Abstract
OBJECTIVES:
To describe the association between parents’ attention-deficit/hyperactivity disorder (ADHD) treatment preferences and goals and treatment initiation.
METHODS:
Parents/guardians of children aged 6 to 12 years diagnosed with ADHD in the past 18 months and not currently receiving combined treatment (both medication and behavior therapy [BT]) were recruited from 8 primary care sites and an ADHD treatment center. Parents completed the ADHD Preference and Goal Instrument, a validated measure, and reported treatment receipt at 6 months. Logistic regression was used to analyze the association of baseline preferences and goals with treatment initiation. Using linear regression, we compared the change in preferences and goals over 6 months for children who initiated treatment versus others.
RESULTS:
The study included 148 parents/guardians. Baseline medication and BT preference were associated with treatment initiation (odds ratio [OR]: 2.6 [95% confidence interval (CI):1.2–5.5] and 2.2 [95% CI: 1.0–5.1], respectively). The goal of academic achievement was associated with medication initiation (OR: 2.1 [95% CI: 1.3–3.4]) and the goal of behavioral compliance with initiation of BT (OR: 1.6 [95% CI: 1.1–2.4]). At 6 months, parents whose children initiated medication or BT compared with others had decreased academic and behavioral goals, suggesting their goals were attained. However, only those initiating BT had diminished interpersonal relationship goals.
CONCLUSIONS:
Parental treatment preferences were associated with treatment initiation, and those with distinct goals selected different treatments. Results support the formal measurement of preferences and goals in practice as prioritized in recent national guidelines for ADHD management.
Keywords: ADHD, shared decision-making
What’s Known on This Subject:
Shared decision-making involves the assessment of preferences and goals and has been prioritized in new attention-deficit/hyperactivity disorder treatment guidelines, yet no studies have examined the impact of both preferences and goals on treatment initiation.
What This Study Adds:
Supporting the clinical utility of preference and goal assessment, we found that parental treatment preferences are associated with treatment initiation, and those with distinct goals select different treatments.
Goal-directed and preference-based care, implemented through a process of shared decision-making (SDM), has been increasingly emphasized for those with chronic illness.1 SDM involves 4 steps: the active participation of both clinicians and families in treatment decisions, the exchange of information, discussion of preferences, and a joint determination of the treatment plan.2 While accounting for a family’s values, SDM helps families and clinicians to jointly weigh the evidence regarding the risks and benefits of distinct options and their likelihood of achieving desired outcomes. However, although the Institute of Medicine has prioritized research on SDM in chronic illness3 and the 2010 Patient Protection and Affordable Care Act supports the implementation of SDM,4 barriers including time constraints continue to limit the implementation of SDM.5
Attention-deficit/hyperactivity disorder (ADHD) is ideal to study SDM in pediatrics because this condition results in impaired academic achievement, self-esteem, and interpersonal relationships for >4 million children6,7; there are multiple evidence-based treatment options8,9; and personal and cultural values influence treatment selection and the outcomes families seek.10–13 Fostering SDM, recent national guidelines and a revised toolkit for ADHD treatment from the American Academy of Pediatrics (AAP) emphasize incorporating family preference into the treatment plan and provide forms to document treatment goals.9,14 Although research suggests that assessing preferences may improve ADHD decision-making,15,16 no studies have formally examined the impact of both preferences and goals on ADHD treatment initiation; these data would support clinicians taking time to elicit families’ preferences and goals. Using the first instrument validated to assess both preferences and goals in ADHD (the ADHD Preference and Goal Instrument [ADHD PGI]),17 the current study tested the association of baseline preferences for medication and behavior therapy (BT) as well as goals of improved academic achievement, behavioral compliance, and interpersonal relationships with treatment initiation. We hypothesized that stronger baseline preferences and goals would be associated with the start of treatment.
Methods
Study Population and Setting
As part of a study to develop the ADHD PGI (Fig 1),17 subjects were recruited from 4 urban and 4 suburban primary care practices within The Children’s Hospital of Philadelphia (CHOP) Pediatric Research Consortium, a primary care practice-based research network, and from the CHOP Center for Management of ADHD, a specialty clinic. Eligible parents had children aged between 6 and 12 years who were enrolled in grades kindergarten or higher, had been diagnosed with ADHD within the last 18 months, and were English speaking. Children with autism spectrum disorders and intellectual disability were excluded. Only parents who completed the 6-month follow-up survey and whose children were not receiving combined therapy (medication and BT) at baseline were included in the analysis to explore the association between preferences and goals and treatment initiation. Specifically, our primary analysis of medication and BT initiation included all children not taking medication or not receiving BT, respectively, at the study start.
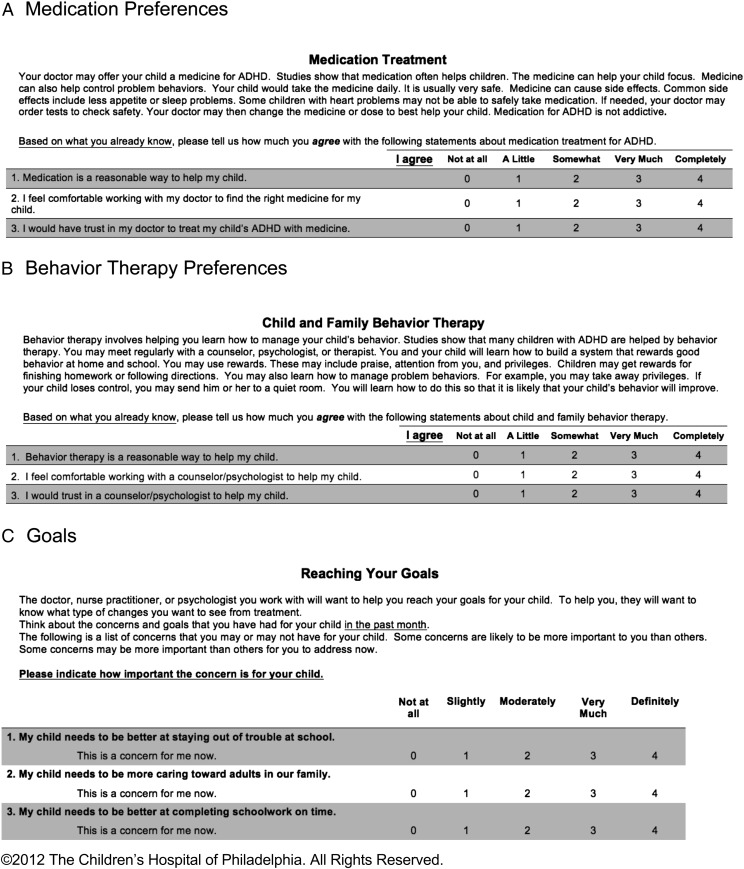
FIGURE 1.
Instructions and format for the ADHD PGI. A sample of items from each scale is shown.
The Preference and Goal Instrument
We previously described the development of 3 distinct scales to elicit parents’ preferences regarding medication and BT and goals for ADHD treatment.17 We conducted exploratory factor analysis,18–20 which elicited specific subscales for the medication preference, BT preference, and goal scales. For medication preference, the final scale included 16 items in 4 domains: acceptability, feasibility, stigma, and side effects. For BT preference, the final scale included 14 items in 3 domains: acceptability, feasibility, and potential adverse effects. Finally, the goal scale included 16 items in 3 domains: academic achievement, behavioral compliance, and interpersonal relationships.
Figure 1 provides a sample of questions from each of the 3 scales. The full scales can be found in the Supplemental Information. Each item on the scales was scored from 0 to 4 according to how much the subject agreed: 0, not at all; 1, a little; 2, somewhat; 3, very much; and 4, completely. The average item score (0–4) was calculated for each scale and domain. Preference items were reversed-scored when necessary so that a higher average score indicated more positive feelings regarding that treatment, and lower preference scores indicated greater concerns in that area (eg, a medication side effects score of 0 would suggest that a parent was very concerned about side effects). For goals, higher average scores indicated a stronger goal. The scales demonstrated acceptable levels of internal consistency (Cronbach’s α of 0.74–0.87) and test–retest reliability (intraclass coefficient: 0.7–0.9) as well as construct, content, and concurrent validity.17
Data Collection
The data collection process has been described in detail elsewhere.17 Parents completed a survey at enrollment and were called 6 months later to again complete the ADHD PGI as well as questions regarding treatment receipt.
Outcome Variables
The primary outcomes were receipt of medication and receipt of BT at the time of the 6-month follow-up interview, assessed by parental report. Specifically, at each follow-up, parents were asked, “Is [your] child currently taking medication for ADHD?” “Is your child currently receiving behavior therapy or counseling for ADHD?”
The secondary outcome was the mean change in preference and goal scores from the study start to the 6-month follow-up.
Covariates
Covariates considered included parent race, child age, child gender, parental education, ADHD subtype, time since diagnosis, receipt of medication or BT at baseline (children not receiving BT might or might not have been receiving medication), and mean score on the Impairment Rating Scale, Parent Version (a reliable and valid instrument that assesses functional impairment in ADHD).21
Statistical Analyses
The study population was described by using descriptive statistics. To avoid collinearity in multivariable models, the correlation between independent variables and among covariates was assessed. If 2 variables were highly correlated, the 1 deemed most clinically meaningful was retained. Bivariate analysis assessed the association between each covariate and each outcome. In final models, covariates were retained if associated with the outcome at the prespecified level of P < .2 to avoid overfitting statistical models. Our primary analysis of medication and BT initiation included all children not taking medication (including those receiving and not receiving BT) as well as all children not receiving BT (including those taking and not taking medication), respectively, at the study start. To assess the stability of results across different subsets of subjects, we conducted additional analyses limited to children receiving neither treatment at baseline (these children might have previously been treated) as well as children who had never received medication, BT, or both. Logistic regression models were then implemented to determine the association of mean preference scores as well as mean goal scores at baseline with treatment initiation within 6 months. We separately analyzed each preference scale overall and each subscale. For goals, models separately included mean scores for academic achievement, behavioral compliance, or interpersonal relationships as the independent variable.
To assess the relative importance of preferences versus goals in explaining treatment initiation, logistic regression models were developed with both mean preference and mean goal scores as independent variables and initiation of medication or BT as the outcome. These analyses were possible because preference and goal scores were not highly correlated (range: –0.11 to 0.14).
For our secondary analysis, we calculated change scores for each scale or subscale separately for families who did and did not initiate treatment; paired t tests or Wilcoxon signed rank tests were used depending on the distribution. The difference in the change between those who initiated treatment and those who did not was then calculated by using linear regression models with covariates included if they were associated with the outcome with P < .2.
All analyses were conducted by using Stata version 11 (StataCorp, College Station, TX). The study was approved by the CHOP institutional review board, and all subjects provided written informed consent.
Results
Study Population
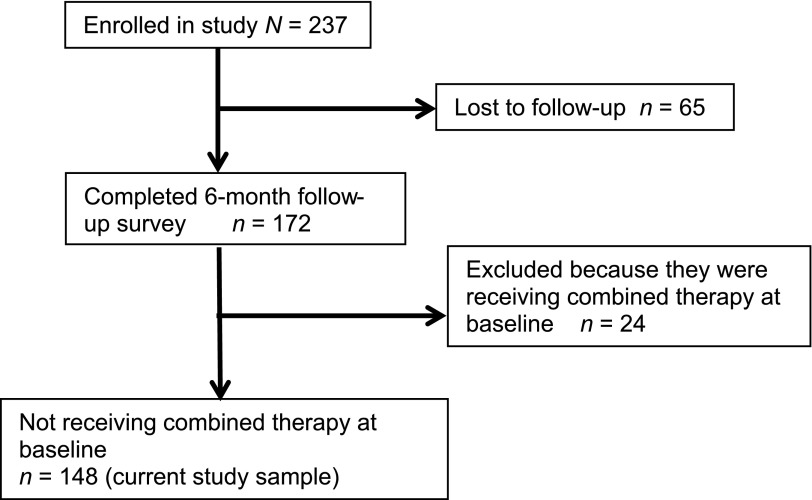
Figure 2 describes the study enrollment. Of 237 parents originally completing the ADHD PGI, 172 (72%) completed the 6-month follow-up survey. Of these 172 parents, 148 (86%) had children who were not receiving combined therapy at the study start and were included in the current analysis. These parents did not differ in demographic or clinical characteristics from parents originally completing the ADHD PGI (P > .1).
FIGURE 2.
Study enrollment and inclusion.
Characteristics of participants are described in Table 1. Participants were evenly divided according to race, and most children had combined-type ADHD. One hundred eight children were not receiving medication at baseline, 124 were not receiving BT, and 84 were not receiving either treatment. The total study population included 148 children because individual children could be included in multiple groups (eg, not on medication, not on BT, not on either). Within these groups, 87 (81%) had never received medication, 97 (78%) had never received BT, and 61 (73%) had never received either treatment, respectively. The mean baseline medication preference scores for these groups were similar (2.5–2.7), as were BT preference scores (2.9–3.0). Among all parents, 25% had a strong preference (mean preference score ≥3) for both treatments, 11% had a strong preference for medication only, 28% had a strong preference for BT only, and 36% for neither.
TABLE 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristica,b | Not Receiving Medication at Baseline | Not Receiving BT at Baseline | Not Receiving Either Treatment at Baseline |
|---|---|---|---|
| Study population, n (%) | 108 (73) | 124 (84) | 84 (57) |
| Race, n (%) | |||
| White | 58 (54) | 66 (53) | 48 (57) |
| Black | 47 (44) | 56 (45) | 34 (41) |
| Other | 3 (2) | 2 (2) | 2 (2) |
| Hispanic | 4 (4) | 4 (3) | 3 (4) |
| Gender, n (%) | |||
| Female | 92 (85) | 111 (90) | 72 (86) |
| Male | 16 (15) | 13 (10) | 12 (14) |
| Education level, n (%) | |||
| High school or less | 27 (25) | 37 (30) | 19 (23) |
| Some college or associate’s degree | 29 (27) | 37 (30) | 25 (30) |
| Bachelor’s degree or higher | 52 (48) | 50 (40) | 40 (47) |
| Mean medication preference score (range) | 2.5 (0.8–3.8) | 2.7 (0.8–4.0) | 2.5 (0.8–3.8) |
| Mean BT preference score (range) | 3.0 (1.6–4.0) | 2.9 (1.5–4.0) | 2.9 (1.6–4.0) |
| Child characteristics | |||
| Age, mean ± SD, y | 8.1 ± 1.6 [range: 6–12] | 8.3 ± 1.7 [range: 6–12] | 8.3 ± 1.6 [range: 6–12] |
| Gender, n (%) | |||
| Male | 76 (70) | 85 (69) | 58 (69) |
| Female | 32 (30) | 39 (31) | 26 (31) |
| Treatment location, n (%) | |||
| Primary care | 48 (44) | 65 (52) | 32 (38) |
| ADHD specialist | 60 (56) | 59 (48) | 52 (62) |
| ADHD diagnosis, n (%) | |||
| Inattentive | 27 (25) | 31 (25) | 24 (29) |
| Hyperactive | 5 (5) | 3 (2) | 3 (3) |
| Combined | 66 (61) | 80 (65) | 51 (61) |
| Not otherwise specified | 10 (9) | 10 (8) | 6 (7) |
| Time since diagnosis, n (%) | |||
| <1 mo | 60 (56) | 60 (48) | 54 (64) |
| 1–3 mo | 4 (4) | 6 (5) | 4 (5) |
| 3–6 mo | 6 (5) | 11 (9) | 5 (6) |
| 6–12 mo | 22 (20) | 20 (16) | 10 (12) |
| 12–18 mo | 16 (15) | 27 (22) | 11 (13) |
| Never received the treatment in question, n (%)c | 87 (81) | 97 (78) | 61 (73) |
| Impairment Rating Scale, Parent Version,d mean ± SD | 2.3 ± 1.2 [range: 0.1–4.8] | 2.8 ± 1.2 [range: 0.1–4.8] | 2.9 ± 1.1 [range: 0.1–4.8] |
| Type of ADHD medication, n (%)e | |||
| Amphetamine compound | NA | 12 (10) | NA |
| Methylphenidate compound | NA | 25 (20) | NA |
| Atypical antipsychotic | NA | 1 (<1) | NA |
| None | 108 (100) | 87 (70) | 108 (100) |
NA, not applicable.
Children receiving combined therapy at baseline were excluded.
The total study population is 148. The groups overlap because some children were receiving neither treatment at baseline and thus were counted in all 3 groups.
For the “Not Receiving Medication at Baseline” group, this means they never received medication. For the “Not Receiving BT at Baseline” group, this means they never received BT. For the “Not Receiving Either Treatment at Baseline” group, this means they never received either medication or BT.
Responses ranged from 0 (no problem) to 6 (extreme problem) for each item.
Children could be receiving multiple types of ADHD medication.
Association of Preferences and Goals With Treatment Receipt
Of the 108 children not receiving medication at baseline, 46 (43%) initiated medication during the study period and, among the 124 children not receiving BT at baseline, 30 (24%) initiated BT. Of those who began medication and BT, only 1 child discontinued medication and 8 discontinued BT during the 6-month follow-up period.
The results of adjusted logistic regression models assessing the association between baseline preference scores and treatment receipt are shown in Tables 2 and 3. In our primary analysis of medication initiation, we found that each 1-point increase in overall medication preference score was associated with 2.6 times greater odds of initiating medication treatment (95% confidence interval [CI]: 1.2–5.5) and that the association for the acceptability subscale was even higher (odds ratio [OR]: 3.1 [95% CI: 1.7–5.8]). In addition, a higher baseline score on the side effects subscale, indicating fewer concerns regarding side effects, was associated with greater odds of medication initiation (OR: 1.5 [95% CI: 1.0−2.2]). Feasibility and stigma scores were not associated with medication initiation. These patterns were consistent across all subgroup analyses, including those limited to children who were on neither treatment at baseline and those limited to children who had never received either medication or BT.
TABLE 2.
Association of Baseline ADHD Preference and Goal Scores With Treatment Initiation at 6-Month Follow-up Among Those Not Receiving a Given Treatment at Baseline
| Scale | Not Receiving Medication at Baseline (Primary Analysis) | Not Receiving BT at Baseline (Primary Analysis) | Not Receiving Either Treatment at Baseline | |
|---|---|---|---|---|
| Adjusted OR of Medication Initiation (95% CI)a,b | Adjusted OR of BT Initiation (95% CI)a,b | Adjusted OR of Medication Initiation (95% CI)a,b | Adjusted OR of BT Initiation (95% CI)a,b | |
| Sample size | 108 | 124 | 84 | 84 |
| Medication preference | 2.6 (1.2–5.5) | – | 2.0 (0.9–4.4) | – |
| Subscalesc | ||||
| Acceptability | 3.1 (1.7–5.8) | – | 2.7 (1.6–15.6) | – |
| Feasibility | 1.1 (0.7–1.8) | – | 1.0 (0.6–1.7) | – |
| Stigma | 0.9 (0.6–1.4) | – | 1.6 (0.8–3.2) | – |
| Side effects | 1.5 (1.0–2.2) | – | 1.4 (0.9–2.2) | – |
| BT preference | – | 2.2 (1.0–5.1) | – | 2.2 (0.8–6.3) |
| Subscalesd | ||||
| Acceptability | – | 5.1 (1.9–13.3) | – | 5.3 (1.3–21.1) |
| Feasibility | – | 1.1 (0.7–1.7) | – | 1.1 (0.6–1.8) |
| Adverse effects | – | 0.9 (0.6–1.5) | – | 1.1 (0.6–2.0) |
| Goals | ||||
| Academic achievement goal | 2.1 (1.3–3.4) | 1.3 (0.8–1.9) | 2.3 (1.3–4.1) | 1.1 (0.7–1.9) |
| Behavioral compliance goal | 1.2 (0.8–1.8) | 1.6 (1.1–2.4) | 1.4 (0.9–2.2) | 1.5 (0.9–2.5) |
| Interpersonal relationship goal | 1.0 (0.7–1.7) | 1.6 (1.1–2.4) | 1.4 (0.8–2.4) | 1.3 (0.8–2.2) |
ORs calculated from multivariable logistic regression adjusting for covariates that were associated with the outcome at the P = .2 level (race, impairment, and baseline BT receipt for medication models; ADHD subtype and child gender for behavior therapy models). ORs reflect the increase in odds of treatment initiation with each 1-point increase in mean preference or goal score.
Medication initiation only assessed for those who were not on medication at baseline (n = 108); BT initiation only assessed for those not on BT at baseline (n = 124). Initiation of both treatments was assessed for those not on either treatment at baseline (n = 84).
Acceptability = overall comfort with and belief in the effectiveness of medication; Feasibility = practical barriers to medication treatment; Stigma = negative perceptions of the child or family by others due to medication treatment; Side effects = side effects of the medication on the child.
Acceptability = overall comfort with and belief in the effectiveness of BT; Feasibility = practical barriers to behavior therapy receipt; Adverse effects = perceived negative consequences of receiving behavior therapy for the child.
, indicates not applicable.
TABLE 3.
Association of Baseline ADHD Preference and Goal Scores With Treatment Initiation at 6-Month Follow-up Among Those Never Receiving a Given Treatment
| Scale | Never Received Medication | Never Received BT | Never Received Either Treatment | |
|---|---|---|---|---|
| Adjusted OR of Medication Initiation (95% CI)a,b | Adjusted OR of BT Initiation (95% CI)a,b | Adjusted OR of Medication Initiation (95% CI)a,b | Adjusted OR of BT Initiation (95% CI)a,b | |
| Sample size | 87 | 97 | 61 | 61 |
| Medication preference | 3.1 (1.3–7.5) | – | 4.9 (1.6–15.6) | – |
| Subscalesc | ||||
| Acceptability | 2.4 (1.3–4.7) | – | 2.9 (1.3–6.7) | – |
| Feasibility | 1.6 (0.9–2.8) | – | 1.6 (0.8–3.2) | – |
| Stigma | 1.2 (0.7–2.0) | – | 1.4 (0.7–2.5) | – |
| Side effects | 1.4 (0.9–2.2) | – | 2.1 (1.1–4.0) | – |
| BT preference | – | 2.9 (0.9–8.8) | – | 4.7 (1.0–21.7) |
| Subscalesd | ||||
| Acceptability | – | 4.2 (1.2–14.1) | – | 20.3 (2.0–200.3) |
| Feasibility | – | 1.3 (0.7–2.2) | – | 1.5 (0.7–3.2) |
| Adverse effects | – | 1.1 (0.6–2.1) | – | 1.3 (0.6–2.9) |
| Goals | ||||
| Academic achievement goal | 2.0 (1.2–3.3) | 1.2 (0.7–2.0) | 2.3 (1.2–4.5) | 1.0 (0.6–1.8) |
| Behavioral compliance goal | 1.3 (0.9–2.0) | 1.7 (1.0–2.6) | 1.3 (0.8–2.2) | 1.4 (0.8–2.5) |
| Interpersonal relationship goal | 0.8 (0.5–1.4) | 1.4 (0.8–2.4) | 0.8 (0.4–1.7) | 1.1 (0.5–2.2) |
ORs calculated from multivariable logistic regression adjusting for covariates that were associated with the outcome at the P = .2 level (race, impairment, and baseline BT receipt for medication models; ADHD subtype and child gender for behavior therapy models). ORs reflect the increase in odds of treatment initiation with each 1-point increase in mean preference or goal score.
Medication initiation only assessed for those who were not on medication at baseline (n = 108); BT initiation only assessed for those not on BT at baseline (n = 124). Initiation of both treatments was assessed for those not on either treatment at baseline (n = 84).
Acceptability = overall comfort with and belief in the effectiveness of medication; Feasibility = practical barriers to medication treatment; Stigma = negative perceptions of the child or family by others due to medication treatment; Side effects = side effects of the medication on the child.
Acceptability = overall comfort with and belief in the effectiveness of BT; Feasibility = practical barriers to behavior therapy receipt; Adverse effects = perceived negative consequences of receiving behavior therapy for the child.
, indicates not applicable.
We next examined BT and found that each 1-point increase in the overall BT preference score was associated with 2.2 times greater odds of initiating BT (95% CI: 1.0−5.1). A higher baseline acceptability score was strongly associated with BT initiation (OR: 5.1 [95% CI: 1.9−13.3]). There was no association between the feasibility and adverse effects subscales and BT initiation. Once again, patterns were similar in all subgroup analyses.
Using logistic regression and accounting for covariates, we also examined whether preference scores for 1 treatment were associated with initiation of the other treatment. We found that medication preference scores were not associated with BT initiation (OR: 1.3 [95% CI: 0.6−2.8]), and BT preferences scores were not associated with medication initiation (OR: 0.8 [95% CI: 0.4−1.6]).
For goals, results of our primary analysis showed that higher endorsement of the goal of improved academic achievement was associated with greater odds of medication (OR: 2.2 [95% CI: 1.3−3.4]) but not BT initiation. This finding was consistent across all subgroups. In contrast, a stronger endorsement of the goals of behavioral compliance and interpersonal relationships was associated with BT (OR: 1.6 [95% CI: 1.1−2.4] for each) but not with medication initiation. Although significance varied, the strength of the association between the goal of behavioral compliance and BT initiation was similar across all subgroup analyses. However, interpersonal goals were not associated with BT initiation among those receiving neither medication nor BT at baseline.
In the analysis that included both preferences and goals as independent variables and treatment initiation as the dependent variable, we found that results were unchanged.
Changes in Preferences and Goals as a Function of Treatment Initiation
In a secondary analysis, we examined the absolute and relative change in preference and goal scores over 6 months for parents whose children initiated medication and BT versus those who did not (Table 4). Parents initiating medication and those initiating BT reported slightly higher adjusted preferences for the treatment they initiated at 6 months versus baseline compared with those who did not initiate the treatment (0.2 [0.0−0.5] and 0.2 [0.0−0.4] for medication and BT, respectively). In terms of medication preference domains, parents whose children initiated medication had a statistically significant increase in medication side effects score of 0.5 (0.1−0.9) points relative to parents whose children did not initiate medication, suggesting a decreased concern about side effects. Changes in other domains were not significant. Among domains of BT preference, parents initiating BT reported higher scores for feasibility and adverse effects than those who did not (0.5 [0.0−1.0] and 0.4 [0.1−0.8], respectively), indicating fewer concerns in these areas.
TABLE 4.
Change in ADHD Preference and Goal Scores From Baseline to 6 Months Among Those Initiating Medication and BT
| Treatment Pattern | Initiated Medication (n = 46) | Did Not Initiate Medication (n = 62) | Relative Difference Between Groupsa | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Score at Study Start | Mean Score at 6 mo | Change (95% CI) | Mean Score at Study Start | Mean Score at 6 mo | Change (95% CI) | Unadjusted (95% CI) | Adjusted (95% CI) | |
| Medication | ||||||||
| Medication preference | 2.7 | 3.0 | 0.3 (0.1 to 0.5)* | 2.4 | 2.4 | 0.0 (–0.1 to 0.2) | 0.3 (0.0 to 0.5)* | 0.2 (0.0 to 0.5)* |
| Acceptability | 2.7 | 3.2 | 0.5 (0.2 to 0.8)* | 1.9 | 2.2 | 0.3 (0.1 to 0.5)* | 0.2 (–0.2 to 0.5) | 0.1 (-0.2 to 0.5) |
| Feasibility | 3.1 | 3.2 | 0.1 (–0.2 to 0.5) | 3.1 | 3.0 | −0.1 (–0.3 to 0.2) | 0.2 (–0.2 to 0.6) | 0.2 (–0.2 to 0.6) |
| Stigma | 2.9 | 2.8 | −0.1 (–0.5 to 0.3) | 3.1 | 2.9 | −0.2 (–0.4 to 0.1) | 0.1 (–0.3 to 0.5) | 0.2 (–0.2 to 0.6) |
| Side effects | 1.8 | 2.3 | 0.5 (0.2 to 0.8)* | 1.5 | 1.5 | 0.0 (–0.4 to 0.2) | 0.5 (0.1 to 1.0)* | 0.5 (0.0 to 0.9)* |
| Goals | ||||||||
| Academics | 2.9 | 2.2 | −0.7 (–1.0 to –0.3)* | 2.1 | 2.1 | 0.0 (–0.2 to 0.2) | −0.7 (–1.0 to –0.3)* | −0.5 (–0.9 to –0.1)* |
| Behavior | 2.3 | 1.9 | −0.4 (–0.8 to –0.1)* | 1.9 | 2.1 | 0.2 (–0.1 to 0.4) | −0.6 (–1.0 to –0.2)* | −0.6 (–1.0 to –0.2)* |
| Interpersonal | 1.3 | 1.3 | 0.0 (–0.3 to 0.3) | 1.0 | 1.2 | 0.2 (0.0 to 0.4)* | −0.2 (–0.5 to 0.1) | −0.1 (–0.5 to 0.2) |
| Treatment Pattern | Initiated BT (n = 30) | Did Not Initiate BT (n = 94) | Relative Difference Between Groupsa | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Score at Study Start | Mean Score at 6 mo | Change (95% CI) | Mean Score at Study Start | Mean Score at 6 mo | Change (95% CI) | Unadjusted (95% CI) | Adjusted (95% CI) | |
| BT | ||||||||
| BT preference | 3.1 | 3.3 | 0.2 (0.1 to 0.5)* | 2.9 | 2.9 | 0.0 (–0.1 to 0.1) | 0.2 (0.1 to 0.5)* | 0.2 (0.0 to 0.4)* |
| Acceptability | 3.3 | 3.3 | 0.0 (–0.2 to 0.3) | 2.8 | 2.9 | 0.1 (–0.1 to 0.2) | −0.1 (–0.4 to 0.3) | −0.1 (–0.4 to 0.2) |
| Feasibility | 2.7 | 3.0 | 0.3 (0.0 to 0.7)* | 2.6 | 2.4 | −0.2 (–0.4 to 0.1) | 0.5 (0.0 to 1.0)* | 0.5 (0.0 to 1.0)* |
| Adverse effects | 3.0 | 3.5 | 0.5 (0.2 to 0.8)* | 3.1 | 3.2 | 0.1 (–0.1 to 0.2) | 0.4 (0.1 to 0.8)* | 0.4 (0.1 to 0.8)* |
| Goals | ||||||||
| Academics | 2.7 | 2.0 | −0.7 (–1.1 to –0.3)* | 2.4 | 2.2 | −0.2 (–0.4 to –0.1)* | −0.5 (–0.9 to –0.1)* | −0.4 (–0.9 to –0.1)* |
| Behavior | 2.6 | 2.1 | −0.5 (–0.9 to –0.1)* | 1.8 | 1.8 | 0.0 (–0.2 to 0.2) | −0.5 (–1.0 to –0.1)* | −0.5 (–0.9 to –0.1)* |
| Interpersonal | 1.5 | 1.2 | −0.3 (–0.6 to 0.1)* | 1.0 | 1.2 | 0.2 (0.1 to 0.3)* | −0.5 (–0.8 to –0.1)* | −0.4 (–0.7 to –0.1)* |
Relative difference was calculated through linear regression, with change in score as the outcome and treatment pattern as the independent variable. Models were adjusted for covariates associated with the outcome at the P < .2 level.
Indicates results significant at the P < .05 level.
The strength of goals also decreased significantly for those initiating versus not initiating treatment. Parents whose children initiated medication versus those who did not had a relative decrease of 0.5 (0.1−0.9) points for the goals of academic achievement and 0.6 (0.2−1.0) points for the goal of behavioral compliance, suggesting fewer concerns in these areas. Medication initiation was not associated with a significant reduction in the goal of improved interpersonal relationships. In addition, parents whose children initiated BT versus those who did not had a statistically significant decrease of 0.4 (0.1−0.9) points for the goals of academic achievement, 0.5 (0.1−0.9) point for behavioral compliance, and 0.4 (0.1–0.7) points for interpersonal relationships.
Discussion
Although the recently published AAP ADHD guidelines and toolkit prioritize the assessment of families’ preferences and goals as part of treatment decision-making, this is the first study, to the best of our knowledge, to examine the association between formally measured treatment preferences as well as goals and subsequent treatment receipt.14 In a diverse sample and consistent with our hypothesis, we found that preferences for medication and BT were associated with subsequent receipt of each treatment. In addition, our results indicate that those with distinct goals select different treatments: academic goals were associated with initiation of medication whereas the goal of improved behavioral compliance was associated with BT receipt. It is noteworthy that the same pattern of findings was replicated when we examined the subset of cases who were receiving neither treatment at baseline and when we examined those who had never received either treatment. We also found that parents whose children initiated medication or BT compared with others had decreased academic and behavioral goals, suggesting that goals were attained. However, only those initiating BT had diminished interpersonal relationship goals.
These findings support the utility of formally assessing preferences and goals as part of ADHD decision-making. Despite the impact of families’ personal and cultural values on treatment decisions,10–13 clinicians sometimes fail to explore families’ preferences and goals regarding treatment.15,16,22 We found that by formally assessing preferences, we were able to identify families who had more than twice the odds of initiating medication or BT. In addition, although the instrument used in this study included multiple preference domains, acceptability (ie, the notion that a particular treatment is a reasonable and effective way to treat a child) was most strongly associated with treatment initiation for both medication and BT. Previous studies examining the relationship of treatment acceptability with treatment receipt have had mixed results.23–26 None of these studies considered acceptability as conceptualized by the ADHD PGI, which includes items related to general acceptability, trust in the provider, family support, and perceived effectiveness of both treatments, which may explain why BT preference was associated with treatment receipt in this study but not in previous research.23 Although the findings warrant confirmation, our results suggest that clinicians should account for treatment preference, especially in the domain of acceptability, as they discuss options with families. In practice, clinicians could use the ADHD PGI before visits to tailor subsequent conversations based on preference to more efficiently and effectively use limited consultation time to address treatment barriers. Our previous work suggests that, in discussing ADHD treatment, clinicians sometimes advocate for just 1 option.16 Use of the ADHD PGI would ensure that parents have the opportunity to communicate their preferences about both sets of evidence-based treatments (medication and BT).
To our knowledge, this is the first study to demonstrate that parental goals affect ADHD treatment initiation. Goals remained a statistically significant predictor of treatment receipt in models that included both preferences and goals as independent variables. Because both the extent to which parents desire improvement (goals) as well as beliefs regarding the specific attributes of the treatment (preference) shape decisions, both warrant discussion in planning ADHD treatment. In addition, given that preferences and goals are associated with treatment receipt, prospective study is needed to understand whether addressing both factors in decision-making mitigates the low rates of adherence found in studies of behavioral interventions and medication treatment.27,28
Our results also demonstrated that those with distinct goals pursue distinct treatments, with the goal of improved academic performance associated with medication initiation and the goal of improved behavioral compliance associated with the start of BT. Perhaps explaining these findings, previous studies have found that some parents find medication to be a less reasonable approach for problems they perceive as resulting from poor parenting or a lack of discipline.29–31 As a result, medication may be perceived as more compatible with addressing academic difficulties and BT with discipline or social problems.
Addressing a gap in the literature,32 our study design enabled us to track changes in preferences and goals over time among those initiating treatment versus those not initiating treatment. Consistent with previous studies which show that experience with medication can improve perceptions of this treatment,33,34 we found that medication and BT preferences increased among those initiating each treatment. We also found fewer concerns regarding side effects of medication and adverse effects of BT among those initiating each treatment, an association not previously described in detail. Results indicated that academic and behavioral goals decreased to a similar extent among those starting medication and BT, and interpersonal goals diminished with BT. Although further study is needed, these findings offer preliminary data to suggest that academic and behavioral goals prioritized by parents before treatment may be addressed to some extent with the start of either medication or BT.
The current study has several limitations. Although the study included parents of children treated both in the subspecialty setting and multiple primary care practices, all subjects were part of 1 health system. Further study is needed to assess how results generalize to other health settings and patient populations. Second, this study focused only on parents’ preferences and goals for treatment. Given data that show the unique perspectives of children and adolescents regarding ADHD treatment, research is needed to understand the impact of their preferences and goals on treatment initiation and outcomes.35–40 Although the instrument used in this study was validated as a measure of goals, we did not simultaneously administer a validated measure of goal attainment. Additional work is needed to confirm that a decrease in goal scores on the ADHD PGI definitively represents goal attainment. In addition, we did not collect longitudinal data on medication dose, the extent of BT receipt, or symptom scores, and we were therefore unable to determine whether children’s symptoms changed, how medication was titrated, or how many BT visits were received after initiating treatment. These factors will be a focus of future work. Finally, we prospectively collected data to track preferences and goals over time but did not share these results with parents or clinicians. Trials are needed that test the impact of sharing information on preferences and goals with parents and clinicians on the ADHD treatment decision-making process and outcomes.
Conclusions
This study demonstrated the association between parental preferences and goals, as measured by the ADHD PGI, and subsequent ADHD treatment receipt. Results suggest that assessing parents’ preferences and goals, as prioritized in the AAP ADHD treatment guidelines, is useful for clinicians in understanding which treatment, if any, parents are likely to initiate for their children. Although these results are preliminary and require confirmation, they also suggest that, at least for achieving academic and behavioral goals, treatment initiation may be more important than the specific treatment selected in helping parents address their goals for their children. Overall, these findings support a process of SDM as a strategy to pair parents and children with treatment they are likely to begin to achieve goals that are salient for the family.
Supplementary Material
Acknowledgments
We thank James Massey, Cayce Hughes, Mark Ramos, Russell Localio, and the clinicians at CHOP’s ADHD Center for their help with the conduct and analysis of this research. We also thank the network of primary care physicians, the patients, and the families for their contributions to clinical research through the Pediatric Research Consortium at CHOP.
Glossary
- AAP
American Academy of Pediatrics
- ADHD
attention-deficit/hyperactivity disorder
- ADHD PGI
ADHD Preference and Goal Instrument
- BT
behavior therapy
- CHOP
The Children’s Hospital of Philadelphia
- CI
confidence interval
- OR
odds ratio
- SDM
shared decision-making
Footnotes
Dr Fiks conceptualized and designed the study, contributed to the analysis and interpretation of data, and drafted the manuscript; Ms Mayne contributed to the acquisition of data, the analysis and interpretation of data, and critically reviewed the manuscript; Ms DeBartolo contributed to the acquisition of data and critically reviewed the manuscript; and Drs Power and Guevara contributed to the conception and design of the study, analysis and interpretation of data, and critically reviewed the manuscript. All authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This research was supported by award K23HD059919 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777–779 [DOI] [PubMed] [Google Scholar]
- 2.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–692 [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine (U.S.) Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009 [Google Scholar]
- 4.The Patient Protection and Affordable Care Act. Public Law No:111-148, 124 Stat 1025 (2010)
- 5.Gravel K, Légaré F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) . Mental health in the United States. Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54(34):842–847 [PubMed] [Google Scholar]
- 7.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. Ambul Pediatr. 2007;7(suppl 1):82–90 [DOI] [PubMed] [Google Scholar]
- 8.The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD . A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–1086 [DOI] [PubMed] [Google Scholar]
- 9.Wolraich M, Brown L, Brown RT, et al. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Steering Committee on Quality Improvement and Management . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olfson M, Gameroff MJ, Marcus SC, Jensen PS. National trends in the treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160(6):1071–1077 [DOI] [PubMed] [Google Scholar]
- 11.Bussing R, Schoenberg NE, Perwien AR. Knowledge and information about ADHD: evidence of cultural differences among African-American and white parents. Soc Sci Med. 1998;46(7):919–928 [DOI] [PubMed] [Google Scholar]
- 12.LeFever GB, Dawson KV, Morrow AL. The extent of drug therapy for attention deficit-hyperactivity disorder among children in public schools. Am J Public Health. 1999;89(9):1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zito JM, Safer DJ, Zuckerman IH, Gardner JF, Soeken K. Effect of Medicaid eligibility category on racial disparities in the use of psychotropic medications among youths. Psychiatr Serv. 2005;56(2):157–163 [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Caring for Children With ADHD: A Resource Toolkit for Clinicians. 2nd ed. Elk Grove Village, IL: ; American Academy of Pediatrics; 2011 [Google Scholar]
- 15.Brinkman WB, Hartl J, Rawe LM, Sucharew H, Britto MT, Epstein JN. Physicians’ shared decision-making behaviors in attention-deficit/hyperactivity disorder care. Arch Pediatr Adolesc Med. 2011;165(11):1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiks AG, Hughes CC, Gafen A, Guevara JP, Barg FK. Contrasting parents’ and pediatricians’ perspectives on shared decision-making in ADHD. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiks AG, Mayne S, Hughes CC, et al. Development of an instrument to measure parents’ preferences and goals for the treatment of attention deficit-hyperactivity disorder. Acad Pediatr. 2012;12(5):445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegener D, Fabrigar L. Analysis and Design for Nonexperimental Data. Handbook of Research Methods in Social and Personality Psychology. New York, NY: Cambridge University Press; 2000:412–450 [Google Scholar]
- 19.Gorsuch R. Factor analysis. In: Shinka J, Velicer F, eds. Handbook of Psychology. Hoboken, NJ: John Wiley & Sons, Inc; 2003:143–164 [Google Scholar]
- 20.Browne M. An overview of analytic rotation in exploratory factor analysis. Multivariate Behav Res. 2001;36(1):111–150 [Google Scholar]
- 21.Fabiano GA, Pelham WE, Jr, Waschbusch DA, et al. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. J Clin Child Adolesc Psychol. 2006;35(3):369–385 [DOI] [PubMed] [Google Scholar]
- 22.Homer CJ, Horvitz L, Heinrich P, Forbes P, Lesneski C, Phillips J. Improving care for children with attention deficit hyperactivity disorder: assessing the impact of self-assessment and targeted training on practice performance. Ambul Pediatr. 2004;4(5):436–441 [DOI] [PubMed] [Google Scholar]
- 23.Krain AL, Kendall PC, Power TJ. The role of treatment acceptability in the initiation of treatment for ADHD. J Atten Disord. 2005;9(2):425–434 [DOI] [PubMed] [Google Scholar]
- 24.Bussing R, Koro-Ljungberg M, Noguchi K, Mason D, Mayerson G, Garvan CW. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corkum P, Rimer P, Schachar R. Parental knowledge of attention-deficit hyperactivity disorder and opinions of treatment options: impact on enrollment and adherence to a 12-month treatment trial. Can J Psychiatry. 1999;44(10):1043–1048 [DOI] [PubMed] [Google Scholar]
- 26.Bennett DS, Power TJ, Rostain AL, Carr DE. Parent acceptability and feasibility of ADHD interventions: assessment, correlates, and predictive validity. J Pediatr Psychol. 1996;21(5):643–657 [DOI] [PubMed] [Google Scholar]
- 27.Barkley RA, Shelton TL, Crosswait C, et al. Multi-method psycho-educational intervention for preschool children with disruptive behavior: preliminary results at post-treatment. J Child Psychol Psychiatry. 2000;41(3):319–332 [PubMed] [Google Scholar]
- 28.Pappadopulos E, Jensen PS, Chait AR, et al. Medication adherence in the MTA: saliva methylphenidate samples versus parent report and mediating effects of concomitant behaviors treatment. J Am Acad Child Adolesc Psychiatry. 2009;48(5):501−510 [DOI] [PubMed]
- 29.Olaniyan O, dosReis S, Garriett V, et al. Community perspectives of childhood behavioral problems and ADHD among African American parents. Ambul Pediatr. 2007;7(3):226–231 [DOI] [PubMed] [Google Scholar]
- 30.Davis CC, Claudius M, Palinkas LA, Wong JB, Leslie LK. Putting families in the center: family perspectives on decision making and ADHD and implications for ADHD care. J Atten Disord. 2012;16(8):675–684 [DOI] [PubMed] [Google Scholar]
- 31.Bussing R, Gary FA, Mills TL, Garvan CW. Parental explanatory models of ADHD: gender and cultural variations. Soc Psychiatry Psychiatr Epidemiol. 2003;38(10):563–575 [DOI] [PubMed] [Google Scholar]
- 32.Brinkman WB, Epstein JN. Treatment planning for children with attention-deficit/hyperactivity disorder: treatment utilization and family preferences. Patient Prefer Adherence. 2011;5:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Robin AL, Brenner S, Eastman J. Social acceptability of methylphenidate and behavior modification for treating attention deficit hyperactivity disorder. Pediatrics. 1991;88(3):560–565 [PubMed] [Google Scholar]
- 34.Johnston C, Fine S. Methods of evaluating methylphenidate in children with attention deficit hyperactivity disorder: acceptability, satisfaction, and compliance. J Pediatr Psychol. 1993;18(6):717–730 [DOI] [PubMed] [Google Scholar]
- 35.Singh I. Clinical implications of ethical concepts: moral self-understandings in children taking methylphenidate for ADHD. Clin Child Psychol Psychiatry. 2007;12(2):167–182 [DOI] [PubMed] [Google Scholar]
- 36.Hawley KM, Weisz JR. Child, parent, and therapist (dis)agreement on target problems in outpatient therapy: the therapist’s dilemma and its implications. J Consult Clin Psychol. 2003;71(1):62–70 [DOI] [PubMed] [Google Scholar]
- 37.Bowen J, Fenton T, Rappaport L. Stimulant medication and attention deficit-hyperactivity disorder. The child’s perspective. Am J Dis Child. 1991;145(3):291–295 [DOI] [PubMed] [Google Scholar]
- 38.Kendall J, Hatton D, Beckett A, Leo M. Children’s accounts of attention-deficit/hyperactivity disorder. ANS Adv Nurs Sci. 2003;26(2):114–130 [DOI] [PubMed] [Google Scholar]
- 39.Baxley GB, Turner PT, Greenwold WE. Hyperactive children’s knowledge and attitudes concerning drug treatment. J Pediatr Psychol. 1978;3(4):172–176 [Google Scholar]
- 40.Sleator EK, Ullmann RK, von Neumann A. How do hyperactive children feel about taking stimulants and will they tell the doctor? Clin Pediatr (Phila). 1982;21(8):474–479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.