Abstract
A key mechanism for mesenchymal stem cells/bone marrow stromal cells (MSCs) to promote tissue repair is by secretion of soluble growth factors (GFs). Therefore, clinical application could be optimized by a combination of cell and gene therapies, where MSCs are genetically modified to express higher levels of a specific factor. However, it remains unknown how this overexpression may alter the fate of the MSCs. Here, we show effects of overexpressing the growth factors, such as basic fibroblast growth factor (bFGF), platelet derived growth factor B (PDGF-BB), transforming growth factor β1 (TGF-β1), and vascular endothelial growth factor (VEGF), in human bone marrow-derived MSCs. Ectopic expression of bFGF or PDGF-B lead to highly proliferating MSCs and lead to a robust increase in osteogenesis. In contrast, adipogenesis was strongly inhibited in MSCs overexpressing PDGF-B and only mildly affected in MSCs overexpressing bFGF. Overexpression of TGF-β1 blocked both osteogenic and adipogenic differentiation while inducing the formation of stress fibers and increasing the expression of the smooth muscle marker calponin-1 and the chondrogenic marker collagen type II. In contrast, MSCs overexpressing VEGF did not vary from control MSCs in any parameters, likely due to the lack of VEGF receptor expression on MSCs. MSCs engineered to overexpress VEGF strongly induced the migration of endothelial cells and enhanced blood flow restoration in a xenograft model of hind limb ischemia. These data support the rationale for genetically modifying MSCs to enhance their therapeutically relevant trophic signals, when safety and efficacy can be demonstrated, and when it can be shown that there are no unwanted effects on their proliferation and differentiation.
Keywords: Growth factors, Mesenchymal stem cells, Bone marrow stromal cells, Angiogenesis
Introduction
Increasing evidence suggests that multipotent mesenchymal stem cells/bone marrow stromal cells (MSCs) represent an ontologic and phylogenetic vestige of ancestors with regenerative potential, as found during early development of mammals [1] or adult newts, salamanders and fishes [2]. MSCs can be isolated from virtually all vascularized tissues [3] and are proposed to correspond with the pericyte compartment [4]. Bone marrow-derived MSCs can reconstitute bone and bone marrow stroma at ectopic sites in immunodeficient mice [5] and have been used for various cell therapies to treat, among many others, graft versus host disease [6], cardiac infarction [7], and epidermal fistulas [8]. In these applications, currently undergoing phase III clinical trials, MSCs are considered not to contribute significantly by direct differentiation and replacement of the damaged tissue, but rather to perform as trophic mediators [9], promoting tissue repair by production and release of soluble factors that inhibit inflammation [10], reduce fibrosis, and induce angiogenesis [11] among other functions.
The regeneration process requires orchestration of various signals including basic fibroblast growth factor (bFGF or FGF-2), platelet derived growth factor B (PDGF-BB), transforming growth factor β1 (TGF-β1), and vascular endothelial growth factor (VEGF) [2]. MSCs do produce and secrete bFGF, PDGF-BB, TGF-β1, and VEGF [12]; however, the expression levels are below those expected to have therapeutic relevance [11, 13, 14]. Therefore, an optimal design to achieve tissue regeneration could combine cell and gene therapy, where MSCs are genetically modified to overexpress these GFs (reviewed by Hodgkinson et al. [15] and Meyerrose et al. [16]). In this context, it is essential to evaluate the effects of overexpressing GFs in MSCs. This notion is strongly supported by a recent study that specifically identified bFGF, PDGF, and TGF-β signaling as critical pathways during proliferation and differentiation of MSCs [17]. Overexpression of GF in MSCs may cause similar effects to those previously described when recombinant GF are supplemented in the culture media. Nevertheless, different dynamics of GF production and receptor binding may lead to unforeseen outcomes. To address this hypothesis remains essential for the planning of a combined cell and gene therapy application. In addition, the comparative analysis of overexpressing different GF in MSCs allows a better understanding of related and nonrelated effects.
Materials and Methods
Cell Isolation and Culture
Bone marrow aspirates from healthy human donors were purchased from Lonza (Allendale, NJ, www.lonza.com). For MSC isolation and expansion, bone marrow aspirates were passed through 90 μm pore strainers for isolation of bone spicules. Then, the strained bone marrow aspirates were diluted with equal volume of phosphate-buffered saline (PBS) and centrifuged over Ficoll (GE Healthcare, Waukesha, WI, www.ge-healthcare.com) for 30 minutes at 700g. Next, mononuclear cells and bone spicules were plated in plastic culture flasks, using minimum essential media α (MEM-α) (HyClone Thermo Scientific, Waltham, MA, http://www.hyclone.com) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, www.atlantabio.com) that had been screened for optimal MSC growth. After 2 days, nonadherent cells were removed by 2–3 washing steps with PBS. MSCs from passages 2–6 were used for experimentation. Using these methods, we have demonstrated that hematopoietic stem cells and monocytes are not present in the cultures after passage 2–3 [16] [18]. Human umbilical vein endothelial cells (HUVECs) were isolated as previously described [19, 20]. Briefly, full-term fetal umbilical cords obtained from the UC Davis Medical Center (processed following designation of the tissue as biological waste released for disposal under institutional review board [IRB] approval) were filled in the vein with 0.2% collagenase type IV solution (Worthington, Lakewood, NJ, www.worthington-biochem.com), incubated at 37°C for 10 minutes, then flushed. Isolated cells were cultured using endothelial growth media 2 (Lonza), with medium changes every 2–3 days. After 1 week in culture, more than 95% of cells were CD31+ as detected by flow cytometry (not shown).
Lentiviral Vectors and MSC Transduction
MSCs were transduced with third-generation lentiviral vectors with the form pCCLc-MNDU3-X-IRES-EGFP, where X is the insertion site for the full length cDNA of bFGF, PDGF-B, TGF-β1, or VEGF-A (165) or without insertion (as control). bFGF cDNA was subcloned from pBLAST45-hFGF2 (Invivogen, San Diego, CA, www.invivogen.com), while cDNAs for PDGF-B, TGF-β1, and VEGF-A (165) were derived from pCMV-SPORT6 vectors (Open Biosystems, Huntsville, AL, www.openbiosystems.com). MSCs were transduced with 2 mg/ml protamine sulfate. The volume of lentivirus used for each transduction was determined by titration as the required volume to generate 80%–95% GFP positive MSCs after 3 days.
Measurement of GF Protein Levels
MSCs cultured in six-well plates (5,000 cells per square centimeter) were transduced with the respective lentiviral vectors. After 4 days, medium was changed to 1 ml per well of MEM-α supplemented with 2% bovine serum albumin and incubated for additional 24 hours. Then, supernatants were collected to confirm overexpression and secretion of each factor using a human angiogenesis array (cat# AAH-ANG-1-8), following manufacturer’s instructions (RayBiotech, Inc. Norcross, GA, www.raybiotech.com). To determine protein secretion by enzyme-linked immunosorbent assay (ELISA), MSCs were plated in 75 cm2 culture flasks (5,000 cells per square centimeter) with 8 ml of MEM-α supplemented with 10% FBS. After 24 hours, supernatants were collected and cell number determined for normalization. Then, protein levels of bFGF, PDGF-BB, TGF-β1, and VEGF were determined by Quantikine Colorimetric Sandwich ELISAs (R&D Systems, Minneapolis, MN, http://www.rndsystems.com), following their provided protocols.
Cell Proliferation
Three days after transduction with the respective lentiviral vectors, 20,000 MSCs per well were plated in duplicate in 12-well plates, with a final concentration of 2,000 cells per square centimeter. The day after and every second day, cells were detached by trypsinization treatment and counted with trypan blue exclusion dye using a hemocytometer.
Western Blots
For detection of activated ERK1/2 and AKT signaling pathways in MSCs, conditioned media of transduced cells was prepared by incubation of cells in 8 ml MEM-α + 10% FBS per 106 cells per 75 cm2 flasks for 24 hours and stored at −80°C. Then, nontransduced MSCs (20,000 cells per square centimeter) were incubated for 2 hours with the conditioned media that had been previously prepared, and proteins were immediately extracted using radio immunoprecipitation assay buffer (Pierce, Rockford, IL, http://www.piercenet.com) supplemented with Halt protease and phosphatase inhibitor cocktail (Pierce). Proteins were loaded in 10% bis-acrylamide gels and transferred to nitrocellulose membranes. After blocking for 1 hour, membranes were incubated with first antibodies overnight. Antibodies against phosphorylated and total Akt and MAPK44/42 (ERK1/2) were purchased from Cell Signaling Technology (Danvers, MA, www.cellsignal.com).
RNA Extraction and Real Time Polymerase Chain Reaction
Total RNA was extracted with RNA-Stat 60 (Iso-Test Diagnostics, Friendswood, TX, www.isotexdiagnostics.com), following manufacturer’s instructions. Reverse transcription using 1 μg of RNA was performed using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA, www.appliedbiosystems.com). For the semiquantification of mRNA levels of bFGF and PDGF-B, a pre-made taqman primers/probe mix was used as provided by Applied Biosystems (accession numbers Hs00960934_m1 and Hs00234042_m1 respectively), using GAPDH as an internal control for these taqman assays (accession number Hs99999905_m1). For all other mRNAs detected, SYBR Green Master mix (Applied Biosystems) was used for real-time reverse-transcription polymerase chain reaction, using primers listed in Table 1. Primers were designed using OligoPerfect software (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), unless denoted by a reference.
Table 1.
Primers used for real-time reverse-transcription polymerase chain reaction
| Gene | Forward primer | Reverse primer | References |
|---|---|---|---|
| ACTA2 | 5′-TCAATGTCCCAGCCATGTAT-3′ | 5′-CAGCACGATGCCAGTTGT-3′ | 21 |
| BSP | 5′-ATGGCCTGTGCTTTCTCAATG-3′ | 5′-AGGATAAAAGTAGGCATGCTT-3′ | 22 |
| CBFA1 | 5′-CGGAATGCCTCTGCTGTTAT-3′ | 5′-TTCCCGAGGTCCATCTACTG-3′ | 23 |
| CNN1 | 5′-GCTGTCAGCCGAGGTTAAGAA-3′ | 5′-TGAGGCCGTCCATGAAGTTG-3′ | 24 |
| FABP4 | 5′-TGAAAGAAGTAGGAGTGGGCTT-3′ | 5′-ATCCCCATTCACACTGATGATC-3′ | 25 |
| GAPDH | 5′-CTCAGTGTAGCCCAGGATGC-3′ | 5′-ACCACCATGGAGAAGGCTGG-3′ | |
| PPAR-γ | 5′-TGCAGGTGATCAAGAAGACG-3′ | 5′-TGGAAGAAGGGAAATGTTGG-3′ | |
| SM22 | 5′-ATGGAGCAGGTGGCTCAGTTC-3′ | 5′-ACTGCCAAGCTGCCCAAAG-3′ | 24 |
| TGF-β1 | 5′-GGGACTATCCACCTGCAAGA-3′ | 5′-CCTCCTTGGCGTAGTAGTCG-3′ | |
| VEGF-A | 5′-AGGCCAGCACATAGGAGAGA-3′ | 5′-TTTCTTGCGCTTTCGTTTTT-3′ |
Osteogenic Differentiation
For osteogenic induction assay, 10,000 MSCs per square centimeter were cultured for 14 days in osteogenic media (MEM-α + 10% FBS supplemented with 0.2 mM ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycerolphosphate), with a medium change every 3–4 days. To measure alkaline phosphatase (ALP) activity, at day 14, the cells were trypsinized and lysed for protein extraction, with 1.5 mM Tris-HCl solution containing 1.0 mM ZnCl2, 1.0 mM MgCl2, and 1% Triton X-100 for 10 minutes. Lysates were then centrifuged at 16,100g for 30 minutes and incubated with p-nitrophenylphosphate liquid substrate solution (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 30 minutes. Released p-nitrophenolate was determined spectrophotometrically at 405 nm, while total protein concentration was determined with Coomassie staining (595 nm). Calcium precipitation was measured based on a previously described protocol [26]. Briefly, cells were fixed with 10% v/v formalin solution for 15 minutes, washed once with PBS, stained for 20 minutes with gentle shaking with 1% w/v Alizarin Red S (ARS) indicator (Ricca Chemicals Company, Arlington, TX www.riccachemical.com), washed twice with PBS, and photographed with a Powershot A2000IS camera (Canon, Lake Success, NY, http://www.usa.canon.com). Then, samples were incubated with 10% v/v acetic acid for 30 minutes, scraped for further dissociation of cell layers, vortexed for 30 seconds and centrifuged at 16,100g for 10 minutes. Optic density of the supernatants was measured at 405 nm. To ensure that variances in calcium precipitation were not due to differences in cell number, protein concentration from control wells was determined with Coomassie staining as described above. For gene expression of osteogenic markers, RNA was extracted at day 14 as described above.
Adipogenic Differentiation
MSCs were cultured in six-well plates to confluence (approximately 15,000 cells per square centimeter) and cultured for 14 or 21 days, with medium change every 3–4 days, in adipogenic medium (MEM-α + 10% FBS supplemented with 0.5 mM isobutilmethylxantine, 50 μM indomethacin, and 0.5 μM dexamethasone). For oil red O staining, cells were fixed after 14 days with 10% v/v formalin solution for 15 minutes, washed once with PBS, and stained for 30 minutes with oil red O (Electron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.com/microscopy). After washing twice with PBS, adipocytes were photographed under a phase contrast microscope. For detection of gene expression of adipogenic markers, RNA was extracted at day 14 as described above. For adipocyte quantification, each unstained well was photographed at day 21 in 10 randomly chosen areas. For Nile Red quantification, MSCs were trypsinized after 21 days in differentiation media and directly stained for 5 minutes with 10 μg/ml Nile Red (MP Biomedicals, Illkirch, France, http://www.mpbio.com) as originally described [27]. Then, samples were washed once with PBS and measured by flow cytometry at 580 nm.
Cell Morphology
Transduced MSCs were plated at a concentration of 5,000 cells per square centimeter on glass coverslips and cultured for 24 hours to allow attachment and maximal spreading. Then, samples were fixed for 15 minutes with 4% paraforomaldehyde, permeabilized for 5 minutes with 0.05% Triton X-100, blocked for 1 hour with PBS + 2% FBS, and incubated with TRITC-labeled phalloidin (1:400, Sigma-Aldrich). Finally, samples were mounted using Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) and inspected under a fluorescent microscope (Axioscope 2 plus, Zeiss, Goettingen, Germany, www.zeiss.com).
HUVEC Migration Assay
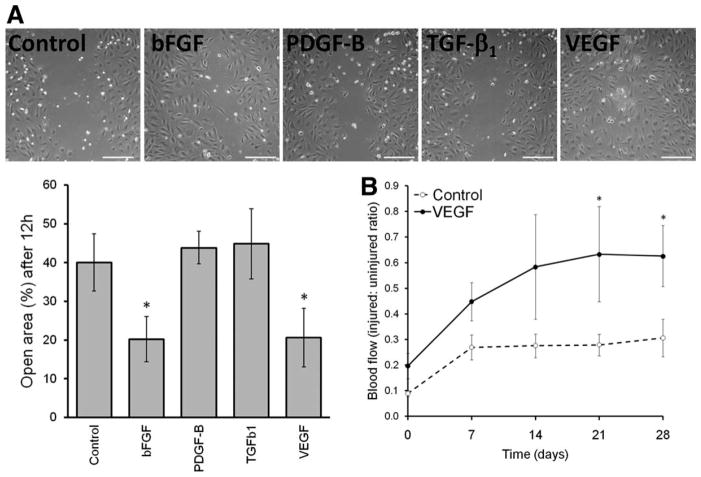
MSCs that had been engineered to overexpress each GF were cultured in six-well plates for 24 hours in standard media (105 cells per 2 ml per well). Then, supernatants were collected and tested for their effect on migration of HUVECs. For the migration assays, HUVEC were plated in 24-well plates (1.5 × 105cells per well) containing inserts from CytoSelect 24-well wound healing assay (Cell Biolabs Inc, San Diego, CA, www.cellbiolabs.com). After overnight incubation, inserts were removed creating a homogenous gap (or “scratch”) in the monolayer of cells. Then, medium was changed to the collected supernatants from each type of GF-engineered MSCs. Wells were photographed under an inverted phase contrast microscope at time 0 hour and after 12 hours. Finally, the open area on acquired pictures was quantified using TScratch Software [28] (ETH, Zurich, Switzerland, http://www.cse-lab.ethz.ch). The percentages of the open areas were calculated as the ratio of the area after 12 and 0 hours.
Hind Limb Ischemia Model and Blood Flow Restoration
All rodent work was performed under an approved animal care protocol in the UC Davis Stem Cell Program immune deficient mouse core. Under anesthesia, nonobese diabetic/severe combined immune deficient (NOD/SCID)/β-2-micro-globulin-deficient mice (Jackson Laboratories—West, Sacramento, CA, http://jaxmice.jax.org/jaxwest) were subjected to unilateral hind limb ischemia surgeries as we have described previously [29, 30]. In brief, the mice were shaved and prepped, the right femoral artery and vein were exposed and dissected from the femoral nerve, and the proximal portion of the femoral artery was ligated with 6-0 braided silk sutures. The distal portion of the saphenous artery and the remaining collateral arteries were ligated and removed from the hind limb. The wound was closed with 6-0 braided silk sutures. MSCs transduced with an empty vector or with VEGF encoding vector, as described above, were injected into the tail vein 24 hours after surgery (MSC group: n = 8, VEGF group: n = 6; 1 × 106 cells per animal). Care was taken to reduce the time from lifting the cells from the plate, washing, and injection, because MSCs can clump with time and could then form emboli when injected. Cells were injected within 1 hour of harvesting from the plate, with a syringe filter to remove any clumps as we have described previously [29, 31].
Blood flow to the ischemic limb was measured immediately before cell transplantation (day 0) and again on days 4, 7, 14, 21, and 28 using a laser Doppler imager (Moor Instruments Ltd, Devon, U.K., www.moor.co.uk) as described previously [29–31]. For imaging, under general anesthesia, both the ischemic and the healthy legs were shaved and the animal was placed on a 37°C heating pad for 2–5 minutes before imaging to allow acclimation to the ambient conditions before blood flow to both legs was simultaneously measured. The blood flow to the ischemic leg is expressed as a ratio relative to the contralateral healthy leg.
Data Presentation and Statistical Analysis
All values in figures represent averages with the standard error of mean as error bars. The number of experiments performed with MSCs derived from different bone marrow donors is shown in the legend of the respective figures. All significant differences were evaluated using a paired-Student’s t test, comparing raw data (not normalized) of conditions with control (MSCs transduced with control lentiviral vector). Throughout this manuscript, the following nomenclature is used: *, p < .05, **, p < .005, ***, p < .0005.
Results
Overexpression of GF Leads to Activation of Specific Signaling Pathways in MSCs
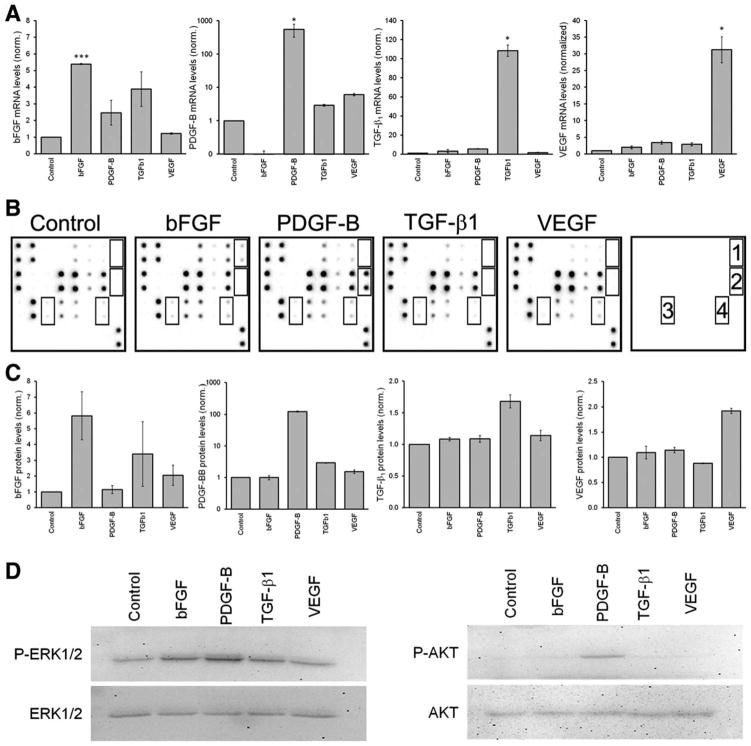
First, we confirmed that lentiviral transduction with bFGF, PDGF-B, TGF-β1, and VEGF-A lead to enhanced production and secretion of these GFs in MSCs. As shown in Figure 1A, the mRNA of each of the four GFs was increased upon over-expression, although in different magnitudes. Each GF was also found to be increased on a protein level, as measured in supernatants of MSCs transduced with the respective GF, as shown using an angiogenesis array (Fig. 1B) and ELISA (Fig. 1C). Remarkably, overexpression of TGF-β1 induced an increase of bFGF at both the mRNA and protein levels. Of note, overexpression of PDGF-B lead to an over 100-fold increase of PDGF-B at both mRNA and protein levels, while overexpression of the other GFs remained within a linear range. The overexpression of these GFs lead to the activation of specific signaling pathways in MSCs (Fig. 1C). This was tested in nontransduced MSCs incubated for 1 hour in conditioned media collected from the GF overexpressing MSCs. Conditioned media of MSCs overexpressing bFGF or PDGF-B induced phosphorylation of ERK1/2, while only PDGF-B also activated AKT. Under these conditions, we do not observe phosphorylation of Smad2/3 induced by TGF-β1. However, we observed an increased accumulation of Smad2/3 in the nucleus of MSCs overexpressing TGF-β1, when compared with all other conditions (data not shown). These results demonstrate effective increases of both mRNA and protein levels of each GF after lentiviral transduction, which lead to the activation of specific signaling pathways in MSCs.
Figure 1.
Overexpression of growth factors (GF) leads to activation of specific signaling pathways in mesenchymal stem cells/bone marrow stromal cells (MSCs). MSCs were transduced with control lentiviral vectors or those designed to overexpress GF. Overexpression of GF was then confirmed at both mRNA and protein levels. (A): mRNA was extracted from MSCs 3 days after transduction and measured by real-time reverse-transcription polymerase chain reaction (n = 3). (B): Protein levels of GF were measured in supernatant of MSCs using a human angiogenesis array, where 1 = basic fibroblast growth factor, 2 = platelet derived growth factor B, 3 = active transforming growth factor β1, and 4 = vascular endothelial growth factor. (C): Protein levels of GF measured in supernatants of MSCs using enzyme-linked immunosorbent assay. (D): Activation of ERK1/2 and AKT1/2 was measured by western blot in MSCs as described. Abbreviations: bFGF, basic fibroblast growth factor; PDGF-B, platelet derived growth factor B; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
Increased Proliferation in MSCs Overexpressing bFGF or PDGF-B
We next sought to determine whether overexpression of any of the GFs had a significant effect on MSC proliferation. Three days after transduction with the GF expression vectors, and every 2nd day, a viable cell count was done (n = 4 normal MSC donors). As shown in Figure 2, overexpression of bFGF and PDGF-B lead to rapid proliferation with a reduction of about 50% in the doubling time of MSCs, when compared with MSCs transduced with a control lentiviral vector. In contrast, overexpression of TGF-β1 and VEGF did not significantly affect MSC growth.
Figure 2.
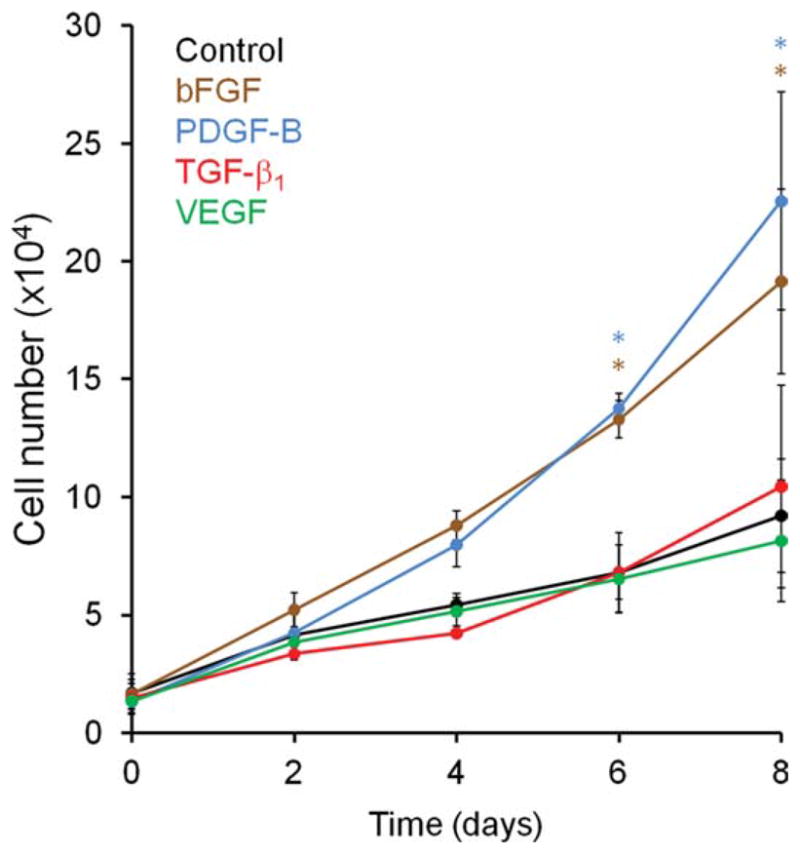
Increased proliferation in mesenchymal stem cells/bone marrow stromal cells (MSCs) overexpressing basic fibroblast growth factor or platelet derived growth factor B. Proliferation of MSCs overexpressing growth factor was measured by counting cells using trypan blue exclusion dye in a hemocytometer, as described (n = 3). Abbreviations: bFGF, basic fibroblast growth factor; PDGF-B, platelet derived growth factor B; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
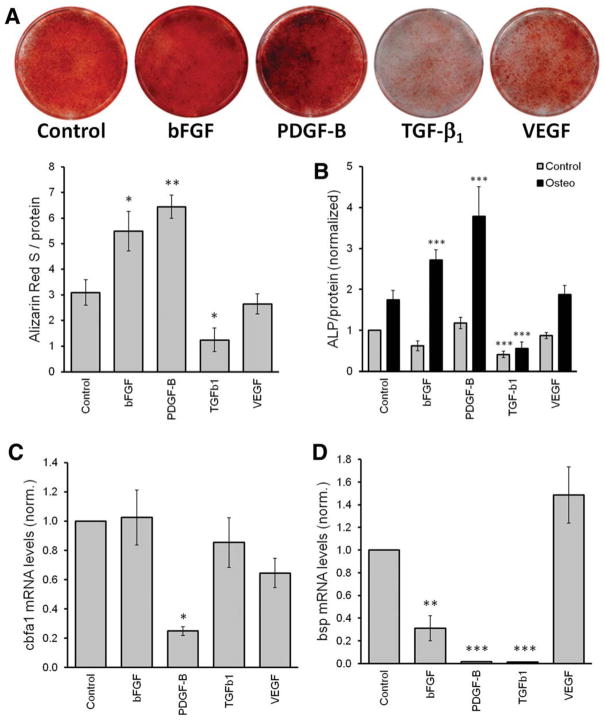
Osteogenic Differentiation of MSCs Is Increased by Overexpression of bFGF and PDGF-B and Inhibited by TGF-β1
To determine the effect of overexpressing GF on the osteogenic differentiation potential of MSCs, transduced cells were cultured for 14 days in osteogenic media, then calcium precipitation, ALP activity, and gene expression of osteogenic markers was measured. Calcium precipitation as determined by ARS staining was enhanced upon overexpression of bFGF and PDGF-B, while overexpression of TGF-β1 strongly inhibited it (Fig. 3A). This was quantified using a previously described protocol [26], which we modified to use the total protein content as an internal loading control. This modification was introduced to confirm that the higher calcium precipitation is not due to the increased cell numbers. We used ALP activity as a second method to measure osteogenesis. As we noticed that significant levels of ALP were also found in MSCs cultured under standard conditions (i.e., no differentiation media), we included this condition as an additional control in this study. In agreement with our results on calcium precipitation, ALP increased with the overexpression of both bFGF and PDGF-B and decreased with the overexpression of TGF-β1 (Fig. 3B). Of note, ALP levels in MSCs engineered to overexpress TGF-β1 were also significantly lower under standard culture conditions, suggesting that MSCs overexpressing TGF-β1 were not maintained in their primitive basal state, but may have differentiated into another cell type.
Figure 3.
Overexpression of basic fibroblast growth factor and platelet derived growth factor B increases the osteogenic differentiation of mesenchymal stem cells/bone marrow stromal cells (MSCs), while transforming growth factor β1 inhibits. Transduced MSCs were cultured in osteogenic media for 14 days. Then, the following assays were performed: (A): Alizarin Red S staining. Upper panel shows representative wells and lower panel the quantification (n = 4)). (B): Alkaline phosphatase activity. Statistical differences are established comparing MSCs in cultured in control media (gray bars) withtheir respective control and MSCs in osteogenic media (black bars) with control cells cultured in osteogenic media (n = 4). (C, D): Semiquantification of osteogenic markers cbfa1 and bsp, respectively (n = 7). Abbreviations: ALP, alkaline phosphatase; bsp, bone sialoprotein; bFGF, basic fibroblast growth factor; cbfa1, core binding factor α 1; PDGF-B, platelet derived growth factor B; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
Next, we measured the expression levels of mRNA known to be associated with osteogenesis. No consistent differences in the mRNA levels of osteopontin, osterix, or osteocalcin were found (data not shown). However, the levels of core binding factor-α1 (cbfa1) were surprisingly downregulated upon overexpression of PDGF-B (Fig. 3C). Also, bone sialo-protein (bsp) was unexpectedly decreased in MSCs that had been engineered to overexpress bFGF or PDGF-B (Fig. 3D). Both cbfa1 and bsp mRNA levels were also measured at days 4 and 10 during osteogenesis and demonstrated the same tendencies (not shown). Also overexpression of TGF-β1 strongly reduced bsp mRNA levels during osteogenesis.
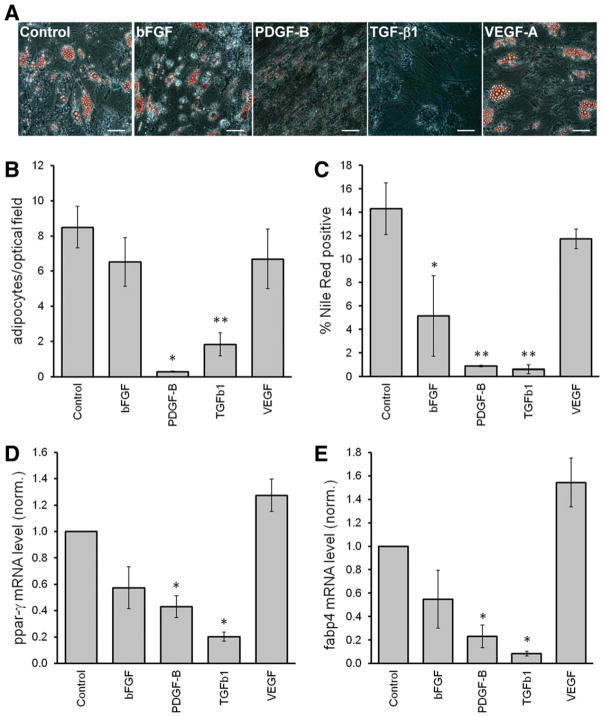
Over-Expression of PDGF-B or TGF-β1 Inhibited the Adipogenic Differentiation of MSCs
The adipogenic differentiation capacity from each GF-overexpressing MSC population was evaluated using three different methods: microscopic count of adipocyte-like cells based on morphology and oil droplet accumulation, quantification by flow cytometry of cells with high triglyceride content, and gene expression of adipogenic markers. After culturing MSCs under adipogenic induction medium for 21 days, cells with large lipid droplets were observed, except in conditions of MSCs overexpressing either PDGF-B or TGF-β1 (Fig. 4A). This was further quantified by staining the cells with Nile Red and measuring the percentage of Nile Red positive cells by flow cytometry (Fig. 4B). Similarly, overexpression of PDGF-B or TGF-β1 strongly decreased the number of cells with high triglyceride content. MSCs overexpressing bFGF also showed a significant reduction of Nile Red+ cells, although with greater variation among donors.
Figure 4.
Overexpression of platelet derived growth factor B or transforming growth factor β1 inhibits the adipogenic differentiation of mesenchymal stem cells/bone marrow stromal cells (MSCs). Transduced MSCs were cultured in adipogenic medium for 21 days. (A): Cells stained with Oil-Red O and pictured in representative areas. Scale bar = 100 mm. (B): Number of adipocytes counted microscopically (n = 4). (C): Quantification of cells with high triglyceride, by means of Nile Red stained cells, using flow cytometry (n = 3). (D, E): Quantification after 14 days under adipogenic media of mRNA levels of adipogenic markers ppar-g and fabp4, respectively (n = 4). Abbreviations: bFGF, basic fibroblast growth factor; fabp4, fatty acid binding protein 4; PDGF-B, platelet derived growth factor B; ppar, peroxisome proliferator-activated receptor γ; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
Next, mRNA levels of the adipogenic markers peroxisome proliferator-activated receptor γ (pparγ) and fatty acid binding protein 4 (fabp4) were measured in transduced MSCs after 14 days in culture under adipogenic media. Consistently, over-expression of PDGF-B or TGF-β1 lead to reduced pparγ and fabp4 mRNA levels, while enforced bFGF expression in MSCs lead to only a minor, but nonsignificant, effect (Fig. 4C, 4D).
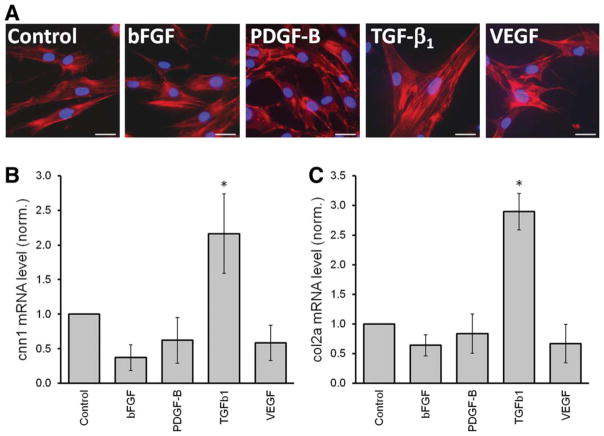
TGF-β1-Mediated Effects on MSCs
Ectopic expression of TGF-β1 in MSCs strongly inhibited their osteogenic and adipogenic differentiation potential (Figs. 3 and 4). As mentioned above, parameters such as the ALP activity were found even below basal levels (i.e., MSCs incubated in normal culture media, Fig. 3B), suggesting that TGF-β1 does not block MSC differentiation to retain them in an immature, undifferentiated state, but rather induces the differentiation of MSCs to a different cell type. We observed that overexpression of TGF-β1 directed strong morphological changes in MSCs that suggest that they are undergoing senescence; large, flattened polygonal shapes with actin bundles characteristic of stress fibers (Fig. 5A). However, MSCs overexpressing TGF-β1 did not appear to undergo true senescence, because the proliferation of cells was found to be normal. In addition, we observed a spontaneous (i.e., in absence of a specific differentiation media) increase of the smooth muscle gene calponin-1 (Fig. 5B) and the chondrogenic marker Col2A (Fig. 5C). However, other smooth muscle/chondrogenic markers including α-smooth muscle actin (ACTA-2), 22 kDa smooth muscle protein (SM22), Sox9, Aggrecan, and Collagen type-X were not significantly affected by overexpression of TGF-β1 (not shown). These results suggest that the changes acquired by MSCs that had been engineered to overexpress TGF-β1 did not lead to a bona fide differentiation process in vitro.
Figure 5.
Transforming growth factor β1 (TGF-β1) induces morphologic alterations in mesenchymal stem cells/bone marrow stromal cells (MSCs). (A): Changes in morphology of MSCs overexpressing growth factors were analyzed using phalloidin to stain actin protein (red). Cells overexpressing basic fibroblast growth factor or vascular endothelial growth factor did not differ from control cells. In contrast, MSCs overexpressing platelet derived growth factor B overlap and create extended protrusions. MSCs overexpressing TGF-β1 were more flattened and larger than control cells and presented abundant actin bundles characteristic of stress fibers. These changes were evident 3 days after transduction. (B) and (C) shows Quantification of mRNA levels of cnn1 and col2a, respectively, 5–7 days after culture of transduced MSCs under standard conditions (without differentiation medium; n = 4). Abbreviations: bFGF, basic fibroblast growth factor; PDGF-B, platelet derived growth factor B; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
Overexpression of VEGF from MSCs Enhanced Migration of Endothelial Cells and Blood Flow Restoration After Hind Limb Ischemia
Our results demonstrated that overexpressing VEGF in MSCs did not exert any significant effects, which is in line with the observation that MSCs do not express VEGF receptors [32]. However, we wanted to rule the possibility of the lack of effects exerted by VEGF potentially being due a nonfunctional protein product. Therefore, we performed a relevant bioassay to test the effects of supernatants collected from the different types of GF overexpressing MSCs on the migration of endothelial cells (HUVEC), which are well known to be responsive to VEGF. As shown in Figure 6A, overexpression of bFGF or VEGF from MSCs strongly induced migration of HUVEC in a wound/scratch assay, demonstrating that the protein products of the gene constructs were fully functional and biologically active. Finally, we tested whether MSCs overexpressing VEGF would also improve restoration of blood flow in mice after induction of unilateral hind limb ischemia. For this, 1-day after creating a hind limb ischemia in NOD/SCID-MPSVII mice as described above, one million MSCs transduced with either control or VEGF vectors were injected into the tail vein and blood flow on the ischemic limb was measured using laser Doppler imaging. As shown in Figure 6B, under these experimental conditions, control MSCs showed only a limited improvement of blood flow, while MSCs over-expressing VEGF showed a clear improvement in revascularization over time. These in vivo data, in conjunction with the enhanced migration of endothelial cells mediated by the MSCs engineered to express VEGF, and the lack of effects on the proliferation or differentiation of the MSCs themselves, position this MSC/VEGF cell population as the best preclinical development candidate tested, to be considered for further testing for future revascularization studies.
Figure 6.
Overexpression of basic fibroblast growth factor and vascular endothelial growth factor (VEGF) enhances migration of endothelial cells. (A): A wound healing assay was used to assess the effect of conditioned media from mesenchymal stem cells/bone marrow stromal cells (MSCs) overexpressing growth factor, on the migration of human umbilical vein endothelial cell. Both representative pictures (phase contrast and upper panels) and quantification (n = 4) were acquired after 12 hours. Scale bar = 200 mm. (B): Unilateral hind limb ischemia was induced in nonobese diabetic/severe combined immune deficient/β-2-microglobulin-deficient mice followed by transplantation of control (open circles, n = 8) or VEGF overexpressing MSCs (n = 6, solid circles). Laser Doppler perfusion imaging was used to assess the ratio blood flow in the healthy versus affected legs. Mean group values ± SD are shown. Asterisks denote significant difference (p ≤ .05). Abbreviations: bFGF, basic fibroblast growth factor; PDGF-B, platelet derived growth factor B; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
Discussion
The effects of GFs on MSCs in vitro have been previously studied, commonly by adding GF as recombinant proteins to the culture media, or using small molecules to inhibit the GF receptors, both allowing the study of concentration-dependent effects. Rarely, however, are various GFs studied in a comparative manner. The effects of overexpressing GF in MSCs might be comparable with the effect of adding recombinant GF to MSC cultures, but there can also be unexpected and unwanted effects from producing GFs from a cell type that can respond to them, so this hypothesis remained to be addressed. To our knowledge, this is the first comparative analysis of overexpressing different GFs that might be biologically active in a wound microenvironment in MSCs.
First, we examined the levels of GF overexpression acquired by MSCs after transduction with the respective lenti-viral vectors. According to the angiogenesis cytokine array used, the level of active TGF-β1 found in supernatants of MSCs that had been engineered to overexpress it, was rather low when compared with bFGF, PDGF-B, or VEGF. All four GFs studied were cloned into the same vector backbone, the same multiplicity of infection was used for transduction and comparable levels of GFP (driven under the same promoter, see Materials and Methods section) were reached for all constructs (not shown), suggesting that active TGF-β1 levels could have been regulated on a post-transcriptional level. Mature TGF-β1 peptides associate with latent-TGF-β-binding proteins localizing to the extracellular matrix, therefore reducing active TGF-β1 levels in solution [33]. We speculate that because active TGF-β1 was determined in supernatants, the latent levels of TGF-β1 may have been under-estimated. Consistent with this idea, supernatants of MSCs that had been engineered to overexpress TGF-β1 could not lead to activation of Smad2/3. In contrast, Smad2/3 was found to be constitutively active in MSCs engineered to overexpress TGF-β1, possibly through the constant exposure to it. We also noted significant effects on the biology and morphology of the MSCs engineered to express TGF-β1, indicating that although the levels of protein were low, they were biologically active.
The effect of TGF-β1 on proliferation and differentiation has been shown to be cell type and concentration dependent [34]. In our experimental setting, we observed that overexpression of TGF-β1 did not significantly affect cell growth, but strongly inhibited both osteogenic and adipogenic differentiation. As previously described, TGF-β1 induces the formation of stress fibers in MSCs and increases the expression of smooth muscle markers [24]. It has also been suggested that, upon contact with endothelial cells, newly recruited MSCs are induced toward a mural cell fate, in a process mediated by the activation of TGF-β [35, 36]. On the other hand, TGF-β1 induces differentiation of MSCs into chondrocytes and is commonly used to prove the chondrogenic potential of MSCs in vitro [37]. However, the differentiation of MSCs into chondrocytes requires the growth of cells in a micromass pellet [38]. In accordance with these data, we observed that MSCs engineered to overexpress TGF-β1 acquired a complex phenotype, characterized by the expression of some smooth muscle and chondrogenic-associated genes, but not others.
The activation of signaling pathways and cell proliferation induced by these GFs clearly correlates with previous experiments using recombinant GF [17, 39–42]. A recent report described bFGF, PDGF-B, and TGF-β1 signaling as critical for MSCs proliferation and differentiation [17]. As expected, bFGF and PDGF-B exerted potent mitogenic effects and enhanced osteogenesis of MSCs. These results correlate with the activation of the ERK1/2 signaling pathway, because it is described to promote proliferation, increase osteogenesis, and inhibit adipogenesis [43]. However, in our studies MSCs engineered to overexpress PDGF-B strongly inhibited adipogenesis, while overexpression of bFGF caused only minor effects. This difference might be associated with the activation of Akt or other signaling pathways by PDGF-B. Thus, the effects of overexpression of the GFs in our study appear to differ in some ways than in previous reports, where the factors were simply added into the medium.
Overexpression of VEGF did not affect MSCs in terms of proliferation, differentiation, and morphology, but provided strong paracrine effects to other target cells. Others have shown enhanced angiogenesis and heart repair with MSCs overexpressing VEGF [14, 44–46], but to our knowledge, none of these groups have reported an autocrine effect induced by overexpressing VEGF. This is not surprising because MSCs do not express VEGF receptors [32]. However, as VEGF has been shown to induce migration of MSCs by activation of PDGF receptors [32], it was important to assess the possibility that the migration of MSCs overexpressing VEGF might be altered. Although there were no significant effects on the MSCs themselves upon transduction with the VEGF expression vectors, there were highly significant effects on migration of human endothelial cells. These data support the potential of these VEGF-producing MSCs to assist in therapeutic angiogenesis.
Our work closely compares the expression of four different GFs that were predicted to be biologically active in a wound microenvironment. We compared the effects on proliferation, differentiation, and bioactivity on endothelial cells. The study demonstrates that, in particular, MSCs engineered to express VEGF did not have abnormalities in proliferation and differentiation, but were potent inducers of endothelial migration and enhanced revascularization in vivo. These data suggest that MSCs engineered to overproduce VEGF in a controlled manner might be a future candidate for augmentation of revascularization. Taken together, this work supports the rationale for genetically modifying MSCs to affect their proliferation and direct their differentiation fates, while enhancing therapeutically relevant signals, such as their angiogenic potential.
Our results are most relevant in the context of combining cell and gene therapy. For example, administration of plasmids coding for bFGF and VEGF for the treatment of coronary artery disease and critical limb ischemia [47, 48] and PDGF-BB to treat chronic wounds for diabetic patients have been performed in human clinical trials [49]. However, the results have been unsatisfactory due to the low sustainability of the GFs at the required sites. As MSCs show that a positive tropism to hypoxic sites [29, 30] are safe (nontumorigenic) [50] and well tolerated in allogeneic transplants, it is well conceivable to use MSCs as vehicles for the delivery of the required GF [16]. However, extensive safety and efficacy testing must be done before this type of cell/gene therapy could ever be considered. This study provide detailed molecular and physical characterization of MSCs engineered to produce four GFs that could potentially have been considered as development candidates. Through these studies, we have ruled out three of the cell populations due to unwanted effects of the engineered GFs on the biology for the MSCs, and have identified one candidate, MSCs engineered to produce VEGF, that is eligible and promising to go forward into further, more detailed translational studies for revascularization therapies.
Conclusion
The therapeutic potential of MSCs could be significantly improved by overexpression of regenerative signals such as growth factors (i.e., combining cell and gene therapies). However, here we show that overexpression of certain growth factors can severely affect the biology of cells, in a therapeutically undesired manner: overexpression of bFGF and PDGF-B increased the proliferation of MSCs and altered their differentiation potential. Overexpression of TGF-β1 induced a spontaneous differentiation of MSCs into an aberrant cell type. In contrast, MSCs that had been engineered to overexpress VEGF did not differ from control MSCs in proliferation or differentiation assays, but had significantly greater angiogenic potential in vitro and in vivo, suggesting that this may be a safe and effective strategy to treat vascular complications.
Acknowledgments
We thank the UC Davis Department of Labor and Delivery for the donation of consented, anonymized and IRB-approved umbilical cord tissue for the human umbilical vein endothelial cell studies. This work was supported by the UC Davis Stem Cell program start-up funding from the UC Davis Health Sciences Deans’ Office (to J.A.N.); the Department of Surgery, UC Davis Health Sciences Campus (to C.S.S.); the California Institute for Regenerative Medicine (CIRM grant TR2-01787 [to J.A.N. and F.A.F.]); National Institutes of Diabetes and Digestive and Kidney Diseases, NIH (NIDDK grants 2R01DK61848 and 2R01DK53041 [to J.A.N.]); and National Heart, Lung and Blood Institute (NHLBI #RO1HL073256 [to J.A.N.]. Funding bodies supported salaries, equipment, mice, and supplies needed for the collection and analysis of the data.
Footnotes
Disclosure of Potential Conflicts of Interest
F.A.F. is a postdoctoral fellow trainee of the California Institute for Regenerative Medicine (CIRM). All other authors indicate no potential conflicts of interest.
Author contributions: F.A.F. and C.S.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; J.A.N.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 3.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 4.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 7.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 12.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song H, Kwon K, Lim S, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19:402–407. [PubMed] [Google Scholar]
- 14.Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgkinson CP, Gomez JA, Mirotsou M, et al. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyerrose T, Olson S, Pontow S, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng F, Boucher S, Koh S, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 18.Meyerrose T, Rosova I, Dao M, et al. Establishment and transduction of primary human stromal/mesenchymal stem cell monolayers. In: Nolta JA, editor. Genetic Engineering of Mesenchymal Stem Cells. Dordrecht, Netherlands: Kluwer Academic Publishers; 2006. pp. 45–58. [Google Scholar]
- 19.Crampton SP, Davis J, Hughes CC. Isolation of human umbilical vein endothelial cells (HUVEC) J Vis Exp. 2007;(3):183. doi: 10.3791/183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baudin B, Bruneel A, Bosselut N, et al. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2:481–485. doi: 10.1038/nprot.2007.54. [DOI] [PubMed] [Google Scholar]
- 21.Popova AP, Bozyk PD, Goldsmith AM, et al. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–L743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welldon KJ, Atkins GJ, Howie DW, et al. Primary human osteoblasts grow into porous tantalum and maintain an osteoblastic phenotype. J Biomed Mater Res A. 2008;84:691–701. doi: 10.1002/jbm.a.31336. [DOI] [PubMed] [Google Scholar]
- 23.Rich JT, Rosova I, Nolta JA, et al. Upregulation of Runx2 and Osterix during in vitro chondrogenesis of human adipose-derived stromal cells. Biochem Biophys Res Commun. 2008;372:230–235. doi: 10.1016/j.bbrc.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narita Y, Yamawaki A, Kagami H, et al. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449–459. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 25.Ugarte F, Ryser M, Thieme S, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867–875. e1. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Gregory CA, Gunn WG, Peister A, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetyl-pyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Greenspan P, Mayer EP, Fowler SD. Nile red: A selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geback T, Schulz MM, Koumoutsakos P, et al. TScratch: A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- 29.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosová I, Link D, Nolta JA. Small interfering RNA-mediated decreases in c-Met levels affect the differentiation potential of human mesenchymal stem cells and reduce their capacity for tissue repair. Tissue Eng Part A. 2010;16:2627–2639. doi: 10.1089/ten.tea.2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 34.Janssens K, ten Dijke P, Janssens S, et al. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 35.Hirschi KK, Skalak TC, Peirce SM, et al. Vascular assembly in natural and engineered tissues. Ann N Y Acad Sci. 2002;961:223–242. doi: 10.1111/j.1749-6632.2002.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 36.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152–1167. doi: 10.1634/stemcells.2004-0062. [DOI] [PubMed] [Google Scholar]
- 38.Mackay AM, Beck SC, Murphy JM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 39.Solchaga LA, Penick K, Porter JD, et al. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 40.Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 41.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary LR, Hruska KA. The cell survival signal Akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells. J Cell Biochem. 2001;81:304–311. [PubMed] [Google Scholar]
- 43.Xiao G, Jiang D, Thomas P, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Hu Q, Mansoor A, et al. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290:H1393–H1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto R, Omura T, Yoshiyama M, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 46.Gao F, He T, Wang H, et al. A promising strategy for the treatment of ischemic heart disease: Mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol. 2007;23:891–898. doi: 10.1016/s0828-282x(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 48.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 49.Mulder G, Tallis AJ, Marshall VT, et al. Treatment of nonhealing diabetic foot ulcers with a platelet-derived growth factor gene-activated matrix (GAM501): Results of a phase 1/2 trial. Wound Repair Regen. 2009;17:772–779. doi: 10.1111/j.1524-475X.2009.00541.x. [DOI] [PubMed] [Google Scholar]
- 50.Bauer G, Dao MA, Case SS, et al. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308–1315. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]