs in the late stage of severe systemic inflammation. To confirm AP-induced fibrin formation, Western blot analysis was performed on total protein that was extracted from the kidney and lung of young and aged mice sacrificed 1 or 24 h after the final (9th) caerulein injection. While fibrin formation in the kidney of young mice during AP was barely detected, fibrin levels in the aged clearly increased after caerulein injection (Fig. 4C). In the lung, fibrin formation during AP was more dramatic in the aged compared to young at 1 and 24 h after the final caerulein injection (Fig. 4D). Minimal amounts of fibrin formation were detected in the lungs from non-treated aged mice. Fibrin formation in the pancreas of all mice was insignificant and below our detection limit (data not shown).
Figure 4. Fibrin formation in the kidney and lung was augmented in the aged after caerulein induced AP.
Immunohistochemical analysis of fibrin in the kidney (A) and lung (B) harvested from mice 1 or 24 h after the last caerulein injection. Arrowheads indicate fibrin positive areas (original magnification, 100x, scale bar indicates 200µm). Images are representative of 5 mice per group. Western blot analysis assessing fibrin formation in the kidney (C) and lung (D) of young and aged mice that were sacrificed 1 or 24 h after the final (9th) caerulein injection. n=4–8 in each group. Relative intensity of fibrin/β-actin in each sample, calculated after densitometric analysis, is shown under each lane.
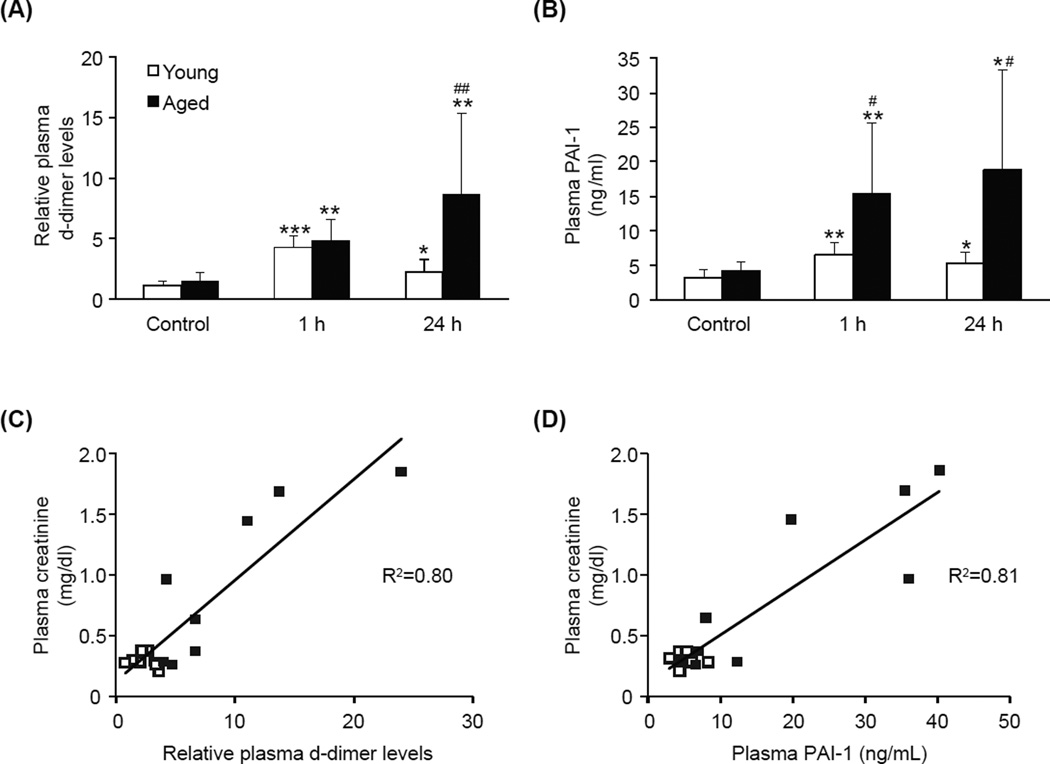
Age-associated increase in plasma d-dimer and PAI-1 levels during AP
To further examine the age-dependent increase in coagulation during AP, we assessed plasma levels of d-dimer, a fibrin degradation product and widely used diagnostic marker of DIC. D-dimer levels were equally elevated in both young and aged mice 1 h after the final caerulein injection (Fig. 5A). While d-dimer levels in young mice decreased to normal levels by 24 h, the levels in aged mice became further elevated, thus, d-dimer levels in aged mice were significantly higher than those in young mice at this time point (p=0.012). We also measured plasma concentration of plasminogen activator inhibitor-1 (PAI-1), a major pro-coagulant factor which functions to inhibit fibrinolysis. Although PAI-1 concentrations were significantly increased in both young and aged mice after caerulein injection, the average concentration was 4-fold higher in the aged (1 h p=0.014; 24 h p=0.003) (Fig. 5B). These results suggest that age-dependent DIC during AP is at least partly caused by augmented PAI-1 production in the aged. Plotting analysis revealed a strong positive correlation between plasma d-dimer levels and creatinine concentration (R2=0.80, Fig 5C); and plasma PAI-1 and creatinine concentration (R2=0.81, Fig 5D), suggesting that enhanced thrombosis in the aged is causally linked to renal dysfunction.
Figure 5. Age-associated increase in plasma d-dimer and PAI-1 levels.
Plasma levels of d-dimer (A) and PAI-1 (B) were analyzed from young and aged mice that were sacrificed either without treatment (Control), 1 or 24 h after the final (9th) caerulein injection. Plasma creatinine concentration plotted with either plasma d-dimer levels (C) or plasma PAI-1 concentration (D) of mice sacrificed at the 24 h time point. Open and closed squares indicate young and aged mice, respectively. Plasma d-dimer levels are relative to the average level of young control. Data are mean ± SD; * p<0.05, ** p<0.01, and *** p<0.001, compared to control of the same age group; # p<0.05 compared to the young group, n=4–9 in each group.
DISCUSSION
The present study describes, for the first time, age-associated severity of acute pancreatitis (AP) in an animal model; these newly defined characteristics of murine AP are similar to the human clinical condition. In this model, aged mice exhibit increased mortality rates with severe systemic inflammation and multiple extra-pancreatic organ injuries. Previous studies by a large number of investigators agree that young mice with caerulein-induced AP exhibit relatively mild symptoms without mortality (Su et al. 2006; Hartwig et al. 2007). Our current study is the first to demonstrate that caerulein-induced AP causes severe and sustained symptoms in aged C57BL/6 mice (23 to 25-months old) with a 9 to10 % mortality rate within 24 h. Young and middle-aged mice exhibited a 100% survival rate in all experiments. Aged (23–25 months old) C57BL/6 mice are equivalent to humans in the 6th – 7th decade of life, and young (4–5 months old) and middle-aged (12–14 months old) mice are equivalent to humans in the 3rd and 4th decade of life, respectively (Flurkey et al. 2007). The mortality rate of aged mice in this AP model mimics the clinical condition as described by a recent study in England which found mortality rates of < 1%, 5.9%, 9.2%, and 12.9% in AP patients at the ages of < 40, 60–69, 70–79, and >80 years, respectively (Ellis et al. 2009).
Other investigators have previously attempted to develop animal models that reflect clinical characteristics of the aged with AP (Miyasaka et al. 1992; Kimura et al. 1996); however these studies were largely unsuccessful mainly due to suboptimal choices for AP induction and time points for analyses. The first study (Miyasaka et al. 1992), using young and aged rats with caerulein-induced AP, found no age-associated changes in plasma amylase concentration, pancreatic histology or tissue weight, and macroscopic assessment of multiple organs. The failure of finding age-associated changes in this study can be explained by the low amount of caerulein injected (40µg/kg, twice), the choice of time points (6 h after the second injection), and the limited types of analyses performed. Our current study examined multiple time points including 1 h and 24 h after 9 injections of caerulein (50µg/kg). In fact, had we only studied the 1 h time point after the 9th injection, we would not have found age-associated increases in plasma amylase activity or histological tissue damage as these parameters did not show age-associated increases until the 24 h time point (see Fig 1B and Fig 2B). Miyasaka et al. (Miyasaka et al. 1992) utilized a second model of AP in the same study in which deoxycholic acid was injected, via the bile duct, into the pancreatic main duct in young and aged rats; this experiment also did not show any significant evidence that susceptibility to pancreatitis increases with age. The authors stated that their failure could be explained by an age-associated increase in the ductal diameter which allowed infused deoxycholic acid to quickly leak out from the pancreatic duct of aged animals. The second study attempting to characterize the effects of aging on the severity of AP used a different approach by infusing taurocholate into the pancreas followed by duct ligation to prevent leakage. This study also failed to find any histological evidence that the severity of AP increases with age, again likely because the time points (6 h after infusion) measured were too early in the course of AP. This study reported an age-associated increase in plasma amylase activity which is consistent with our results that plasma amylase activity was initially high in young but rapidly reduced to normal levels whereas the levels remained high in the aged animals during later time points.
Plasma amylase activity has been considered a good marker to assess the severity of pancreatitis in animal models (Bhatia et al. 2005); however, clinical studies have not been able to correlate the degree of hyperamylasemia with the severity or duration of AP (Matull et al. 2006). Although plasma amylase activity may be a good indicator of the “presence” of AP, the data presented in our study as well as the results of others (Zyromski et al. 2008) discourage using it as a marker of the “severity” of AP. Plasma IL-6 concentration has also been commonly used as a prognostic marker to assess the severity of pancreatitis (Aoun et al. 2009) or other inflammatory conditions (Kelly & Cross 1992). Our results support the use of IL-6 as an indicator of the severity of AP as aged mice exhibited significantly higher concentrations of IL-6 compared to young and middle-aged mice. Though TNFα plays a pivotal role in the early phase of severe AP, the half-life of this cytokine is very short, and thus, its plasma concentration is less useful as biomarkers of early events than downstream cytokines (Papachristou 2008). TNFα induction often precedes that of IL-6 and is frequently thought to be the major inducer of IL-6. Our data indicates that TNFα concentrations are not increased in an age-associated manner during AP which argue against the possibility that increases in this cytokine are the direct cause of elevated plasma IL-6 concentrations in aged animals.
Activation of the hemostatic system and coagulation abnormalities have also been reported in clinical cases of AP (Salomone et al. 2003; Maeda et al. 2006) and are related to its severity (Ranson et al. 1977; Maeda et al. 2006). We previously demonstrated that aged mice are significantly more prone to endotoxemia-induced coagulation than young mice and that strong fibrin formation seen only in aged mice is not the result of increased severity of the disease but rather an age-dependent phenomenon which led to increased susceptibility (Starr et al. 2010). In the current model of caerulein-induced AP, fibrin deposition and increased levels of circulating d-dimer (fibrin degradation product) and PAI-1 (inhibitor of fibrinolysis) were higher in the aged compared to young. Furthermore, plasma d-dimer levels were strongly correlated to plasma concentrations of creatinine, suggesting that enhanced thrombosis in the aged is causally linked to renal dysfunction. It was recently reported that plasma d-dimer concentrations of AP patients on admission accurately identifies patients who will later develop organ failure during the course of their disease (Radenkovic et al. 2009). During AP, young mice showed significantly increased d-dimer and little fibrin formation, indicating that a sufficient level of fibrinolysis prevented accumulation of fibrin. On the other hand, aged mice showed both significantly elevated d-dimer levels and fibrin formation, indicating that, although strong fibrinolysis occurred, the fibrinolytic function was not sufficient to prevent accumulation of fibrin. Age-associated increases in PAI-1 support this premise.
An important remaining question is whether the age-associated severity of AP is caused by initial pancreatic inflammation or subsequent systemic inflammation with manifestations of coagulation and MODS. During the early phase of caerulein-induced AP (1 h after the 9th injection), pancreatic MPO levels and cell death were modestly but significantly higher in aged compared to young mice, indicating the presence of an age-associated increase in initial local pancreatic inflammation which may be the main trigger of later augmented systemic inflammation and MODS in the aged. Normal physiological function of the exocrine pancreas is altered by aging (Khalil et al. 1985; Majumdar et al. 1997). Our previous studies on mice showed that aging causes significant loss of pancreatic acinar cell proliferation both in vivo, during tissue regeneration (Watanabe et al. 2005), and in vitro, upon stimulation with growth factors (Takahashi et al. 2012). These previous studies also indicate that decreased acinar cell proliferation is largely due to an age-dependent loss of phosphatidylinositol-3 kinase (PI3K) signaling in the pancreas; thus, it is possible that such age-associated changes in exocrine pancreatic signaling contribute to the increase in local pancreatic inflammation during the initial phase of AP. On the other hand, histological scoring showed significant age-associated increases in pancreatic tissue damage at 24 h when plasma IL-6 concentration also showed an age-associated increase, raising the possibility that increased pancreatic tissue damage in aged mice is secondary to age-associated augmentation of systemic inflammation. Our previous studies also indicate that, upon abdominal administration of equal amount of bacterial endotoxin, aged mice compared to young, show significantly augmented systemic inflammation in multiple organs including lungs, kidney, fat, and heart (Saito & Papaconstantinou 2001; Saito et al. 2003; Starr et al. 2009; Starr et al. 2011). This fact supports our speculation that aged mice would have exhibited more severe systemic inflammation than young mice even if the severity of initial local inflammation in the pancreas were equivalent in both age groups. Thus, the degree of systemic inflammation rather than the initial pancreatic insult may be a more important contributor to the progression and outcome of AP, particularly in the aged where cases of systemic inflammation tend to be more severe.
In conclusion, the present study describes an animal model that replicates clinical characteristics of AP in the aged. The severity of caerulein-induced AP is more pronounced in aged mice which exhibit higher mortality rates, increased markers of local pancreatic and systemic inflammation, elevated levels of circulating coagulation factors and signs of microvascular coagulation, and tissue damage within distant extra-pancreatic organs. These conditions closely resemble observed clinical characteristics of elderly patients with AP, making this newly described aged animal model an important tool for investigating the progression and causes of age-associated severity during AP.
EXPERIMENTAL PROCEDURES
Animals
Young (4–5 month-old), middle-aged (12–14 month-old) and aged (23–25 month-old) male C57BL/6 mice were obtained from a colony at the National Institute on Aging. Before experiments, all mice were maintained for at least 7 days in an environment under controlled temperature (21–23°C), humidity (30–70%), and lighting (14 hours light/10 hours dark) with free access to water and chow (Rodent Diet No. 2500, LabDiet, St. Louis, MO). Experimental acute pancreatitis (AP) was induced by a commonly used method using supramaximal concentrations (50 µg/kg) of caerulein (American Peptide Company, Sunnyvale, CA), a stable analogue of gastrointestinal peptide hormone cholecystokinin (CCK), given intraperitoneally (i.p.) at hourly intervals for up to 8 hours (up to 9 injections total) as previously described (Ethridge et al. 2002; Hashimoto et al. 2003). Control mice received no injection. At multiple time points including 1 h after the 4th injection and 1, 12, and 24 h after the final (9th) injection, mice were anesthetized by isoflurane (Butler Schein, Dublin, OH) inhalation (2–5% in air) and blood was collected from the inferior vena cava. For analysis of fibrin, mice were injected with heparin (50 units/mouse, i.p., APP Pharmaceuticals, Schaumburg, IL) 5 minutes prior to sacrifice and blood was collected with a heparin-coated syringe. For analysis of plasma coagulation factors, such as plasma d-dimer and plasminogen activator inhibitor-1 (PAI-1), heparin was not injected to mice, and blood was collected with 10% volume of 0.1 M sodium citrate. The collected blood was immediately centrifuged at 4°C to obtain plasma samples which were then stored at −80°C. For histological analysis, whole pancreas and kidney were dissected and fixed in 10% buffered formalin phosphate (Fisher Scientific, Fair Lawn, NJ) for 24 h. Lungs were slowly infused with 10% buffered formalin phosphate from the trachea to maintain tissue structure before being fixed for 24 h. For tissue protein analyses, the entire vasculature was perfused with physiological saline through the cardiac ventricles, for the purpose of eliminating circulating cells, and each tissue was harvested, flash frozen in liquid nitrogen, and stored at −80°C. All mice were examined for the presence of tumors; 10.4% of aged mice with evident signs of tumor were excluded from the study.
Blood protein analyses
Plasma amylase activity was measured using Phadebas Amylase Test kit (Magle Life Sciences, Cambridge, MA) according to the manufacturer’s protocol. Plasma creatinine concentration was measured by colorimetric method using QuantiChrom™ Creatinine assay kit (Bioassay systems, Hayward, CA). Plasma concentrations of TNFα, IL-6, d-dimer and PAI-1 were measured by enzyme-linked immunosorbent assay (ELISA) using kits purchased from R&D Systems (Minneapolis, MN), Thermo Scientific (Rockford, IL), Molecular Innovations (Novi, MI), and Stago (Asnières, France), respectively.
Myeloperoxidase (MPO) assay
Frozen tissue samples were pulverized in liquid nitrogen and homogenized in ice-cold lysis buffer containing 10 mM Tris (pH 7.4), 5 mM EDTA, 200 mM NaCl, 10% glycerol, 1 mM PMSF, and a protease inhibitor cocktail tablet (cOmplete ULTRA Tablets, Roche Diagnostics, Mannheim, Germany). The homogenates were purified by two rounds of centrifugation (1,500 × g for 15 minutes at 4°C) and the supernatant used for measuring MPO levels (MPO ELISA kit, Hycult Biotechnology, Uden, Netherlands). The levels of MPO were expressed as nanogram per milligram of tissue protein.
Histological analysis
The collected pancreas, kidney, and lung tissues, fixed in 10% buffered formalin phosphate for 24 hours, were stored in 70% ethanol and embedded in paraffin. Tissue sections with 5µm thickness were prepared on glass slides and stained with hematoxylin and eosin (H&E). The photomicrographs were taken using Nikon Eclipse E200 microscope and Nikon Digital Sight DS-U3/DSFi1 digital camera system with NIS Elements F3.2 Imaging Software. The degrees of edema, inflammation, and tissue degeneration/necrosis in the pancreas, were scored under the microscope on a scale of 0–3 (0 being normal and 3 being severe) by a certified pathologist (E.Y.L.) primarily using our previously described method (Ethridge et al. 2002). Apoptotic cell death was examined in pancreas, kidney, and lung tissue sections by detecting DNA fragments using terminal deoxynucleotidyl transferase (TdT) with ApopTag® Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA).
Immunohistochemical staining
Fibrin deposition was evaluated by immunohistochemical staining using anti-fibrin monoclonal antibody 59D8 (Runge et al. 1987). In brief, paraffin-embedded sections were deparaffinized and incubated with 0.3% H2O2 solution to remove any endogenous peroxidase activity. The sections were incubated with diluted (1:100) anti-fibrin monoclonal antibody 59D8 and washed. The sections were then incubated with DAKO EnVision+ reagent (Dako, Carpinteria, CA) and washed. The peroxidase substrate was added for stain development. The sections were counterstained with hematoxylin, dehydrated, and permanently mounted. The photomicrographs were taken using Nikon Eclipse E200 microscope and Nikon Digital Sight DS-U3/DSFi1 digital camera system with NIS Elements F3.2 Imaging Software.
Western blot analysis
Protein was extracted from individual frozen tissues, and Western blot analysis for fibrin was performed as previously described (Starr et al. 2010). Protein samples (n=5 per age and timepoint) were pooled prior to analysis and each membrane was reprobed for β-actin to assure equal protein (40µg) loading (Sigma-Aldrich, St. Louis, MO).
Statistical Analysis
Means and standard deviations were used to describe all continuous variables. Two-way analysis of variance (ANOVA) was originally performed to compare the differences between age and treatment groups. Due to heterogeneity observed in mice due to age, treatment groups were stratified by age and compared using one-way ANOVA. Significant outcomes were further compared by Fisher’s least significance difference (LSD) test, for all pair-wise comparisons. All continuous measurements (except cell death) were log-transformed to meet the model assumptions for statistical analysis and are reported back-transformed in their original units. For the mortality experiment, results were analyzed by Fisher’s Exact Test. P values less than 0.05 were considered statistically significant for all statistical tests. Data were analyzed using the Statistical Analysis Software, SAS version 9.2.
ACKNOWLEDGEMENTS
This work was supported by grants P01 DK035608, R01 AG 025908, R01 AG 039732, and R01 AG025273. We thank Ms. Hsin-Fang (Grace) Li and Ms. Qishan (Shelley) Wu for statistical analysis, and Dr. Jun Ienaga and Miss Mizuki Saito for technical assistance. We also thank Mrs. Donna Gilbreath for illustrative assistance and Dr. Hitoshi Takahashi for technical instruction. The 59D8 anti-fibrin monoclonal antibody was kindly provided by Dr. Charles T. Esmon at Oklahoma Medical Research Foundation.
Abbreviations
- AP
acute pancreatitis
- DIC
disseminated intravascular coagulation
- IL
interleukin
- MODS
multiple organ dysfunction syndrome
- PAI-1
plasminogen activator inhibitor-1
- SIRS
systemic inflammatory response syndrome
- TNF
tumor necrosis factor
Footnotes
AUTHOR CONTRIBUTIONS
The research was designed by H.S. and D.O., all experiments were performed by D.O., data analysis was performed by D.O. and M.E.S., pathological scoring was performed by E.Y.L., statistical analysis was performed by A.S., manuscript was written by D.O., M.E.S., B.M.E., and H.S. Funding for this research was obtained by B.M.E. and H.S.
Contributor Information
Daiki Okamura, Email: daiki.okamura@uky.edu.
Marlene E. Starr, Email: marlene.starr@uky.edu.
Eun Y. Lee, Email: eylee@email.uky.edu.
Arnold Stromberg, Email: astro11@email.uky.edu.
B. Mark Evers, Email: mark.evers@uky.edu.
REFERENCES
- Andersson B, Olin H, Eckerwall G, Andersson R. Severe acute pancreatitis--outcome following a primarily non-surgical regime. Pancreatology. 2006;6:536–541. doi: 10.1159/000096977. [DOI] [PubMed] [Google Scholar]
- Aoun E, Chen J, Reighard D, Gleeson FC, Whitcomb DC, Papachristou GI. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology. 2009;9:777–785. doi: 10.1159/000214191. [DOI] [PubMed] [Google Scholar]
- Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259–G1265. doi: 10.1152/ajpgi.00435.2004. [DOI] [PubMed] [Google Scholar]
- Biczo G, Hegyi P, Sinervirta R, Berczi S, Dosa S, Siska A, Ivanyi B, Venglovecz V, Takacs T, Alhonen L, Rakonczay Z., Jr Characterization of polyamine homeostasis in l-ornithine-induced acute pancreatitis in rats. Pancreas. 2010;39:1047–1056. doi: 10.1097/MPA.0b013e3181d3cdf0. [DOI] [PubMed] [Google Scholar]
- Eland IA, Sturkenboom MJ, Wilson JH, Stricker BH. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand J Gastroenterol. 2000;35:1110–1116. doi: 10.1080/003655200451261. [DOI] [PubMed] [Google Scholar]
- Ellis MP, French JJ, Charnley RM. Acute pancreatitis and the influence of socioeconomic deprivation. Br J Surg. 2009;96:74–80. doi: 10.1002/bjs.6414. [DOI] [PubMed] [Google Scholar]
- Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322. doi: 10.1053/gast.2002.35951. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Brandvain Y, Klebanov S, Austad SN, Miller RA, Yuan R, Harrison DE. PohnB6F1: a cross of wild and domestic mice that is a new model of extended female reproductive life span. J Gerontol A Biol Sci Med Sci. 2007;62:1187–1198. doi: 10.1093/gerona/62.11.1187. [DOI] [PubMed] [Google Scholar]
- Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas. 2006;33:336–344. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ. 2004;328:1466–1469. doi: 10.1136/bmj.328.7454.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig W, Kolvenbach M, Hackert T, Fortunato F, Schneider L, Buchler MW, Werner J. Enterokinase induces severe necrosis and rapid mortality in cerulein pancreatitis: characterization of a novel noninvasive rat model of necro-hemorrhagic pancreatitis. Surgery. 2007;142:327–336. doi: 10.1016/j.surg.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58–66. doi: 10.1097/00006676-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Kelly NM, Cross AS. Interleukin-6 is a better marker of lethality than tumor necrosis factor in endotoxin treated mice. FEMS Microbiol Immunol. 1992;4:317–322. doi: 10.1111/j.1574-6968.1992.tb05011.x. [DOI] [PubMed] [Google Scholar]
- Khalil T, Fujimura M, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Effect of aging on pancreatic secretion in rats. Am J Surg. 1985;149:120–125. doi: 10.1016/s0002-9610(85)80020-9. [DOI] [PubMed] [Google Scholar]
- Kimura W, Okubo K, Han I, Kanai S, Matsushita A, Muto T, Miyasaka K. Effects of pancreatic duct ligation and aging on acute taurocholate-induced pancreatitis. Experiments in the perfused pancreas in rats. Int J Pancreatol. 1996;19:117–127. doi: 10.1007/BF02805225. [DOI] [PubMed] [Google Scholar]
- Levi M, de Jonge E, van der Poll T, ten Cate H. Disseminated intravascular coagulation. Thromb Haemost. 1999;82:695–705. [PubMed] [Google Scholar]
- Lindkvist B, Appelros S, Manjer J, Borgstrom A. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol. 2004;2:831–837. doi: 10.1016/s1542-3565(04)00355-6. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hirota M, Ichihara A, Ohmuraya M, Hashimoto D, Sugita H, Takamori H, Kanemitsu K, Baba H. Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitis. Pancreas. 2006;32:87–92. doi: 10.1097/01.mpa.0000186248.89081.44. [DOI] [PubMed] [Google Scholar]
- Majumdar AP, Jaszewski R, Dubick MA. Effect of aging on the gastrointestinal tract and the pancreas. Proc Soc Exp Biol Med. 1997;215:134–144. doi: 10.3181/00379727-215-44120. [DOI] [PubMed] [Google Scholar]
- Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130–140. doi: 10.1097/shk.0b013e3180487ba1. [DOI] [PubMed] [Google Scholar]
- Matull WR, Pereira SP, O'Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006;59:340–344. doi: 10.1136/jcp.2002.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K, Funakoshi A, Jimi A, Sazaki N, Kitani K. Manifestations of experimental acute pancreatitis in young and old rats. Arch Gerontol Geriatr. 1992;14:167–174. doi: 10.1016/0167-4943(92)90051-5. [DOI] [PubMed] [Google Scholar]
- Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–744. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- Papachristou GI. Prediction of severe acute pancreatitis: current knowledge and novel insights. World J Gastroenterol. 2008;14:6273–6275. doi: 10.3748/wjg.14.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkovic D, Bajec D, Ivancevic N, Milic N, Bumbasirevic V, Jeremic V, Djukic V, Stefanovic B, Milosevic-Zbutega G, Gregoric P. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas. 2009;38:655–660. doi: 10.1097/MPA.0b013e3181a66860. [DOI] [PubMed] [Google Scholar]
- Ranson JH, Lackner H, Berman IR, Schinella R. The relationship of coagulation factors to clinical complications of acute pancreatitis. Surgery. 1977;81:502–511. [PubMed] [Google Scholar]
- Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep. 2004;6:99–103. doi: 10.1007/s11894-004-0035-0. [DOI] [PubMed] [Google Scholar]
- Runge MS, Bode C, Matsueda GR, Haber E. Antibody-enhanced thrombolysis: targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci U S A. 1987;84:7659–7662. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, Shaheen NJ, Sandler RS. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448–1453. doi: 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Saito H, Papaconstantinou J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J Biol Chem. 2001;276:29307–29312. doi: 10.1074/jbc.M103740200. [DOI] [PubMed] [Google Scholar]
- Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Salomone T, Tosi P, Palareti G, Tomassetti P, Migliori M, Guariento A, Saieva C, Raiti C, Romboli M, Gullo L. Coagulative disorders in human acute pancreatitis: role for the D-dimer. Pancreas. 2003;26:111–116. doi: 10.1097/00006676-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Singh VP, Bhagat L, Navina S, Sharif R, Dawra RK, Saluja AK. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut. 2007;56:958–964. doi: 10.1136/gut.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1166–G1174. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr ME, Ueda J, Takahashi H, Weiler H, Esmon CT, Evers BM, Saito H. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood. 2010;115:4886–4893. doi: 10.1182/blood-2009-10-246678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med. 2011;50:371–380. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Okamura D, Starr ME, Saito H, Evers BM. Age-dependent reduction of the PI3K regulatory subunit p85alpha suppresses pancreatic acinar cell proliferation. Aging Cell. 2012;11:305–314. doi: 10.1111/j.1474-9726.2011.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonsi AF, Bacchion M, Crippa S, Malleo G, Bassi C. Acute pancreatitis at the beginning of the 21st century: the state of the art. World J Gastroenterol. 2009;15:2945–2959. doi: 10.3748/wjg.15.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Rychahou PG, Uchida T, Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128:1391–1404. doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Xin MJ, Chen H, Luo B, Sun JB. Severe acute pancreatitis in the elderly: etiology and clinical characteristics. World J Gastroenterol. 2008;14:2517–2521. doi: 10.3748/wjg.14.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyromski NJ, Mathur A, Pitt HA, Lu D, Gripe JT, Walker JJ, Yancey K, Wade TE, Swartz-Basile DA. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G552–G558. doi: 10.1152/ajpgi.90278.2008. [DOI] [PubMed] [Google Scholar]