Abstract
Contemporary research has revealed a great deal of information on the behaviours of microtubules that underlie critical events in the lives of neurons. Microtubules in the neuron undergo dynamic assembly and disassembly, bundling and splaying, severing, and rapid transport as well as integration with other cytoskeletal elements such as actin filaments. These various behaviours are regulated by signalling pathways that affect microtubule-related proteins such as molecular motor proteins and microtubule severing enzymes, as well as a variety of proteins that promote the assembly, stabilization and bundling of microtubules. In recent years, translational neuroscientists have earmarked microtubules as a promising target for therapy of injury and disease of the nervous system. Proof-of-principle has come mainly from studies using taxol and related drugs to pharmacologically stabilize microtubules in animal models of nerve injury and disease. However, concerns persist that the negative consequences of abnormal microtubule stabilization may outweigh the positive effects. Other potential approaches include microtubule-active drugs with somewhat different properties, but also expanding the therapeutic toolkit to include intervention at the level of microtubule regulatory proteins.
Keywords: axon, dendrite, microtubule, neuron, taxol
Introduction
Decades of research on neuronal microtubules have yielded an extensive body of knowledge on the behaviours of microtubules that underlie the growth and maintenance of the axon, the development and plasticity of the dendritic arbours, and the migration of developing neurons to their destinations (Conde and Caceres, 2009). Microtubules undergo dynamic assembly and disassembly, bundling and splaying, severing, and rapid transport as well as other manifestations of forces imposed upon them (Baas and Buster, 2004). They also integrate at many levels with other cytoskeletal elements, most notably actin filaments (Myers and Baas, 2011). These various behaviours are regulated by signalling pathways that affect microtubule-related proteins such as molecular motor proteins, microtubule severing enzymes, microtubule depolymerizing enzymes as well as a variety of proteins that promote the assembly, stabilization and bundling of microtubules. In recent years, translational neuroscientists have earmarked microtubules as a promising target for therapy of nerve injury, neurodegenerative diseases, and cancer of the nervous system. Proof-of-principle that pharmacological stabilization of microtubules can have positive benefits on all of these conditions has come from studies using taxol, the most potent and well-defined microtubule-stabilizing drug, as well as its derivatives and analogues.
The theory behind microtubule-stabilizing drugs as therapy for the nervous system is that stabilization will prevent the microtubule array from degrading in the face of disease and injury, and may even fortify the microtubule array in a manner conducive to repair and regeneration. Not unexpectedly, however, the positive benefits do not come without potential risks. The dynamic nature of the microtubule array is quintessential to its normal array of functions, and tight regulation over when and where microtubule assembly, nucleation, stabilization and de-stabilization occur are key to much of the work the microtubule array needs to do, during adult life as well as development. Advocates for the use of taxol and related compounds have argued that potential negative effects may be avoidable or at least minimized by using very low concentrations of the drug, by targeting it to where it is needed, limiting exposure to discrete frames of time and/or exploiting the somewhat different properties of available microtubule-stabilizing drugs (Michaelis et al., 2006; Brunden et al., 2011; Sengottuvel and Fischer, 2011; Shemesh and Spira, 2011; Ballatore et al., 2012; Das and Miller, 2012). This may well be correct, and certainly if a drug produces favourable results, it should be exploited to its full benefit.
Taxol has been given to cancer patients for many years as the drug of preference, especially for breast cancer. Given systemically at cancer-reducing dosages, taxol and related drugs produce painful and often debilitating peripheral neuropathies (Mielke et al., 2006; Scripture et al., 2006; Reyes-Gibby et al., 2009). This being the case, there is concern that even very low concentrations of the drug used to treat nerve injury or disease might ultimately cause negative effects in nervous tissue, even in situations where the effects initially appear to be positive. Other microtubule-stabilizing drugs with somewhat different properties are already under consideration, and these may offer notable improvements over taxol. Moreover, the knowledge now available on neuronal microtubules and the plethora of their behaviours and regulatory pathways indicates that more sophisticated and/or subtle interventions should theoretically be possible, without the associated risks of abnormally stabilizing microtubules.
Here we discuss the results from several recent studies using taxol and related drugs in experimental models of nerve injury and disease. We note the positive effects, but also raise potential concerns to be considered or overcome if this approach is to be translated into the clinic. In addition, we ponder strategies that exploit microtubules in other ways as a means to enhance nerve regeneration and preserve neurons against degeneration. In so doing, we hope to shine new light on microtubules as a potentially powerful target for the focus of therapies for patients suffering from injuries or disease of the nervous system.
Microtubule dynamics and stabilization
Microtubules are dynamic polymers composed of tubulin subunits, each of which is a dimer of alpha and beta tubulin. The dynamics of microtubule populations are governed by a mechanism known as dynamic instability (Kirschner and Mitchison, 1986). In brief, this mechanism depends upon the fact that free tubulin exists with GTP rather than GDP associated with beta tubulin. The hydrolysis of GTP-tubulin to GDP-tubulin occurs only after the tubulin has been incorporated into a polymer. Hence, the older region of the microtubule toward the minus end will be richer in GDP-tubulin than the newer region of the microtubule toward the plus end. If GTP hydrolysis stochastically catches up to the addition of new subunits such that there is no longer a ring of GTP-tubulins at the plus end, the microtubule undergoes rapid catastrophic disassembly. As long as there is GTP-tubulin forming a ‘cap’ at the plus end of the microtubule, it will keep assembling. Because of the stochastic nature of this mechanism, different microtubules within a population will simultaneously undergo either assembly or disassembly. In living cells, the most dynamic microtubules can and do display dynamic instability events, with rapid bouts of assembly and disassembly. However, another feature of the model is also at play in living cells and this is called selective stabilization. Microtubules can be stabilized either by the capture of their plus ends, for example by proteins and structures in the cell cortex, or by binding along the length of the microtubule of stabilizing proteins. When a microtubule or a region of a microtubule is stabilized, it may still undergo subunit exchange with the soluble tubulin pool, but such exchange is slow compared to the rapid bouts of assembly and catastrophe characteristic of dynamically unstable microtubules.
Taxol binds to beta tubulin in a pocket on the luminal surface of the microtubule and counteracts the effects of GTP hydrolysis taking place on the other side of the beta tubulin molecule (Amos and Löwe, 1999; Prota et al., 2013). In so doing, the drug suppresses the disassembly of the polymer and thereby promotes its assembly. Different concentrations of taxol can be used, as determined empirically for given situations, that can slow disassembly, completely prohibit disassembly, or promote microtubule assembly. Typically, such concentrations would be in the nanomolar or low micromolar range. With increasing concentrations, taxol can exhibit some effects not readily explained only by its mechanism described above. Taxol can result in dense bundling of microtubules for example, which often consist of very short individual microtubules within the bundle. Bundling of microtubules by taxol appears to be dependent upon recruiting other factors in cells, as the affect is not observed with purified tubulin (Turner and Margolis, 1984).
Interestingly, drugs that promote microtubule depolymerization at higher concentrations can ‘kinetically stabilize’ microtubules when used at very low concentrations (Jordan, 2002). These drugs, such as nocodazole and vinblastine, interact with free tubulin subunits and lower their capacity to assemble onto the microtubule polymer. This effectively decreases the concentration of free tubulin in solution available to participate in microtubule dynamics, thus shifting the balance between polymer and free subunits toward depolymerization. However, very low concentrations of such drugs can hold the balance steady, thus curtailing both assembly and disassembly of the polymer, and hence kinetically stabilizing it. Microtubules stabilized in this fashion still undergo some level of subunit exchange, and therefore are not stabilized in the same way as taxol-stabilized microtubules. The ongoing search for drugs that affect microtubules in various ways is driven in large part by the need for new and better approaches for cancer therapy. With appropriate knowledge of their mechanism of action, any of these drugs could have affects conducive to recovery of the nervous system from injury or disease with perhaps more favourable properties than taxol.
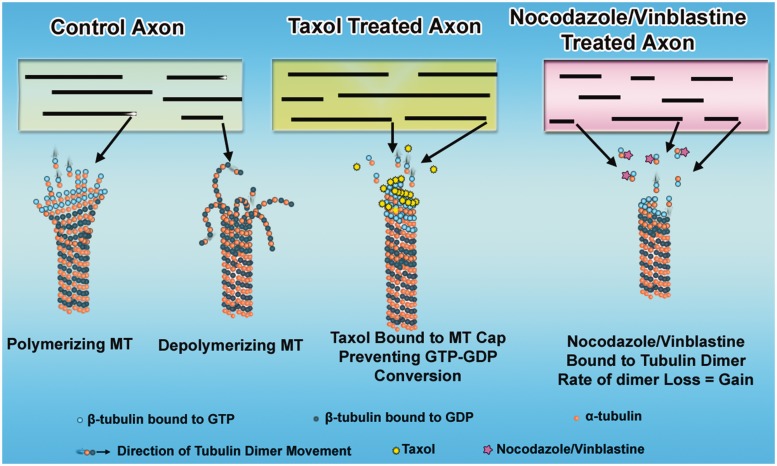
Figure 1 schematically illustrates a control axon as well as axons treated with either taxol or nanomolar levels of nocodazole or vinblastine. In the control axon, different microtubules in the population undergo either assembly or disassembly from their plus ends, according to the principles of dynamic instability. In axons treated with taxol, disassembly is prohibited such that microtubules continue to add subunits to their plus ends. In the case of the nocodazole or vinblastine, the microtubules are kinetically stabilized such that assembly and disassembly are roughly equal and the microtubules remain the same length.
Figure 1.
Effects of microtubule-active drugs on microtubule dynamics. Shown are a control axon and axons during the first moments of exposure to microtubule-active drugs. In the control axon, the microtubules (MT) display dynamic instability at their plus ends. In taxol-treated axons, the microtubules are stabilized (no longer lose subunits) and no longer show dynamic instability at their plus ends. Hence, they continue to assemble. In axons treated with low concentrations of microtubule depolymerizing drugs, the microtubules become ‘kinetically stabilized,’ which means they lose and gain subunits at the same rate, resulting in no length change.
Neuronal microtubules
Best known as the spindle fibres that compose the mitotic spindle of dividing cells, microtubules are equally critical for neurons. Vertebrate neurons in their post-migratory configuration generally consist of a single axon and multiple dendrites. These are elongated processes (often complex in their branching patterns) that require microtubules as shape-sustaining architectural struts. In addition, the microtubules of axons and dendrites serve as the major railways for organelle and other cargo transport, in both directions. Especially critical to their role in transport, the organization of microtubules in axons and dendrites is tightly regulated. In a typical vertebrate neuron, nearly all of the microtubules in the axon are oriented with their plus ends directed away from the cell body (Heidemann et al., 1981), whereas the dendrites have a mixed orientation of microtubules (Baas et al., 1988). We have recently discussed in detail how these patterns (illustrated in Fig. 2A) were discovered, and the contributions of these microtubule polarity patterns to the distinct morphological and compositional features that define the identity of the axon and the dendrites (Baas and Lin, 2011). Because different types of organelles engage either plus-end-directed or minus-end-directed molecular motor proteins, the polarity pattern of the microtubule array is a major determinant of which organelles/cargoes will be transported into each type of process from the cell body, as well as the efficiency and character of anterograde and retrograde organelle movements within the axon and dendrites. This simple scenario satisfactorily explains, for example, why Golgi outposts appear in dendrites but not the axon. Axons are long and thin and grow indefinitely (until they reach a target) because the nearly uniformly oriented microtubule array provides a unidirectional vector for membrane transport and addition to the axon’s tip, which is not the case for dendrites, which remain short and stout and taper with distance from the cell body without reaching any target.
Figure 2.
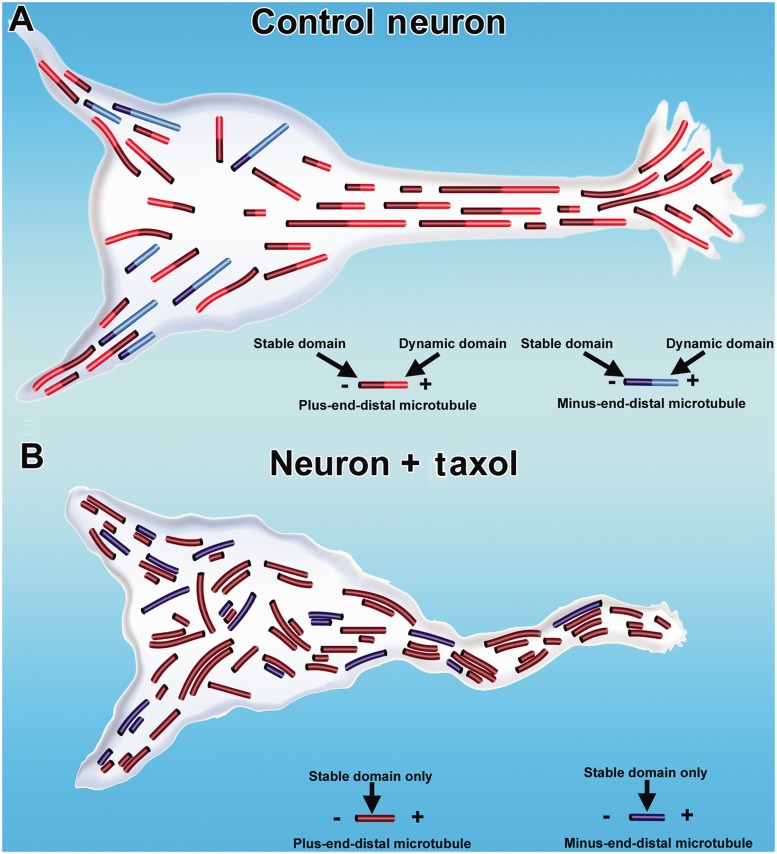
Effects of taxol on microtubule organization in axons and dendrites. In control neurons, microtubules are nearly uniformly plus-end-distal in the axon. In the dendrite, microtubules have a mixed orientation (A). Each microtubule consists of a stable domain toward the minus end of the microtubule, with most microtubules also consisting of a dynamic (labile) domain toward the plus end of the microtubule. Dendritic microtubules are less stable than axonal microtubules, as indicated by shorter stable domains on dendritic microtubules in the illustration. In neurons treated with taxol (B), the density of microtubules increases, the normal domain structure of individual microtubules is lost because the microtubules are stabilized all along their lengths, and flaws arise in the normal polarity patterns of the microtubules. Such abnormalities can lead to degeneration of axons and dendrites.
Neurons are often thought of as having very stable microtubules, with this being important for the maintenance of the stable wiring of the nervous system (Brady et al., 1984). In fact, although a fraction of the microtubule array of the axon is indeed quite stable, a fraction is not, with dendritic microtubules having an even lower fraction of stable microtubule polymer than axons (Baas et al., 1991). Younger developing neurons also have more labile microtubules in general than mature neurons, especially in the growth cones of their axons, in which highly dynamic microtubules are essential for normal pathfinding (Challacombe et al., 1997; Geraldo and Gordon-Weeks, 2009). Substantial evidence exists that the stable and labile microtubule fractions in the neuron are not separate microtubules but rather that each microtubule has a stable region toward its minus end with most of these microtubules extending a highly dynamic labile region from its plus end (Baas and Black, 1990; Brown et al., 1993). Because of this configuration (shown in Fig. 2A) and related experimental data, the stable region has been likened to a microtubule nucleating structure that controls the distribution and polarity orientation of the dynamic microtubule polymer (Baas and Ahmad, 1992). Without that level of control to restrict where new assembly arises, new microtubules would presumably arise with haphazard organization, thus corrupting the all-important microtubule polarity patterns of axons and dendrites. This applies just as well to the dendrite, because its normal pattern is mixed but it is not random (Baas et al., 1989).
All of this is relevant to the use of drugs that affect microtubule stabilization because such drugs also promote abnormal microtubule nucleation and assembly, and even subtle alterations in the microtubule polarity patterns of axons and dendrites could have profoundly negative consequences over time (Kuznetsov, 2010; Baas and Mozgova, 2012). Shown in Fig. 2B is a neuron treated with taxol, displaying abnormal accumulation and bundling of microtubules, loss of microtubule domain structure, and corruption of normal microtubule polarity patterns. Such negative effects potentially include traffic jams in organelle transport, and the mislocalization of organelles and proteins that should be enriched or exclusive to one type of process or the other. In fact, major changes in axonal microtubule polarity were recently reported in cultured neurons treated with taxol, and of particular concern, the abnormalities persisted even after washing out the drug (Shemesh and Spira, 2010). Thus, Fig. 2B also indicates degeneration of the neuron, as a result of the effect of taxol treatment.
In recent years, a powerful method has arisen for directly observing microtubule assembly events in living cells. This method exploits the fact that cells express a category of proteins called +tips, which interact with the plus end of the microtubule only when it undergoes a bout of rapid assembly (Akhmanova and Steinmetz, 2008). When fluorescent fusion proteins for +tips are expressed in cells, they appear as comet-shaped bursts of fluorescence at the plus end of a growing microtubule, with the tail of the comet directed toward the minus end of the microtubule. In cultured neurons and in vivo, it can be observed that all compartments of the neuron display these microtubule comets (Stepanova et al., 2003; Rolls, 2011), indicating that ongoing microtubule dynamics occur throughout the neuron and presumably throughout its entire life. Even very low levels of microtubule-active drugs can almost completely obliterate the appearance of these comets, even when there is no net loss of microtubule mass. This is important not only because a fraction of the microtubule array is normally dynamic, but also because the +tips are important in their own right, with myriad functions for axons and dendrites. For example, a +tip called EB3 is critically important for microtubules to interact with appropriate partner proteins in dendritic spines (Jaworski et al., 2009). There is reason for concern that microtubule-stabilizing drugs such as taxol could have negative effects on a variety of important events that depend on these +tips. For example, learning and memory have been associated with the normal functioning of dendritic spines and could be compromised if microtubules do not appropriately engage proteins in the spines.
Another aspect of microtubule regulation in neurons worthy of note is the accumulation on microtubules of certain tubulin post-translational modifications (Janke and Kneussel, 2010; Wloga and Gaertig, 2010; Janke and Bulinski, 2011; Garnham and Roll-Mecak, 2012). The best studied of these are detyrosination and acetylation, although others exist such as polyglutamylation. Both detyrosination and acetylation occur on the alpha tubulin component of the tubulin heterodimer and both occur only after a tubulin subunit is incorporated into microtubule polymer. Both of these modifications are induced by enzymes and both modifications are reversed by other enzymes after the tubulin subunit has been liberated from the microtubule due to depolymerization. Detyrosination is the removal of the C-terminal tubulin residue from alpha tubulin, whereas acetylation is the addition of an acetyl moiety to lysine 40 of alpha tubulin, which lies at the luminal surface of the microtubule polymer. Because these modifications only occur on the polymer, they accumulate with time on the microtubule, so that the older the microtubule is, the more modified it becomes. Other factors contribute, such as availability of the relevant enzymes, but the levels of the modified subunits are generally considered a good indication of the stability of the microtubule, although they do not cause stability, at least not directly.
These tubulin modifications had been known for decades, with no apparently function, although it was always logical to conclude that they must do something important because they are so tightly controlled by enzymatically-driven cycles that are conserved from primitive to complex organisms. Several excellent review papers have recently been published on these modifications (see above), so we will summarize the recent functional progress briefly to say that these modifications affect the lattice of the microtubule in such a way as to heighten or reduce its capacity to interact with a variety of microtubule-related proteins. For example, the microtubule-severing protein katanin interacts better with acetylated microtubules (Sudo and Baas, 2010), the depolymerizing kinesin family termed kinesin-13 interacts better with tyrosinated microtubules (Peris et al., 2009), and kinesin-1 (conventional kinesin) interacts better with detyrosinated microtubules (Dunn et al., 2008; Konishi and Setou, 2009; Hammond et al., 2010). As expected, the +tips interact better with unmodified tubulins, and this promotes the association of these proteins with rapidly growing plus ends of microtubules (Peris et al., 2006).
One of the most pronounced effects of taxol treatment is that the microtubules accumulate more post-translationally modified subunits, as would be expected with greater stability. This means that the microtubules would be richer in acetylated and detyrosinated tubulin and poorer in tyrosinated and unacetylated tubulin, which would notably change the proclivity of the microtubule to interact with various proteins such as mentioned above. This is not necessarily a bad thing, but it could be. For example, taxol might be applied to a degenerating axon to preserve the microtubule array against loss, but over time, the effect might actually be a greater loss of microtubule mass because the microtubules would become more acetylated and hence more sensitive to severing by katanin. Interestingly, and for unknown reasons, taxol treatment seems to augment the accumulation of modified subunits in microtubules even faster and more thoroughly than would be predicted on the basis of enhanced stability alone, which suggests that concern about this issue should be taken especially seriously.
It is also worth noting that dendritic microtubules are generally less stable and hence less rich in post-translationally modified tubulins compared with axonal microtubules (Baas et al., 1991) (Fig. 2A), and this plays a role in the normal regulation of motor-driven traffic in each type of process. For example, kinesin-1 strongly favours transport of cargo into the axon relative to the dendrite, due to the higher levels of detyrosinated tubulin in axonal microtubules (see references above). Treatment with taxol would presumably eliminate or at least lesson this normal difference between axons and dendrites, and hence disturb the normal mechanisms that direct traffic and thereby preserve neuronal polarity.
One of the oldest literatures on the neuronal cytoskeleton deals with the complement of fibrous microtubule-associated proteins that decorate microtubules in the different compartments of the neuron (Matus, 1988). These microtubule-associated proteins are expressed in generous levels, as they are straightforward to detect by blotting or immunocytochemistry. They are considered to promote microtubule assembly and stabilization, and many of them also promote microtubule bundling. Some caution is due, however, as such conclusions on their properties are drawn in large part from biochemical studies and/or overexpression studies (see Baas et al., 1994). Hence, there is still some mystery as to what these proteins do at physiological levels in cells. For example, the idea that loss of tau from axonal microtubules destabilizes them still lacks experimental support (Tint et al., 1998). It is known that the various microtubule-associated proteins have tightly controlled patterns of expression and also intracellular distribution in the neuron. These include traditional microtubule-associated proteins such as tau, MAP2 and MAP1B, as well as less traditional microtubule-associated proteins such as doublecortin and STOP (stable tubule-only peptide, now known as MAP6) (Slaughter and Black, 2003; Tint et al., 2009; Jean et al., 2012). As neurons develop, a juvenile isoform of MAP2 that is widespread in axons and dendrites is downregulated while the adult isoforms become dendrite-enriched and virtually absent from the axon (Dehmelt and Halpain, 2005). Tau, on the other hand, accumulates in axons because it must be phosphorylated at certain sites to bind microtubules, and this does not occur to any appreciable degree in dendrites. MAP1B tends to accumulate in the distal regions of growing axons (Black et al., 1994). All of the microtubule-associated proteins have multiple phosphorylation sites that regulate various properties, such as the extension outward of tau’s projection domain that is thought to contribute to microtubule-microtubule spacing (Gustke et al., 1994). Some of the microtubule-associated proteins also interact with actin, providing a potential link between microtubules and actin filaments (Myers and Baas, 2011). There is not an extensive literature on the effect of taxol on microtubule-associated protein association with microtubules, but some in vitro evidence exists that at least for some microtubule-associated proteins, taxol can affect the binding of the microtubule-associated protein to the microtubule lattice (Black, 1987; Ross et al., 2004; Lin et al., 2011a). This could be further exacerbated if the microtubule-associated proteins bind differentially to microtubules that are more or less rich in post-translationally modified subunits (see above). Thus, it is advisable to be wary of this when considering the use of taxol for therapeutic purposes.
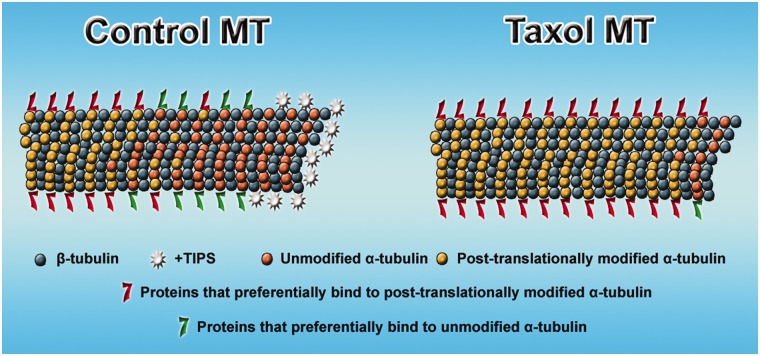
Figure 3 summarizes the effect of taxol treatment on microtubules, indicating not only cessation of dynamics, but a loss of the +tips from the plus end of the microtubule, an increase in acetylated and detyrosinated tubulin, and an alteration in the association of microtubule-related proteins with the lattice of the microtubule.
Figure 3.
Effects of taxol on microtubule composition. Control microtubule (MT) shows more post-translationally modified subunits toward the minus end of the microtubule and more unmodified subunits toward the plus end. Different complements of microtubule-related proteins associate with regions of the microtubule that are richer in modified or unmodified subunits. At the plus end of the microtubule is an enrichment of +tips. In the case of microtubules stabilized with taxol, the subunits become predominantly modified all along the length of the microtubule, and hence the complement of microtubule-related proteins favours those that are associated with modified subunits. Also, in the presence of taxol, the +tips no longer appear at the plus end of the microtubule.
Finally, it is worth mentioning that neurons express an isotype (primary gene product) of beta tubulin called beta-III tubulin that (other than Sertoli cells) is normally neuron-specific (Katsetos et al., 2003). Whether or not specific isotypes of tubulin contribute to a microtubule’s functional properties remains controversial in most cells, but some evidence exists that incorporation of beta-III tubulin into a microtubule can keep it somewhat more labile than it would otherwise be, in the face of the various microtubule-stabilizing proteins that exist in the neuron (Panda et al., 1994). This may be important, for example, as discussed earlier in growth cones, where microtubules need to be dynamic during turning events crucial for axonal navigation. The presence of beta-III tubulin renders microtubules less sensitive to the stabilizing effects of taxol (Kamath et al., 2005; Katsetos et al., 2007), which is relevant to the cancer field because tumours that express beta-III tubulin at high levels are aggressive and not as responsive to taxol as other types of tumours (Seve et al., 2005; Ganguly et al., 2011). The levels of beta-III tubulin in neurons normally diminish somewhat during development but increase in response to injury (Moskowitz et al., 1993), and this may be important when designing treatment regimes if taxol is to be used on the injured or diseased nervous system.
Neurodegenerative diseases
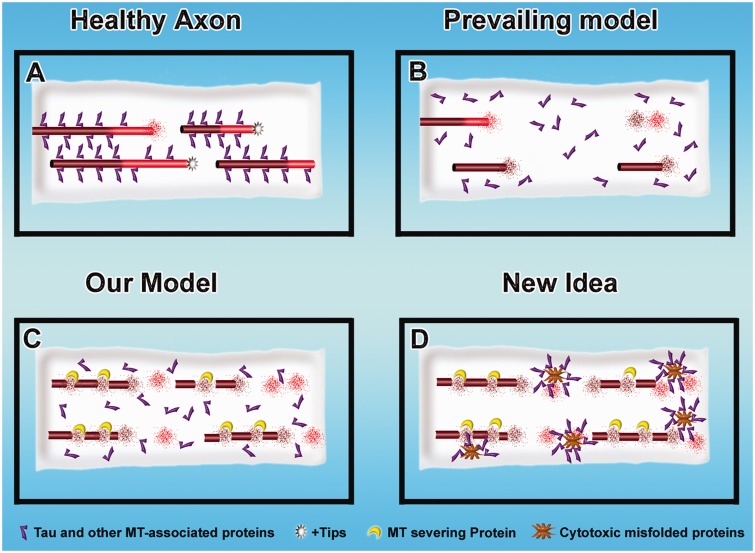
Neurodegenerative diseases are often associated with a gradual loss of microtubule mass from axons, and there are some reports of this also occurring in dendrites (for discussion and references, see Sudo and Baas, 2011). Such microtubule loss is most notably documented in a category of diseases called tauopathies, in which tau is hyper-phosphorylated and as a result becomes progressively dissociated from microtubules (Duan et al., 2012; Yoshiyama et al., 2012). Pure tauopathies are caused by mutations in tau. Alzheimer’s disease, the most common neurodegenerative disease, does not involve mutations in tau, but rather tau becomes hyper-phosphorylated in response to abnormal amyloid-β. Popular schematic illustrations indicate that microtubules ‘fall apart’ as they lose tau, presumably by a loss-of-function mechanism, as tau is classically considered a microtubule-stabilizing protein (see above). We have posited a loss-of-function mechanism as well, but with a variation. In our model, loss of tau from microtubules causes them to degrade not by their normal dynamic properties but rather because they become more sensitive to proteins that sever microtubules, mainly katanin (Qiang et al., 2006; Sudo and Baas, 2011). These models are shown in Fig. 4A–C.
Figure 4.
Mechanisms of microtubule loss during nerve degeneration. (A) A healthy axon. (B and C) Three mechanistic possibilities for microtubule loss during tau-based neurodegeneration. In the prevailing model (B), when tau detaches from them, the microtubules become less stable and depolymerize by their normal dynamic properties. In our model (C), when tau detaches from them, the microtubules become more sensitive to proteins such as katanin that actively promote microtubule loss (Sudo and Baas, 2011). (C) A ‘gain of function’ mechanism by which cytotoxic mutated proteins associated with neurodegeneration could promote microtubule loss by sequestering proteins such as tau.
Another possibility is a gain-of-function mechanism wherein the abnormal tau, either in the form of soluble hyper-phosphorylated protein or in the form of abnormal filaments, produces toxicity that can have myriad ill effects on the axon, including microtubule loss (Kanaan et al., 2012). This could be due, for example, to the hyper-activation of kinases that regulate microtubule-regulatory proteins, or the abnormal tau filaments could sequester other proteins that normally contribute to microtubule stability (LaPointe et al., 2009). Notably, the gain-of-function scenario could also apply to a variety of other pathogenic proteins. For example, mutations of spastin, a microtubule-severing protein, are the chief cause of hereditary spastic paraplegia, but a loss-of-function scenario for a severing protein would not logically comport with a loss in microtubule mass. However, if mutant spastin has a gain-of-function pathogenic mechanism (Solowska et al., 2008), it could very well elicit its toxic effects in a manner that would induce microtubule loss. In fact, we find it provocative that loss of microtubules may be a common downstream affect in a variety of degenerative conditions caused by different upstream mechanisms (Fig. 4D).
The taxol strategy for treatment posits that whatever is causing the microtubule loss, the neuron will benefit from a preservation of microtubule mass by treatment with microtubule-stabilizing drugs. Positive effects on axonal transport and motor improvements were observed with a mouse model for Alzheimer’s disease in which taxol was introduced intravenously (Zhang et al., 2005). These improvements reflect the fact that taxol is taken up by motor neurons at the neuromuscular junction and transported retrogradely to the spinal cord. However, taxol does not cross the blood–brain barrier, and for this reason, more recent studies have focused on epithilone D, a drug that stabilizes microtubules by essentially the same mechanism as taxol but crosses the blood–brain barrier. In fact, this drug has the interesting and potentially useful property of accumulating in the CNS, which means that unwanted effects in the PNS or elsewhere in the body will presumably be minimal. Recently, a wide variety of studies were conducted on this drug, ranging from histology to axonal transport to behaviour on a mouse model for Alzheimer’s disease (Brunden et al., 2010; Zhang et al., 2012). Optimizing the best concentration, the authors found impressively positive effects, with the retention of more viable axons, the loss of fewer hippocampal neurons, and improved performance on memory tests compared with controls. Epothilone D is currently in clinical trials, with doses of 0.003 to 0.01 mg/kg delivered intravenously in patients with mild Alzheimer’s disease, 50–90 years of age (ClinicalTrials.gov Identifier: NCT01492374). Other workers have reported positive effects of microtubule-stabilizing drugs on neurons from a mouse model for hereditary spastic paraplegia (Fassier et al., 2013). Collectively, these results are exciting in that they demonstrate that prevention/reversal of microtubule loss associated with neurodegeneration can be therapeutic. Even so, for the various reasons outlined in the previous section, there is reason to remain cautious about whether a microtubule-stabilizing drug even at low concentrations is a good approach for human patients. In fact, a recent study on spartin, another protein that when mutated causes hereditary spastic paraplegia, indicates that axonal degeneration may be due to too much stabilization of microtubules (Nahm et al., 2013); this serves as a reminder that some degree of microtubule instability is actually ‘good for the brain’ (Carillo et al., 2013).
Nerve injury and regeneration/repair
Another important arena of biomedical research is nerve regeneration/repair after injury, which is especially an issue in the CNS, in which axons display far less regenerative capacity than those in the PNS (Thuret et al., 2006; Lim and Tow, 2007). This problem has classically been considered one mainly of environment, as the CNS normally contains molecules that are deleterious to axonal regeneration, and even more such molecules are produced after injury. A ‘glial scar’ forms after the spinal cord is injured wherein activated microglia induce the manufacture of chondroitin sulphate proteoglycans, which strongly deter axonal growth/regeneration. Recent studies have focused on intrinsic factors in adult neurons that limit the growth potential of mature axons after injury compared to the axons of developing neurons (Sun and He, 2010; Blackmore, 2012). Various strategies have been proposed to augment the capacity of injured axons to regenerate, such as treating the neurons with cocktails of growth factors and/or enzymatically digesting components of the glia scar, but such approaches have thus far not lived up to the hope that they would enable human patients to regenerate injured nerves in the CNS. When the regeneration field was younger, a good deal of the research centred on axonal transport and cytoskeleton (Jacob and McQuarrie, 1991), but a dead end was reached in terms of translating the conclusions of these studies into therapy. More recently, however, there has been a resurgence of attention on microtubules as an avenue to augment nerve regeneration, and this is because of new studies conducted with taxol.
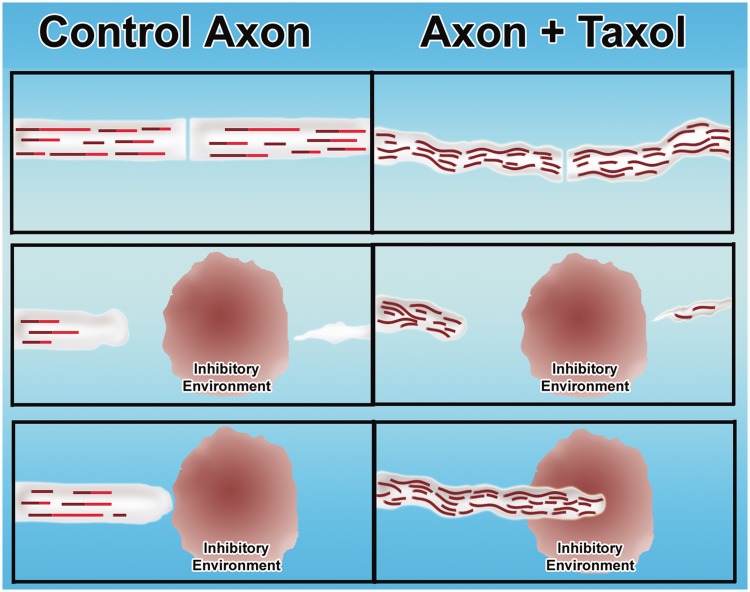
Two important papers were recently published, one using taxol to augment regeneration of injured optic nerve (Sengottuvel et al., 2011) and the other using the drug to augment regeneration of injured spinal cord (Hellal et al., 2011). Both papers came to essentially the same conclusion, namely that taxol applied continuously to the injury site (at very low concentrations) not only positively affected the axons but also reduced scar formation as well as the production of inhibitory molecules by the scar tissue. The positive effects on the axons were presumably because of intrinsic as well as extrinsic factors, as taxol at similarly low doses was also shown to augment regeneration of axons of isolated neurons in culture. In light of these findings, it is curious that other recent studies have now shown that a critical factor in why axons regenerate so much better in the PNS is that these axons have an intrinsic program to deacetylate their axonal microtubules in a gradient-like fashion from the site of injury (Cho and Cavalli, 2012). This property, which is regulated by a signalling pathway involving both calcium and kinases, is not shared by axons in the CNS. The reason why this is so intriguing is that taxol treatment at the injury site would have precisely the opposite effect, namely to heighten the level of microtubule acetylation (see above). Although more work needs to be carried out, it may be that taxol treatment is taking the axon in a very different direction than what would constitute a normal regenerative pathway. In further support of this view, there are studies suggesting that microtubules must be highly dynamic at the cut end of a damaged axon if it is to form a new growth cone capable of doing what growth cones normally do (Bradke et al., 2012). It may be that stabilizing microtubules enables the tip of the axon to become a more powerful ‘battering ram’ (akin to how the growth cone was originally envisioned by Cajal; see Baas and Luo, 2001). Although this can assist regeneration (Fig. 5), a more natural mode of repair would have the tip of the axon behave more dynamically.
Figure 5.
Taxol-based strategy for augmenting regeneration of injured adult axons. (Left) Control situation (not treated with taxol) and situation with taxol treatment (right). (Top) Cut axon. Middle and bottom panels show the degeneration of the distal stump and the failure of the proximal cut end of the axon to regenerate through the inhibitory environment generated at the lesion site. In the control situation, the axon attempts to regenerate but fails as it encounters the inhibitory environment. In the taxol situation, the stabilized microtubules enable the axon to grow through the inhibitory environment.
Taxol and beyond
Ironically, one of the reasons why taxol has been considered an appealing choice for treating nerve injury and disease is that various derivatives of the drug have already been approved for use in human patients, for treatment of cancer. By stabilizing microtubules, taxol prevents the necessary transitions of the microtubule array that must occur during the cell cycle and hence prohibits cell division. In addition, by stabilizing microtubules, taxol is inhibitory to migration and invasion of cancer cells (Terzis et al., 1997). As indicated earlier, the literature is rife with reports on neurodegeneration and neuropathic pain produced in patients undergoing such treatment (Mielke et al., 2006; Scripture et al., 2006; Reyes-Gibby et al., 2009), and this is almost certainly a reflection of the potent effects of stabilizing microtubules in the axon. Although disturbing, at least there is a body of knowledge to build upon, for example with concentrations of the drug (at least when taken systemically) that should clearly be avoided. As noted above, these reports are on the PNS, as taxol does not cross the blood–brain barrier. For this reason, the use of taxol in the CNS is less charted territory, and a matter of more mystery and concern. As noted above, taxol is less effective on tumours that express high levels of beta III tubulin, such as certain forms of lung cancer as well as cancers of the brain, and this has prompted the consideration of other microtubule-stabilizing approaches.
When considering the mechanism of action of taxol and the effect of various concentrations of the drug, it is important to keep in mind that some studies have been conducted on microtubules assembled from pure tubulin, others have been conducted on microtubules together with the assorted microtubule-associated proteins in adult brain, and still others have been conducted on living cells. Biochemical studies suggest that a concentration of taxol of 5 µM decreases the critical concentration of tubulin needed for assembly from 0.2 to <0.1 mg/ml (Orr et al., 2003). Taxol is membrane-permeable and readily enters cultured cells, where in the presence of the normal complement of microtubule-associated proteins, the drug eliminates detectable microtubule disassembly at micromolar levels and promotes assembly. Contemporary studies both in vitro and in living cells generally focus on the effect of various concentrations of taxol on microtubule assembly and disassembly rates, as well as effects on microtubule length and mass. Lower concentrations of taxol can theoretically permit some dynamics to continue to occur, as has been noted in such studies (Jordan and Wilson, 2004). In studies on cultured neurons relevant to axonal regeneration, concentrations are generally used in the nanomolar range, but when applied topically to an injury site in the animal, the concentration has been in the micromolar range, as high as even 1000 micromolar (Sengottuvel et al., 2011). In human cancer patients, taxol is administered intravenously usually at 135–175 mg/m2 (Scripture et al., 2006), whereas a 10th of this concentration was used in the Alzheimer’s mouse studies (Zhang et al., 2005). In these latter approaches, the exposure of individual neurons to the drug is not as direct as it would be in cell culture, so there remains uncertainty as to the actual concentration of taxol to which the relevant axons are exposed (Table 1).
Table 1.
Examples of taxol effects on the nervous system
| Purpose | Application | Introduction method and concentration | Outcome | Reference |
|---|---|---|---|---|
| Cancer therapy | Human patients | Intravenous, (135–175 mg/m2) | Effective at reducing cancer, but side-effects of peripheral neuropathy | Scripture et al., 2006 |
| Regeneration of injured axons | Cultured rodent neurons | Bath applied in culture media, (3–50 nM) | 3 nM enhanced axonal growth even on inhibitory substrates; 50 nM was detrimental to axonal growth | Sengottuvel et al., 2011 |
| Regeneration of injured axons | Adult rodent injured spinal cord | Continuous topical application, (256 ng/day) | Enhancement of nerve regeneration and functional recovery | Hellal et al., 2011; |
| Regeneration of injured axons | Adult rodent injured retinal nerve | Continuous topical application, (1000 µM) | Enhancement of nerve regeneration and functional recovery | Sengottuvel et al., 2011 |
| Alzheimer’s disease and tauopathies | Alzheimer’s rodent model | Intravenous, (10–12 mg/m2) | Axonal transport, histological, and motor improvements | Zhang et al., 2005 |
Cancer being at the top of the list of deadly diseases that afflict the human population, there is an ongoing hunt for novel and better strategies to prevent cancer cell proliferation and tumour invasion, with fewer deleterious side-effects. Because cell division and motility are microtubule-based events, many of the newer approaches being considered could provide a starting place for microtubule-based strategies for treating the injured or diseased nervous system. For example, several years ago the idea arose that mitosis could be inhibited with drugs that suppress a category of kinesin motor proteins believed to be mitosis-specific (Rath and Kozielski, 2012). A motor protein called kinesin-5 (not to be confused with kif5, which is kinesin-1) was chosen as an initial target because inhibition of this motor was known to cause bipolar spindles to collapse. The prototype drug was called monastrol, as it resulted in mono-astral spindles. Drug companies have been working to generate better drugs against kinesin-5 that are suited for use in patients, although problematic results in clinical trials have dampened enthusiasm (Komlodi-Pasztor et al., 2012). There is a plethora of other mitotic motors, and hence there are more opportunities to find drugs with the best properties for stopping cancer proliferation with minimal ill effects. For example, the kinesin-13 family acts as a potent microtubule depolymerizer, and its inhibition may suppress cancer proliferation either by upsetting the balance of factors involved in chromosome segregation or possibly through a more generalized stabilization of microtubules (Sanhaji et al., 2011). Another possibility is to develop drugs that target accessory proteins such as TPX2 (Aguirre-Portoles et al., 2012; Vainio et al., 2012), which is important for the function of kinesin-5 and the mitotic motor called kinesin-12 (Wittmann et al., 2000; Tanenbaum et al., 2009; Ma et al., 2011).
Another avenue that is under consideration for cancer therapy is to target microtubule-severing proteins. These are enzymes that hydrolyze ATP to pull on a tubulin subunit within the wall of the microtubule, thereby breaking the lattice, thus severing the microtubule (Roll-Mecak and McNally, 2010). This is a normal process with physiologically important roles to play across many cell types. If the severing happens at the plus end of the microtubule, the activity essentially acts as a depolymerase (Sharp and Ross, 2012). If the severing happens elsewhere along the microtubule, the activity can potentially result in microtubule loss if the microtubule being severed is relatively labile. Alternatively, the severing can result in the creation of multiple shorter microtubules, if the parent microtubule is relatively stable (Baas et al., 2005). In addition to katanin and spastin (discussed above), it is now known that vertebrate cells express two other katanin-like proteins and three fidgetins. The various severing proteins apparently have different distributions and roles to play during mitosis, suggesting a division of labour as well as different properties and regulatory mechanisms (Zhang et al., 2007). Recent studies show that katanin and spastin can concentrate at the leading edge of some cell types, including cancer cells, and contribute to their motile properties (Baas and Sharma, 2011; Draberova et al., 2011; Zhang et al., 2011). Given that microtubule-severing proteins are important both for cell division and motility, these enzymes could be powerful targets to curtail the proliferation, motility and invasive properties of cancer cells.
At present, to the best of our knowledge, there are no known drugs that affect any of the microtubule-severing proteins. This will undoubtedly change because these proteins are such appealing targets for cancer treatment. In theory, such drugs could be especially useful for treating the aggressive and highly invasive brain tumours for which little can currently be done. Proof-of-principle studies are currently underway using RNA interference to curtail the expression of these proteins (Draberova et al., 2011), and the possibility exists that this technology could also be used in the near future therapeutically. If the different severing proteins target different classes of microtubules (such as those earmarked by different tubulin modifications; Lacroix et al., 2010; Sudo and Baas, 2010), there could be great opportunity to manipulate a cell’s microtubule composition through manipulation of specific severing proteins.
Microtubule-based therapies for injury and disease of the nervous system
We would argue that the main lesson from the work with microtubule-stabilizing drugs is the proof-of-principle that therapeutic intervention at the level of microtubules can have profound effects to enhance nerve regeneration and hinder nerve degeneration. For the reasons outlined in the earlier sections, we recommend keeping the focus on microtubules but expanding the therapeutic toolkit beyond the use of taxol and its analogues and derivatives. One possibility is to explore drugs that might be able to fortify the microtubule array in various ways without stabilizing it in a fashion that would have the panoply of concerns we discussed above. Another possibility is to exploit the potential in manipulating microtubule-related proteins such as molecular motors and microtubule-severing proteins, as well as the enzymes that regulate tubulin post-translational modifications. Such approaches would capitalize on the expanding body of basic science literature on neuronal microtubules as well as contemporary techniques for drug discovery.
With regard to the idea of fortifying the microtubule array, one approach would be to explore tubulin-interacting drugs that directly influence dynamics. As discussed above, very low concentrations of known microtubule depolymerizing drugs such as vinblastine or nocodazole can act as kinetic stabilizers of microtubules by creating a near perfect balance between subunit loss and gain on the polymers (Fig. 1). If this can be achieved in the case of neurons in vivo, it may be possible to establish a buffering effect that would protect against microtubule loss. Kinetically stabilized microtubules still undergo subunit exchange, albeit more slowly than normal, and hence would not be expected to be as abnormal as taxol-stabilized microtubules. For example, in our hands, there was no dramatic increase in acetylation or detyrosination of microtubules in neurons treated with nanomolar levels of vinblastine (Baas and Ahmad, 1993). There are subtle differences in the properties of the various tubulin-interacting drugs (Jordan, 2002; Prota et al., 2013), and these differences may prove useful in providing options for fine-tuning treatment regimes for individual disease or injury scenarios. As with taxol, most of these drugs have been studied in the context of cancer therapy, and hence there is already information on tolerance and side effects from animal models and clinical trials.
In terms of newer drugs with less understood but potentially advantageous properties, two examples from the recent literature come to mind. The laboratory of John Bixby has reported that a drug called F03 augments nerve regeneration in a similarly robust fashion to taxol, and yet does not stabilize microtubules (Usher et al., 2010). Instead, F03 results in an increase in microtubule mass, with both stable and dynamic polymer increasing in roughly equal proportions. Theoretically, this would be better than a strong microtubule stabilizer in that the levels of dynamic polymer would remain robust. Little is known about how F03 elicits its effects, but its structure is similar to neuropsychiatric drugs that are already being used by human patients. The laboratory of Illana Gozes has reported that a short eight amino acid neuroprotective peptide called NAP (drug name, Davunetide) interacts with microtubules in a manner that enables neurons to overcome a number of different disease and injury challenges (Gozes, 2011). The peptide can be effective at femtomolar levels, indicating that it could not influence microtubules in the fashion of a typical drug, but instead probably influences a pathway that regulates the lattice of the microtubule so that it interacts differently with microtubule-related proteins. This would be not unlike the mechanism by which tubulin post-translational modifications influence the properties of microtubules in cells. Indeed, we have shown that NAP protects axonal microtubules from excess severing by katanin when tau is depleted from cultured neurons, and this protection is quite similar to that which is provided by experimentally deacetylating the microtubules (Sudo and Baas, 2011).
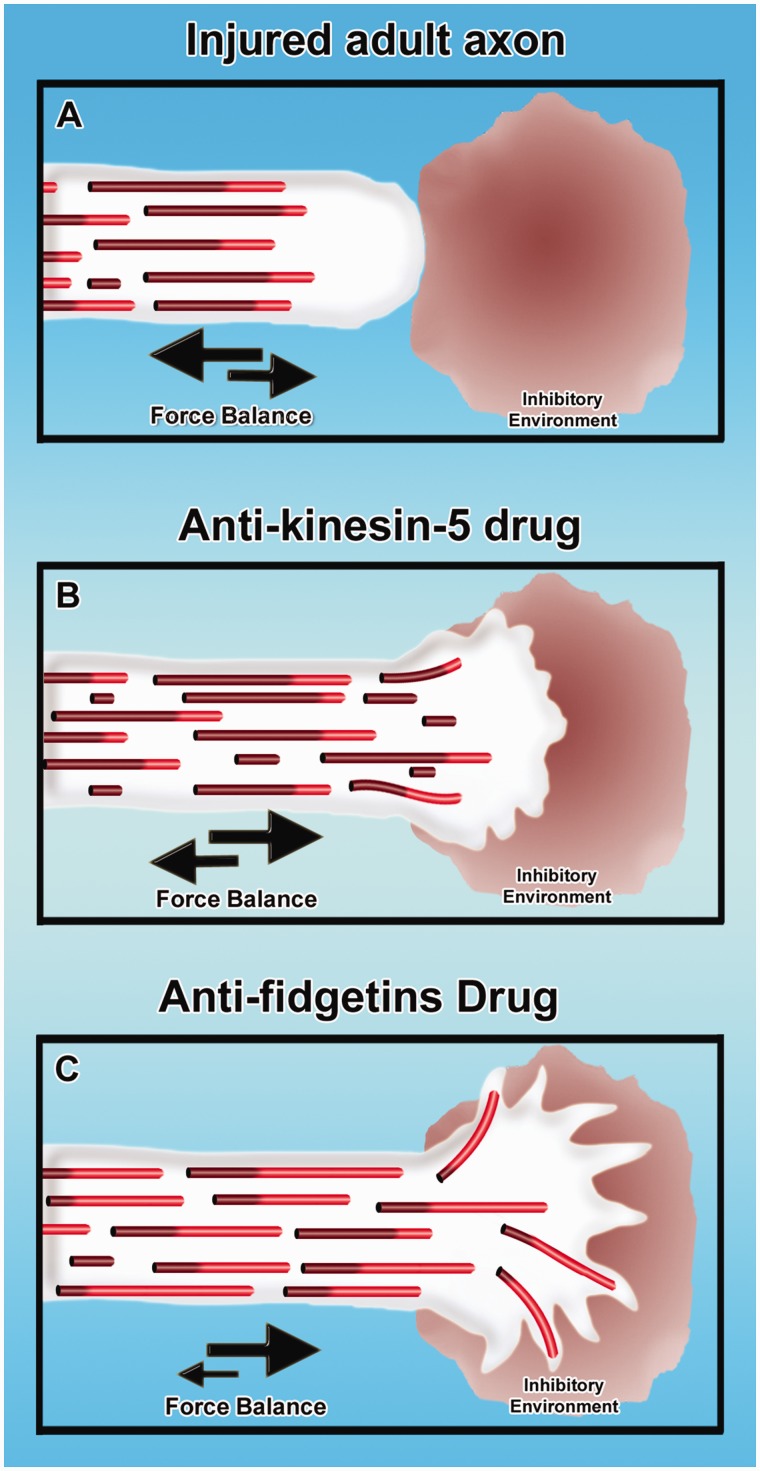
The greatest hope for the future, we believe, is the possibility of intervention at the level of microtubule-related proteins. We have been pursuing this idea in the context of molecular motor proteins. At the time kinesin-5 was being developed as a target for anti-cancer drugs (see above), our laboratory was chiefly focused on seeking out molecular motor proteins with the appropriate properties for regulating the transport of microtubules in axons and dendrites. Mitotic motors such as kinesin-5 seemed ideally suited in that they are specialized to generate forces between microtubules and other microtubules or between microtubules and actin filaments. In fact, we have now found that terminally post-mitotic neurons continue to express many of the mitotic motors, and use these motors for regulating microtubule transport and organization (Baas, 1999; Baas et al., 2006; Liu et al., 2010; Lin et al., 2012). Our studies indicate that kinesin-5 acts as a ‘brake’ on microtubule transport in neurons, and inhibition of this motor causes developing axons to grow faster and retract less, and prevents them from turning properly in response to environmental cues (Myers and Baas, 2007; Nadar et al., 2008, 2012). Interestingly, inhibiting kinesin-5 has the effects on an axon that would be helpful toward regeneration in the case of adult injured nerves (Fig. 6A); if treated with a drug such as monastrol, we wondered whether injured adult axons could be made to grow faster and to ignore inhibitory cues such as those found in the glial scar (Fig. 6B). Using cultured adult dorsal root ganglion neurons from rodents, we found evidence consistent with these predictions, although not as robust as we might have hoped (Lin et al., 2011b). This may be because the levels of kinesin-5 diminish in adult neurons, but we are encouraged by the fact that the levels stay higher in the neurons of the CNS compared to the PNS, and hence the response could be more robust if the drugs were to be applied in vivo to injured axons within the spinal cord.
Figure 6.
New microtubule-based strategies for augmenting regeneration of injured adult axons. Injured adult axon does not regenerate through an inhibitory environment, with the balance of forces favouring retraction rather than growth (A). Treatment with anti-kinesin-5 drugs can shift the balance of forces toward axonal growth, notably increase the frequency of short microtubules in the axon undergoing transport, and enable the axon to enter the inhibitory environment (Lin et al., 2011b). Theoretical at this time is the idea that inhibiting fidgetins (fidgetin and/or fidgetin-like 2) may cause a consequential increase in the fraction of the microtubule mass that is dynamic/labile and restore the axon to a more juvenile state of growth, thus enabling it to grow faster and enter the inhibitory environment.
We anticipate that in the near future cancer researchers will develop drugs against an array of kinesins, with such drugs providing new opportunities for pharmacologically altering the microtubule array of injured axons in a manner conducive to regeneration. For example, we have found the inhibition of kinesin-12, which has partially overlapping functions with kinesin-5, can also enhance axonal growth rates and cause deficits in turning (Liu et al., 2010). Given that kinesin-13 depolymerizes microtubules, inhibition of its activity may provide an intervention with benefits similar to taxol but without the shortcomings of directly stabilizing the microtubules. Caution is due with regard to these and other such approaches, as each has its complications. For example, kinesin-12 levels are very low in adult neurons (Liu et al., 2010), and hence it is unclear whether there is enough of this motor that its inhibition would have any consequential effect. With regard to kinesin-13, recent studies on Caenorhabditis elegans suggest that this motor may actually assist in axonal regeneration by restoring microtubules to a more dynamic state consistent with axonal growth (Ghosh-Roy et al., 2012). Despite such issues that require due consideration, we believe that the future is bright for the use of specific kinesin inhibitors to promote nerve regeneration and possibly to stave off degeneration.
We are especially enthusiastic about the future for microtubule-severing proteins as a centrepiece for therapy. Although a great deal more work needs to be done, our working hypothesis is that the palette of severing proteins expressed in vertebrate neurons (seven in total, to the best of our knowledge) is constantly at work, shaping and pruning the microtubule arrays (Baas and Yu, 2012). Just as katanin has a preference for microtubules richer in acetylated tubulin (Sudo and Baas, 2010) and avoids microtubules rich in tau (Qiang et al., 2006), we posit that the other severing proteins have preferences for microtubules with different complements of modified and unmodified subunits and/or microtubule-associated proteins. For example, fidgetin or fidgetin-like 2 may favour unacetylated microtubules, and may function during neuronal maturation to prune back the levels of these less stable microtubules to enable the acetylated microtubules to dominate as neurons firm up their structure. If this is true, suppressing these fidgetins in adult injured axons may assist them in regenerating by restoring them to a more plastic stage more similar to development (Fig. 6C). In terms of treating tauopathies, a drug that inhibits katanin may notably diminish microtubule loss in brain neurons (Fig. 4C and D), with little or no problem given that there are six other severing proteins to help fulfil the normal functions of katanin. Although all of this is speculation at present, the point is that there may be relatively straightforward things that can be done that set the microtubule array on a path toward a more physiological mode of repair than treatment with drugs that create a highly abnormal stabilization of microtubules.
Of course, all of this depends on new generations of drugs and other therapeutic approaches that target the proteins of interest. There are already drugs being developed that can affect the enzymes that affect tubulin post-translational modifications, which could open the door to combinatorial treatments; for example, enabling the benefits of taxol without the concomitant increase in tubulin acetylation or detyrosination. Until appropriate drugs are available to inhibit the panoply of microtubule-based motors and severing proteins, the possibility exists that therapeutic RNA interference could be used to knock down their expression in particular cells of the nervous system. Another possibility is to target the kinases and phosphatases that regulate microtubule-related proteins and/or other elements of their regulatory pathways. Thus, the potential is vast for a highly flexible kit of tools through which clinicians in the near future can exploit microtubules to diminish axonal degeneration and augment repair and regeneration of injured and diseased axons.
Acknowledgements
We are thankful to past and present members of our laboratory for their contributions to the work presented here. The authors declare no financial or conflicting interests.
Funding
The work in the Baas laboratory has been funded over the years by grants from the National Institutes of Health, the National Science Foundation, the Department of Defense, the Alzheimer’s Association, the Craig H. Neilsen Foundation, the Spastic Paraplegia Foundation, the Christopher and Dana Reeve Foundation, the Philadelphia Institute for Neurodegeneration, and the State of Pennsylvania Tobacco Settlement Funds.
References
- Aguirre-Portoles C, Bird AW, Hyman A, Canamero M, Perez de Castro I, Malumbres M. Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res. 2012;72:1518–28. doi: 10.1158/0008-5472.CAN-11-1971. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–22. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Amos LA, Löwe J. How taxol stabilises microtubule structure. Chem Biol. 1999;6:R65–9. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. doi: 10.1016/s0896-6273(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon. J Cell Biol. 1992;116:1231–41. doi: 10.1083/jcb.116.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. J Cell Biol. 1993;120:1427–37. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–94. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Buster DW. Slow axonal transport and the genesis of neuronal morphology. J Neurobiol. 2004;58:3–17. doi: 10.1002/neu.10281. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–9. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Karabay A, Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–24. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Baas PW, Lin S. Hooks and comets: the story of microtubule polarity orientation in the neuron. Dev Neurobiol. 2011;71:403–18. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Luo L. Signaling at the growth cone: the scientific progeny of Cajal meet in Madrid. Neuron. 2001;32:981–4. doi: 10.1016/s0896-6273(01)00556-6. [DOI] [PubMed] [Google Scholar]
- Baas PW, Mozgova OI. A novel role for retrograde transport of microtubules in the axon. Cytoskeleton (Hoboken) 2012;69:416–25. doi: 10.1002/cm.21013. [DOI] [PubMed] [Google Scholar]
- Baas PW, Pienkowski TP, Cimbalnik KA, Toyama K, Bakalis S, Ahmad FJ, et al. Tau confers drug stability but not cold stability to microtubules in living cells. J Cell Sci. 1994;107(Pt 1):135–43. doi: 10.1242/jcs.107.1.135. [DOI] [PubMed] [Google Scholar]
- Baas PW, Sharma V. Cell migration: katanin gives microtubules a trim. Curr Biol. 2011;21:R302–4. doi: 10.1016/j.cub.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Baas PW, Slaughter T, Brown A, Black MM. Microtubule dynamics in axons and dendrites. J Neurosci Res. 1991;30:134–53. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Baas PW, Vidya Nadar C, Myers KA. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7:490–8. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Baas PW, Yu W. Creative destruction of the microtubule array. Cell Cycle. 2012;11:2420–1. doi: 10.4161/cc.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Brunden KR, Huryn DM, Trojanowski JQ, Lee VM, Smith AB., 3rd Microtubule stabilizing agents as potential treatment for alzheimer's disease and related neurodegenerative tauopathies. J Med Chem. 2012;55:8979–96. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM. Taxol interferes with the interaction of microtubule-associated proteins with microtubules in cultured neurons. J Neurosci. 1987;7:3695–702. doi: 10.1523/JNEUROSCI.07-11-03695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Fischer I. Microtubule-associated protein 1b (MAP1b) is concentrated in the distal region of growing axons. J Neurosci. 1994;14:857–70. doi: 10.1523/JNEUROSCI.14-02-00857.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore MG. Molecular control of axon growth: insights from comparative gene profiling and high-throughput screening. Int Rev Neurobiol. 2012;105:39–70. doi: 10.1016/B978-0-12-398309-1.00004-4. [DOI] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–93. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Brady ST, Tytell M, Lasek RJ. Axonal tubulin and axonal microtubules: biochemical evidence for cold stability. J Cell Biol. 1984;99:1716–24. doi: 10.1083/jcb.99.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Li Y, Slaughter T, Black MM. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J Cell Sci. 1993;104(Pt 2):339–52. doi: 10.1242/jcs.104.2.339. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Yao Y, Potuzak JS, Ferrer NI, Ballatore C, James MJ, et al. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies. Pharmacol Res. 2011;63:341–51. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–6. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challacombe JF, Snow DM, Letourneau PC. Dynamic microtubule ends are required for growth cone turning to avoid an inhibitory guidance cue. J Neurosci. 1997;17:3085–95. doi: 10.1523/JNEUROSCI.17-09-03085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–78. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–32. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Carillo RA, Menon K, Zinn K. Is instability good for the brain? Neuron. 2013;77:599–601. doi: 10.1016/j.neuron.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Das V, Miller JH. Microtubule stabilization by peloruside A and paclitaxel rescues degenerating neurons from okadaic acid-induced tau phosphorylation. Eur J Neurosci. 2012;35:1705–17. doi: 10.1111/j.1460-9568.2012.08084.x. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draberova E, Vinopal S, Morfini G, Liu PS, Sladkova V, Sulimenko T, et al. Microtubule-severing ATPase spastin in glioblastoma: increased expression in human glioblastoma cell lines and inverse roles in cell motility and proliferation. J Neuropathol Exp Neurol. 2011;70:811–26. doi: 10.1097/NEN.0b013e31822c256d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Dong S, Gu F, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer's disease: focusing on tau-mediated neurodegeneration. Transl Neurodegener. 2012;1:24. doi: 10.1186/2047-9158-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Morrison EE, Liverpool TB, Molina-Paris C, Cross RA, Alonso MC, et al. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci. 2008;121:1085–95. doi: 10.1242/jcs.026492. [DOI] [PubMed] [Google Scholar]
- Fassier C, Tarrade A, Peris L, Courageot S, Mailly P, Dalard C, et al. Microtubule-targeting drugs rescue axonal swellings in cortical neurons from spastin knockout mice. Dis Model Mech. 2013;6:72–83. doi: 10.1242/dmm.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Yang H, Cabral F. Class III beta-tubulin counteracts the ability of paclitaxel to inhibit cell migration. Oncotarget. 2011;2:368–77. doi: 10.18632/oncotarget.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham CP, Roll-Mecak A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton (Hoboken) 2012;69:442–63. doi: 10.1002/cm.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy A, Goncharov A, Jin Y, Chisholm AD. Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell. 2012;23:716–28. doi: 10.1016/j.devcel.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I. Microtubules (tau) as an emerging therapeutic target: NAP (davunetide) Curr Pharm Des. 2011;17:3413–7. doi: 10.2174/138161211798072553. [DOI] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33:9511–22. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–83. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–5. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–31. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob JM, McQuarrie IG. Axotomy accelerates slow component b of axonal transport. J Neurobiol. 1991;22:570–82. doi: 10.1002/neu.480220603. [DOI] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33:362–72. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Jean DC, Baas PW, Black MM. A novel role for doublecortin and doublecortin-like kinase in regulating growth cone microtubules. Hum Mol Genet. 2012;21:5511–27. doi: 10.1093/hmg/dds395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Kamath K, Wilson L, Cabral F, Jordan MA. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–7. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, et al. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33:826.e15–30. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos CD, Draberova E, Smejkalova B, Reddy G, Bertrand L, de Chadarevian JP, et al. Class III beta-tubulin and gamma-tubulin are co-expressed and form complexes in human glioblastoma cells. Neurochem Res. 2007;32:1387–98. doi: 10.1007/s11064-007-9321-1. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Legido A, Perentes E, Mork SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol. 2003;18:851–66. doi: 10.1177/088307380301801205. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–42. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–67. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV. Effect of the degree of polar mismatching on traffic jam formation in fast axonal transport. Comput Methods Biomech Biomed Engin. 2010;13:711–22. doi: 10.1080/10255840903505154. [DOI] [PubMed] [Google Scholar]
- Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–54. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, et al. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–51. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PA, Tow AM. Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singapore. 2007;36:49–57. [PubMed] [Google Scholar]
- Lin PC, Chan PM, Hall C, Manser E. Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J Biol Chem. 2011a;286:41466–78. doi: 10.1074/jbc.M111.283580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu M, Mozgova OI, Yu W, Baas PW. Mitotic motors coregulate microtubule patterns in axons and dendrites. J Neurosci. 2012;32:14033–49. doi: 10.1523/JNEUROSCI.3070-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu M, Son YJ, Timothy Himes B, Snow DM, Yu W, et al. Inhibition of kinesin-5, a microtubule-based motor protein, as a strategy for enhancing regeneration of adult axons. Traffic. 2011b;12:269–86. doi: 10.1111/j.1600-0854.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Nadar VC, Kozielski F, Kozlowska M, Yu W, Baas PW. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J Neurosci. 2010;30:14896–906. doi: 10.1523/JNEUROSCI.3739-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Titus J, Gable A, Ross JL, Wadsworth P. TPX2 regulates the localization and activity of Eg5 in the mammalian mitotic spindle. J Cell Biol. 2011;195:87–98. doi: 10.1083/jcb.201106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu Rev Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Georg G, Telikepalli H, McIntosh M, Rajewski RA. Ongoing in vivo studies with cytoskeletal drugs in tau transgenic mice. Curr Alzheimer Res. 2006;3:215–9. doi: 10.2174/156720506777632880. [DOI] [PubMed] [Google Scholar]
- Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer. 2006;42:24–30. doi: 10.1016/j.ejca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Moskowitz PF, Smith R, Pickett J, Frankfurter A, Oblinger MM. Expression of the class III beta-tubulin gene during axonal regeneration of rat dorsal root ganglion neurons. J Neurosci Res. 1993;34:129–34. doi: 10.1002/jnr.490340113. [DOI] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J Cell Biol. 2007;178:1081–91. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Microtubule–actin interactions during neuronal development. Adv Neurobiol. 2011;5:73–96. [Google Scholar]
- Nadar VC, Ketschek A, Myers KA, Gallo G, Baas PW. Kinesin-5 is essential for growth-cone turning. Curr Biol. 2008;18:1972–7. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadar VC, Lin S, Baas PW. Microtubule redistribution in growth cones elicited by focal inactivation of kinesin-5. J Neurosci. 2012;32:5783–94. doi: 10.1523/JNEUROSCI.0144-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M, Lee MJ, Parkinson W, Lee M, Kim H, Kim YH, et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron. 2013;77:680–95. doi: 10.1016/j.neuron.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of taxol resistance related to microtubules. Oncogene. 2003;22:7280–95. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA. 1994;91:11358–62. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-gly proteins at microtubule plus ends. J Cell Biol. 2006;174:839–49. doi: 10.1083/jcb.200512058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–66. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prota AE, Bargsten K, Zurwerra D, Field JJ, Diaz JF, Altmann KH, et al. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587–90. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–9. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12:527–39. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain. 2009;10:1146–50. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls MM. Neuronal polarity in drosophila: sorting out axons and dendrites. Dev Neurobiol. 2011;71:419–29. doi: 10.1002/dneu.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Santangelo CD, Makrides V, Fygenson DK. Tau induces cooperative taxol binding to microtubules. Proc Natl Acad Sci USA. 2004;101:12910–5. doi: 10.1073/pnas.0402928101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhaji M, Friel CT, Wordeman L, Louwen F, Yuan J. Mitotic centromere-associated kinesin (MCAK): a potential cancer drug target. Oncotarget. 2011;2:935–47. doi: 10.18632/oncotarget.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4:165–72. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol facilitates axon regeneration in the mature CNS. J Neurosci. 2011;31:2688–99. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Fischer D. Facilitating axon regeneration in the injured CNS by microtubules stabilization. Commun Integr Biol. 2011;4:391–3. doi: 10.4161/cib.4.4.15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seve P, Mackey J, Isaac S, Tredan O, Souquet PJ, Perol M, et al. Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther. 2005;4:2001–7. doi: 10.1158/1535-7163.MCT-05-0244. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Ross JL. Microtubule-severing enzymes at the cutting edge. J Cell Sci. 2012;125:2561–9. doi: 10.1242/jcs.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh OA, Spira ME. Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta Neuropathol. 2010;119:235–48. doi: 10.1007/s00401-009-0586-0. [DOI] [PubMed] [Google Scholar]
- Shemesh OA, Spira ME. Rescue of neurons from undergoing hallmark tau-induced Alzheimer's disease cell pathologies by the antimitotic drug paclitaxel. Neurobiol Dis. 2011;43:163–75. doi: 10.1016/j.nbd.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Slaughter T, Black MM. STOP (stable-tubule-only-polypeptide) is preferentially associated with the stable domain of axonal microtubules. J Neurocytol. 2003;32:399–413. doi: 10.1023/B:NEUR.0000011334.70648.87. [DOI] [PubMed] [Google Scholar]
- Solowska JM, Morfini G, Falnikar A, Himes BT, Brady ST, Huang D, et al. Quantitative and functional analyses of spastin in the nervous system: implications for hereditary spastic paraplegia. J Neurosci. 2008;28:2147–57. doi: 10.1523/JNEUROSCI.3159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–64. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci. 2010;30:7215–26. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum Mol Genet. 2011;20:763–78. doi: 10.1093/hmg/ddq521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol. 2010;20:510–8. doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Macurek L, Janssen A, Geers EF, Alvarez-Fernandez M, Medema RH. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr Biol. 2009;19:1703–11. doi: 10.1016/j.cub.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Terzis AJ, Thorson F, Heese O, Visted T, Bjerkvig R, Dahl O, et al. Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (taxol) in vitro. Br J Cancer. 1997;75:1744–52. doi: 10.1038/bjc.1997.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Tint I, Jean D, Baas PW, Black MM. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J Neurosci. 2009;29:10995–1010. doi: 10.1523/JNEUROSCI.3399-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tint I, Slaughter T, Fischer I, Black MM. Acute inactivation of tau has no effect on dynamics of microtubules in growing axons of cultured sympathetic neurons. J Neurosci. 1998;18:8660–73. doi: 10.1523/JNEUROSCI.18-21-08660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PF, Margolis RL. Taxol-induced bundling of brain-derived microtubules. J Cell Biol. 1984;99:940–6. doi: 10.1083/jcb.99.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher LC, Johnstone A, Erturk A, Hu Y, Strikis D, Wanner IB, et al. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J Neurosci. 2010;30:4693–706. doi: 10.1523/JNEUROSCI.0302-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio P, Mpindi JP, Kohonen P, Fey V, Mirtti T, Alanen KA, et al. High-throughput transcriptomic and RNAi analysis identifies AIM1, ERGIC1, TMED3 and TPX2 as potential drug targets in prostate cancer. PLoS One. 2012;7:e39801. doi: 10.1371/journal.pone.0039801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Wilm M, Karsenti E, Vernos I. TPX2, A novel xenopus MAP involved in spindle pole organization. J Cell Biol. 2000;149:1405–18. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–55. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. J Neurol Neurosurg Psychiatry. 2012 doi: 10.1136/jnnp-2012-303144. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32:3601–11. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Grode KD, Stewman SF, Diaz-Valencia JD, Liebling E, Rath U, et al. Drosophila katanin is a microtubule depolymerase that regulates cortical-microtubule plus-end interactions and cell migration. Nat Cell Biol. 2011;13:361–70. doi: 10.1038/ncb2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci USA. 2005;102:227–31. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Rogers GC, Buster DW, Sharp DJ. Three microtubule severing enzymes contribute to the “pacman-flux” machinery that moves chromosomes. J Cell Biol. 2007;177:231–42. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]