Abstract
Alcoholism is a pervasive social problem, and thus understanding factors which regulate alcohol (ethanol) reward is important for designing effective therapies. One putative regulatory system includes the kappa opioid receptor (KOR) and its endogenous ligand, dynorphin. Previously we demonstrated that acute ethanol increased preprodynorphin expression via brain-derived neurotrophic factor (BDNF) in striatal neurons, and that blockade of the KOR attenuated decreases in ethanol intake observed following increased expression of BDNF (Logrip et al., 2008). As high doses of KOR agonists can generate an aversive state, we hypothesized that endogenous dynorphin may regulate ethanol intake by interfering with the rewarding properties of ethanol. We found that low, non-aversive doses of the KOR agonist U50,488H blocked the rewarding properties of ethanol during conditioning, thus impairing the acquisition of conditioned place preference. Importantly, we demonstrate that U50,488H also inhibited the conditioned increase in locomotor activation normally observed in the ethanol-paired chamber on test day. Taken together, these data indicate that the KOR/dynorphin system may acutely regulate ethanol intake via inhibition of the rewarding properties of ethanol.
Introduction
Alcoholism, as a chronically relapsing disease, is a major public health issue, and as such increased understanding of the neural mechanisms which drive or inhibit alcohol (ethanol) use is of great importance. One system which may be involved in modulating ethanol intake is the kappa opioid receptor (KOR) and its endogenous ligand, dynorphin (Chavkin et al., 1982). Previous research has found decreased expression of dynorphin and the KOR in rodents known to consume high levels of ethanol, as compared to their low-drinking counterparts (Fadda et al., 1999; Marinelli et al., 2000; Spanagel, 1996; Winkler and Spanagel, 1998). In addition, activation of the KOR by systemic treatment with the agonist U50,488H significantly reduces ethanol drinking in rats under a two-bottle choice paradigm (Lindholm et al., 2001), while blockade of the KOR increases ethanol intake (Mitchell et al., 2005). In addition, mutations in the human dynorphin and KOR genes have been associated with the development of alcohol addiction (Xuei et al., 2006). Recently we demonstrated that dynorphin is a downstream effector of BDNF in the regulation of ethanol intake (Logrip et al., 2008). Specifically, we found that acute ethanol treatment of striatal neurons increases the expression of preprodynorphin, the mRNA precursor of dynorphin, via a BDNF-dependent pathway, and activation of the KOR by dynorphin is required for the reduction of two-bottle choice ethanol drinking by RACK1-mediated increases in BDNF expression (Logrip et al., 2008). Taken together, these data suggest a modulatory role for dynorphin over ethanol drinking, in which the dynorphin/KOR system functions to reduce ethanol drinking.
Contrary to this hypothesis, blockade of the KOR causes a decrease in ethanol intake in rats that are physiologically dependent on ethanol, an effect not observed in nondependent rats (Walker and Koob, 2007). One possible explanation for this discrepancy involves an alteration in dynorphin tone in ethanol withdrawal, which has been shown to increase prodynorphin expression (Przewlocka et al., 1997). Indeed, induction of a dysphoric state following extensive drug intake has been suggested as one mechanism driving compulsive drug use (Koob et al., 2004), resulting in an alteration of the reward value of the drug. These data, taken together with the opposing function of the KOR system in regulating ethanol intake in the absence of dependence, suggest that the system may play an integral role in modulating the hedonic properties of ethanol and thereby regulating ethanol intake. However, ethanol consumption is a complex behavior which may be modulated by multiple factors (e.g. Koob, 2003; Samson and Czachowski, 2003; Weiss and Porrino, 2002), and as such pharmacologically-induced reductions in ethanol intake may indicate either a lower motivation to consume due to decreased reward value or conversely an elevated reward value yielding a lower quantity of ethanol required to achieve the same total level of reward. Thus a direct determination of the directionality of KOR-mediated modulation of ethanol reward would inform significantly on this discrepancy.
One mechanism for investigating the role of the KOR system in modulating the rewarding effects of ethanol is conditioned place preference (CPP), a Pavlovian conditioning paradigm in which the preference for either of two distinct environments – one paired with the drug and one unpaired –is compared during a drug-free test (reviewed in Cunningham et al., 2006; Tzschentke, 1998). KOR agonists, when administered at sufficient doses, result in conditioned aversion to drug-paired environments (Iwamoto, 1985; Shippenberg and Herz, 1986). Activation of the KOR has also been shown to blunt place preference to both morphine and cocaine (Funada et al., 1993; McLaughlin et al., 2006a; Pliakas et al., 2001; Shippenberg et al., 1996; Suzuki et al., 1992; Zhang et al., 2004a, b), while generation of dysphoric states by pre-exposure to stressors results in the opposite effect, that is, enhanced CPP (Matsuzawa et al., 1999; McLaughlin et al., 2006a; McLaughlin et al., 2006b; McLaughlin et al., 2003). These CPP modulations (reduction vs. enhancement) correlate with the effect of KOR activity on ethanol intake in nondependent (Lindholm et al., 2001; Logrip et al., 2008; Mitchell et al., 2005) and dependent (Walker and Koob, 2007) animals, respectively. Together these data suggest a bimodal regulation of ethanol intake via modulation of the rewarding properties of ethanol, relative to the basal hedonic state of the animal. Importantly, these data suggest that in nondependent animals, ethanol reward may be directly regulated by activation of the KOR. Therefore, we hypothesized that activation of the KOR, at a level that does not generate an aversive state, would directly interfere with the acquisition of conditioned ethanol-environmental associations, thereby demonstrating the role of the KOR system in regulating ethanol reward.
Methods
Reagents
trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide methanesulfonate salt (U50,488H) was obtained from Sigma Aldrich (St. Louis, MO). Physiological saline (0.9% sodium chloride) was obtained from Hospira, Inc. (Lake Forest, IL).
Animals
DBA/2J (DBA) mice (6-8 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, ME), and experimental procedures began 1 week after arrival. Mice were group housed, 4 per cage, under a 12 h light/dark cycle, with lights on at 7:00 a.m. and off at 7:00 p.m., and were provided with continuous ad libitum access to food and water. All animal procedures were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council (1996).
Place Conditioning Apparatus
Conditioned place preference experiments were conducted in a 3-chambered place conditioning apparatus housed in a light- and sound-attenuating chamber (Med Associates, Inc., St. Albans, VT). Two 6.6″ × 5″ conditioning chambers – one white with a stainless steel mesh floor and the other black with a stainless steel rod floor – were connected by a smaller (2.85″ × 5″) central access chamber with gray walls and a smooth PVC floor. In addition, each chamber was equipped with a house light, with the luminance adjusted such that the sum total of the environmental (visual and tactile) cues should not produce a significant baseline preference across all mice for a specific chamber. All chambers were separated by automated guillotine doors and equipped with photobeams to measure locomotor activity. The central chamber was consistently used as the point of entry for the animal, with the disruption of center chamber photobeams triggering door opening and, when necessary to confine the mouse to an individual chamber, interruption of two consecutive photobeams in the conditioning chamber causing the guillotine doors to close. All chamber activity, as determined by photobeam breaks, was recorded by a computer using Med Associates software.
Place Conditioning Paradigm
The place conditioning procedure was conducted over 10 consecutive days, during which time mice were trained under a Pavlovian conditioning paradigm to associate the physiologic effects of drug treatment (the unconditioned stimulus) with a specific conditioning chamber (the conditioned stimulus). The alternate conditioning chamber was paired with saline such that mice were equivalently exposed to both chambers but only one was explicitly paired with ethanol (reviewed in Cunningham et al., 2006; Tzschentke, 1998). On the first day, mice were placed in the central chamber, both doors were opened and the mice were allowed free access to all three chambers for the duration of a 30-minute habituation session, referred to as the pretest. The next eight days comprised the conditioning sessions, with one session run daily. On conditioning days 2, 4, 6 and 8, mice were injected with drug or vehicle and immediately confined in the appropriate conditioning chamber for 5 minutes. On alternate days (conditioning days 3, 5, 7 and 9), the treatment condition and conditioning chamber were reversed. On the tenth, day, mice were placed in the central chamber and allowed free access to all chambers for the duration of a 30-minute test session. Both pretest and test sessions were performed in a drug-free state, and total time spent in each chamber, as well as locomotor activity within each chamber, as measured by number of photobeam breaks, were recorded.
Ethanol Place Conditioning
DBA mice underwent place conditioning training as described above, with the following parameters. Mice received two intraperitoneal injections on each conditioning day. On drug treatment days, mice were injected first with U50,488H (1 or 3 mg/kg of a 0.1 or 0.3 mg/mL solution, respectively, in saline) or saline, followed 10 minutes later by a second injection of ethanol (2 g/kg of a 20% (v/v) solution of ethanol in saline) or saline, as determined by a given animal's drug group assignment. On saline treatment days, mice were injected twice, 10 minutes apart, with 0.9% saline at volumes equivalent to those used for the drug injections. Mice were placed in the central access chamber immediately after the second injection and confined in the appropriate conditioning chamber for 5 minutes, followed by immediate replacement in the home cage. Drug treatment day, drug-paired chamber and drug-paired chamber-by-treatment-day were assigned randomly prior to the start of the experiment and counterbalanced across the treatment groups.
Data Analysis
Raw chamber times were analyzed by three-way ANOVA as described in the text, followed by two-way ANOVA by treatment group to further investigate significant interactions. Chamber times were also arithmetically transformed to yield the CPP score, which expresses preference for a given chamber after place conditioning as the difference in time spent in the drug-paired chamber during test as compared to pretest (CPP Score = paired(test) – paired(pretest)). Drug-paired chamber activity data were analyzed using repeated measures ANOVA. In addition, drug-paired chamber activity data were transformed as above to yield an Activity Score (Activity Score = activity in paired(test) – activity in paired(pretest)). Both CPP score and activity score were analyzed by one-way ANOVA. Student-Neuman-Keuls comparisons were used where appropriate as a post-hoc determinant of significance. Correlations between CPP Score and Activity Score were calculated using the Pearson Product-Moment statistic including all treatment groups with significant measures for either score, namely ethanol and ethanol + 3 mg/kg U50,488H.
Results
Acquisition of ethanol CPP is blocked by a low dose of the KOR agonist U50,488H
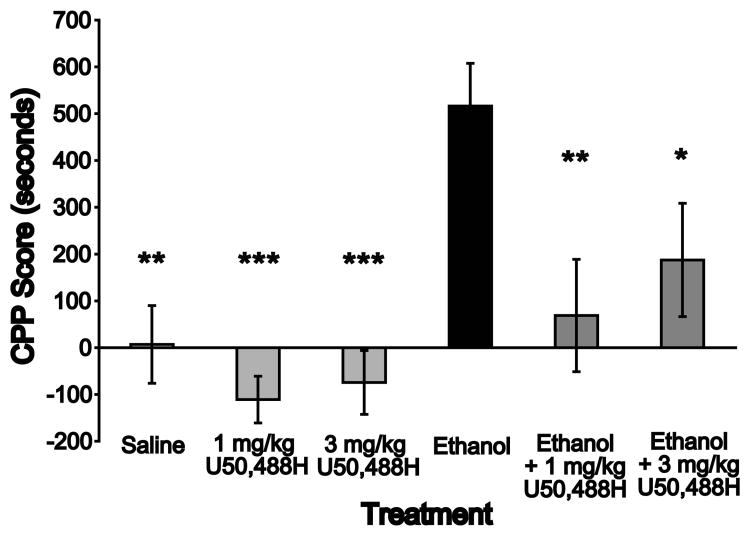
Previously we demonstrated that ethanol, via BDNF, increases expression of preprodynorphin, the mRNA precursor of the dynorphin polypeptide, the endogenous ligand for the kappa opioid receptor (Logrip et al., 2008). We proposed that this upregulation of dynorphin by ethanol may serve as a source of feedback to regulate subsequent ethanol intake. One mechanism by which dynorphin, via activation of the KOR, might decrease ethanol intake could involve a direct inhibition of ethanol reward. We hypothesized that activation of the KOR would reduce the rewarding properties of ethanol and thus block the acquisition of ethanol CPP. To test this, we performed place conditioning studies in which DBA mice were conditioned for 5 minutes in a drug-paired chamber in the presence of saline or ethanol (2 g/kg, i.p.), with administration of the KOR agonist U50,488H (1 or 3 mg/kg, i.p.) or saline 10 minutes prior. On alternate days, mice received two injections of saline, spaced 10 minutes apart, followed by placement in the unpaired chamber. As shown in Figure 1, mice that were administered ethanol developed a significant preference for the ethanol-paired chamber, and acquisition of this preference was completely blocked by co-administration of either dose of U50,488H. Analysis of CPP score, which measures changes in preference for the drug-paired chamber over training, by one-way ANOVA showed a main effect of Treatment (Fig. 1, F(5,42) = 6.19, p < 0.001). Post-hoc analyses confirmed that these differences were entirely due to increased preference in the ethanol group as compared to all other treatment groups (p's < 0.05) and there were no differences among other treatment groups (all p's > 0.17). This was not due to pre-existing chamber biases in any treatment group, since all groups spent equivalent time in both conditioning chambers during the pretest session, as shown in Table 1. Analysis of raw chamber times during the pretest session by two-way repeated measures ANOVA demonstrated main effects of Treatment (F(5,126) = 10.08, p < 0.001) and Chamber (F(2,126) = 3.64, p < 0.05) with no interaction (F(10,126) = 1.39, p = 0.20), due to elevated time spent in the central chamber by the ethanol + 3 mg/kg U50,488H treatment group (p < 0.001 vs. both paired and unpaired chambers). Importantly, no differences were found among the treatment groups in time spent in either the paired (all p's > 0.26) or the unpaired (all p's > 0.40) chambers, indicating no pre-existing bias for either chamber. Additional analyses of raw chamber times by three-way ANOVA with Day as a repeated measure confirmed that the statistical findings for CPP Score were not an artifact of the mathematical transformation used to obtain the score. Specifically, the tests showed no between-subjects effects, but significant interactions between Day and Chamber (F(2,126) = 5.729, p < 0.005) as well as a three-way interaction between Treatment, Chamber and Day (F(10,126) = 5.422, p < 0.0001). To determine the factors influencing these interactions, effects of Day and Chamber within each treatment were analyzed by two-way repeated measures ANOVA, uncovering main effects of chamber in the ethanol treatment group (F(2,14) = 5.84, p < 0.05), due to significant differences between the ethanol-paired chamber and both other chambers (p's < 0.05). In addition, significant interactions between Chamber and Day were found for both the ethanol and the ethanol + 3 mg/kg U50,488H treatment groups. For the ethanol-trained group pretreated with 3 mg/kg U50,488H, this effect was due to a decrease in time spent in the central chamber on test day (p < 0.001), that is, a reversion of the elevation in central chamber time observed during the pretest session. Importantly, the interaction observed for the ethanol treatment group was due to elevated time spent in the ethanol-paired chamber on the test day (p < 0.001) as well as to a decrease in time spent in the central chamber on test day (p < 0.001), as compared to pretest. In addition, time spent in the ethanol-paired chamber was significantly higher than time spent in either of the other chambers on test day. Together these analyses indicate that the interactions observed in the three-way ANOVA resulted from increased time spent in the ethanol-paired chamber by the ethanol treatment group on test day, as well as from decreased time spent in the central chamber by both the ethanol and the ethanol + 3 mg/kg U50,488H treatment groups. Importantly, the lack of change in time spent in the paired chamber for any treatment group other than ethanol confirms that while neither dose of U50,488H generates aversion, both block the formation of preference for the ethanol-paired chamber. Main effects of Day were also found for four of the six treatment groups (1 mg/kg and 3 mg/kg U50,488H, ethanol and ethanol + 1 mg/kg U50,488H, F's >, p's < 0.05); however, post-hoc analyses showed no significant differences for this factor (all p's > 0.12). Together, these results show that ethanol place conditioning produces a robust CPP which can be blocked by concurrent activation of the KOR by low, nonaversive doses of U50,488H.
Figure 1. Activation of the kappa opioid receptor blocks acquisition of ethanol conditioned place preference.
DBA mice were treated with first with saline or U50,488H (1 or 3 mg/kg, i.p.), followed 10 minutes later by treatment with saline or ethanol (2 mg/kg, i.p.) and immediate confinement in the drug-paired chamber on drug conditioning days, or similar treatments with saline only followed by confinement in the unpaired chamber on alternate days. Mice acquire a preference for the ethanol-paired chamber, an effect blocked by pretreatment with either dose of U50,488H during acquisition. Data are expressed as mean +/- S.E.M. CPP Score (difference between time spent in the drug-paired chamber during the 30-minute drug-free test as compared to the same chamber during the 30-minute drug-free pretest period). * p < 0.05, ** p < 0.005, *** p < 0.001 versus ethanol. n = 8 per treatment group.
Table 1. Average time spent per chamber for each treatment group during the pretest and test sessions.
| Treatment | Pretest | Test | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Drug | Center | Saline | Drug | Center | Saline | |
| Saline | 606.92 +/-79.18 | 633.12 +/-42.61 | 559.96 +/-58.48 | 613.76 +/-103.84 | 644.81 +/-81.79 | 541.43 +/-105.09 |
| 1 mg/kg U50,488H | 653.61 +/-87.52 | 544.13 +/-52.51 | 602.25 +/-84.98 | 542.66 +/-103.36 | 661.88 +/-84.92 | 595.46 +/-117.15 |
| 3 mg/kg U50,488H | 608.19 +/-64.22 | 653.45 +/-60.26 | 538.36 +/-56.68 | 533.77 +/-97.00 | 677.93 +/-89.81 | 588.30 +/-116.12 |
| Ethanol | 590.74 +/-64.74 | 646.62 +/-60.26 | 562.64 +/-66.09 | 1107.02 +/-124.66***, b | 299.09 +/-36.33 b | 393.90 +/-120.55 |
| Ethanol + 1 mg/kg U50,488H | 571.92 +/-64.02 | 668.76 +/-37.53 | 559.31 +/-65.69 | 640.58 +/-157.12 | 647.11 +/-110.47 | 512.31 +/-132.15 |
| Ethanol + 3 mg/kg U50,488H | 464.21 +/-71.16 | 878.36 +/-104.28 | 457.43 +/-70.37 | 651.67 +/-154.53 | 590.89 +/-128.44 a | 557.44 +/-151.49 |
Results are presented as mean +/- S.E.M seconds spent in each chamber over the 30-minute sessions.
p < 0.001 vs. other chambers within the same treatment and day.
p < 0.05,
p < 0.001 vs. the same chamber on pretest day
Conditioned increase in locomotor activity in the ethanol-paired chamber is blocked by U50,488H
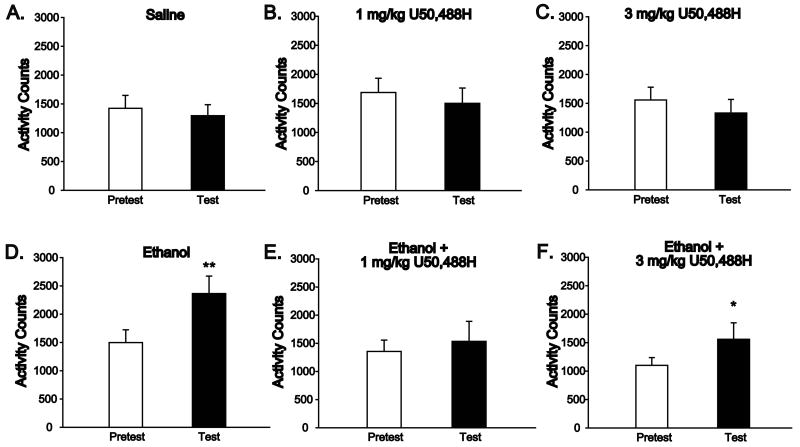
The above data demonstrate that pretreatment with the KOR agonist U50,488H inhibits acquisition of ethanol conditioned place preference. One feature of Pavlovian conditioned responses to a drug-paired environment is elevated locomotor activity within the conditioned environment, which has previously been demonstrated in the conditioned chamber following ethanol CPP training (Cunningham and Noble, 1992). Thus we hypothesized that mice in the ethanol treatment group would demonstrate higher activity levels in the drug-paired chamber during test, while mice conditioned with ethanol in the presence of U50,488H would not display increased locomotor activity, as we had observed no increase in time spent in the ethanol-paired chamber in this group. As shown in Figure 2, ethanol place conditioning resulted in increased locomotor activity in the ethanol-paired chamber, which was not observed in ethanol-naïve groups. Analysis of locomotor activity counts during the 30-minute pretest and test sessions by one-way ANOVA with Day as a repeated factor confirmed that place conditioning resulted in increased locomotor activity in the ethanol-paired chamber for the ethanol treatment group, with a main effect of Day (Fig. 2D, F(1,7) = 15.88, p < 0.01), while no effects were observed for the saline and U50,488H control groups (Fig. 2A-C, all F's < 3.78, all p's > 0.09). These data demonstrate that the Pavlovian conditioned response to the ethanol-paired chamber triggers elevated locomotor activity on test day, an effect specific to ethanol-conditioned subjects.
Figure 2. Ethanol place conditioning increases locomotor activity in the ethanol-paired chamber, an effect which is blocked by U50,488H pretreatment during acquisition.
DBA mice were treated with saline or U50,488H (1 or 3 mg/kg, i.p.), followed 10 minutes later by saline or ethanol (2 mg/kg, i.p.) on drug conditioning days. (A-F) Mean +/- S.E.M. locomotor activity during the pretest (white bars) and test (black bars) sessions for all treatment groups. (AC) Mice in the saline (A), 1 mg/kg U50,488H (B) and 3 mg/kg U50,488H (C) control groups show no change in locomotion following place conditioning training. n = 8 per treatment group. (D) Ethanol conditioning results in increased locomotor activity in the ethanol-paired chamber on test day, as compared to pretest day. ** p = 0.005 vs. pretest. n = 8 per treatment group. (E-F) Administration of 1 mg/kg (E) or 3 mg/kg (F) U50,488H prior to ethanol treatment blocks the conditioned increase in locomotor activity seen with ethanol alone. n = 8 per treatment group.
Next we tested whether pretreatment with U50,488H, which blocked the acquisition of ethanol place preference as measured by time spent in the conditioned chamber, also inhibited increases in locomotor activity in the ethanol-paired chamber on test day. Analyses by one-way ANOVA confirmed that mice pretreated with U50,488H prior to ethanol conditioning sessions displayed no conditioned locomotor activation on test day, as no significant effects of Day were observed for either the 1 mg/kg (Fig. 2E, F(1,7) = 0.70, p = 0.43) or 3 mg/kg (Fig. 2F, F(1,7) = 4.67, p = 0.07) dose. Together these data demonstrate that U50,488H pretreatment during acquisition blocks the increase in locomotor activity in the ethanol-conditioned chamber normally observed on test day.
To further assess ethanol-induced conditioned locomotor responding and its blockade by U50,488H, activity counts for the drug-paired chamber were transformed to generate an activity score, indicating the difference in activity counts between test and pretest. Analysis by one-way ANOVA revealed a significant increase in locomotor activity following place conditioning, with a main effect of Treatment (F(5,42) = 5.46, p < 0.001). Post-hoc comparisons demonstrated a significant difference between ethanol and all treatments (all p's < 0.005) except ethanol + 3 mg/kg U50,488H (p = 0.13) and no differences between other treatments (all p's > 0.08), including the group treated with ethanol + 1 mg/kg U50,488H (p = 0.25 compared to saline control, p = 0.35 compared to 1 mg/kg U50,488H alone) and the group treated with 3 mg/kg U50,488H (p = 0.08 compared to saline control, p = 0.09 compared to 3 mg/kg U50,488H alone). It should be noted that while both doses of U50,488H blocked ethanol place preference, that inhibition was more significant for the lower dose of U50,488H (p = 0.004) than for the higher dose (p = 0.016). As we found high correlation between the CPP score and activity score measures for the ethanol and ethanol + 3 mg/kg U50,488H treatment groups (R = 0.84, p < 0.0001), and 3 mg/kg U50,488H less effectively prevented place conditioning to ethanol, this suggests that the higher dose of U50,488H may lie on the descending limb of the dose-response curve. Nonetheless, these results demonstrate that low, non-aversive doses of U50,488H block the development of ethanol CPP such that neither the duration of time spent nor the locomotor activity in the ethanol-paired chamber are altered by conditioning, suggesting that U50,488H functions to directly inhibit the conditioned rewarding properties of ethanol.
Discussion
Here we demonstrate that non-aversive doses of U50,488H administered 10 minutes prior to ethanol block the acquisition of ethanol CPP, as measured both by time spent and conditioned locomotor activity in the drug-paired chamber. These results indicate that pretreatment with U50,488H on ethanol conditioning days blocks ethanol reward and thus inhibits the acquisition of ethanol CPP.
Modulation of CPP by the KOR system
Previous reports have demonstrated KOR-dependent inhibition of CPP to multiple drugs of abuse, whether by direct application of KOR agonists (Funada et al., 1993; McLaughlin et al., 2006a; Shippenberg and Herz, 1986; Shippenberg et al., 1996; Shippenberg et al., 1998; Suzuki et al., 1992; Zhang et al., 2004a, b) or following genetic manipulations upregulating the endogenous KOR ligand dynorphin (Pliakas et al., 2001). As the KOR agonist doses used in these studies produce a significant conditioned place aversion (Funada et al., 1993; Iwamoto, 1985; Suzuki et al., 1992), it is perhaps not surprising that they inhibit cocaine and morphine CPP, although it should be noted that not all compounds generating aversive responses can functionally antagonize drug-induced place preference (Suzuki et al., 1992). To our knowledge, though, this is the first study demonstrating a direct inhibition of drug reward by KOR activation at nonaversive doses, indicative of a direct modulation of ethanol reward by the KOR system. However, the possibility still exists that the low doses of KOR agonist produce a mild aversion to counteract ethanol reinforcement on conditioning days which may, on its own, be insufficient to produce conditioned place aversion in the control groups, as a similar low dose of U50,488H has been used to condition odorant aversion in C57BL/6 mice (Land et al., 2008). Alternatively, activation of the KOR may block the acquisition of ethanol place preference by preventing memory formation, as direct infusion of dynorphin B into the hippocampus inhibits spatial learning (Sandin et al., 1998). However, given the ability of a range of KOR agonist doses to condition place aversion (McLaughlin, 2006 #537; Pliakas, 2001 #570; Funada, 1993 #803; Suzuki, 1992 #776; Bals-Kubik, 1989 #610), direct impairment of the memories underlying place conditioning seems an unlikely explanation. Regardless, further investigation into the mechanism of action by which U50,488H prevents the acquisition of ethanol place conditioning, perhaps via blockade of ethanol reward, as well as the affective consequences of the low doses of U50,488H used herein, present important future lines of study.
Interestingly, activation of the KOR does not categorically reduce place preference to drugs of abuse. In particular, pretreatment with behavioral stressors – such as forced swim tests or fear conditioning – actually potentiate cocaine CPP (McLaughlin et al., 2006a; McLaughlin et al., 2003) via a KOR-dependent mechanism. McLaughlin and colleagues have demonstrated that U50,488H alters cocaine CPP in a time-dependent bimodal fashion, with short intervals between U50,488H and cocaine treatment blocking acquisition of CPP and increasing delay between treatments resulting in enhancement, rather than blockade, of cocaine CPP (McLaughlin et al., 2006a). Fear conditioning administered 24 h prior to ethanol place conditioning sessions similarly potentiated ethanol CPP in rats (Matsuzawa et al., 1998, 1999). Blocking the development of stress-induced dysphoria by pretreatment with the KOR antagonist nor-BNI concomitantly prevented the stress-induced enhancement of cocaine CPP (McLaughlin et al., 2006a; McLaughlin et al., 2006b; McLaughlin et al., 2003), Taken together, these results indicate that the KOR system can acutely inhibit the development of reward associations for multiple drugs of abuse, including ethanol, but that an overactivation of this system, for instance following stressful experiences, may induce a dysphoric state, thus enhancing the rewarding properties of drugs encountered subsequent to the onset of dysphoria.
The KOR system and regulation of ethanol's rewarding properties
The bimodal regulation of drug reward by the KOR system mirrors its ability to regulate ethanol intake in dependent and nondependent rats (Lindholm et al., 2001; Walker and Koob, 2007). Thus, while activation of the KOR attenuates ethanol intake in nondependent rats (Lindholm et al., 2001), the development of ethanol dependence confers a reversal in the functionality of this receptor system to regulate ethanol intake, whereby the KOR antagonist nor-BNI significantly decreases operant ethanol self-administration only in ethanol-dependent rats (Walker and Koob, 2007). Together with the data reported herein, these results suggest an important role for the KOR system in the regulation of ethanol reward, thereby exerting control over ethanol self-administration (Lindholm et al., 2001; Walker and Koob, 2007). Thus the KOR and its endogenous ligand dynorphin provide possible targets for the development of medications not only to treat alcoholism but also to prevent its development. Further research to expand the understanding of the transition from KOR depression of ethanol intake to its promotion of compulsive drinking would be of great interest for therapies to combat alcohol addiction.
Acknowledgments
The authors would like to thank Drs. Cory Blaiss and Elyssa Margolis for critical reading of this manuscript. This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grant F31 AA015462 (M.L.L.) and by the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (P.H.J and D.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Fadda P, Tronci S, Colombo G, Fratta W. Differences in the opioid system in selected brain regions of alcohol-preferring and alcohol-nonpreferring rats. Alcohol Clin Exp Res. 1999;23:1296–1305. [PubMed] [Google Scholar]
- Funada M, Suzuki T, Narita M, Misawa M, Nagase H. Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology. 1993;32:1315–1323. doi: 10.1016/0028-3908(93)90026-y. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Place-conditioning properties of mu, kappa, and sigma opioid agonists. Alcohol Drug Res. 1985;6:327–339. [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res. 1998;803:169–177. doi: 10.1016/s0006-8993(98)00679-9. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta-and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999;368:9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/s0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Thompson AC. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol. 1998;345:27–34. doi: 10.1016/s0014-2999(97)01614-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R. The influence of opioid antagonists on the discriminative stimulus effects of ethanol. Pharmacol Biochem Behav. 1996;54:645–649. doi: 10.1016/0091-3057(95)02288-0. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu-and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A, Spanagel R. Differences in the kappa opioid receptor mRNA content in distinct brain regions of two inbred mice strains. Neuroreport. 1998;9:1459–1464. doi: 10.1097/00001756-199805110-00039. [DOI] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, Goate A, Bucholz K, Schuckit M, Nurnberger J, Jr, et al. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004a;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004b;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]